Abstract
Although many scholars have recently studied the relationships between the pretreatment neutrophil-to-lymphocyte ratio (NLR) and prognosis in patients with small cell lung cancer (SCLC), the conclusions have been inconsistent. Accordingly, in this meta-analysis, we attempted to assess the clinicopathological and prognostic value of the pretreatment NLR in SCLC. Related literature was searched using PubMed, Embase, Cochrane Library, Web of Science, Chinese Biomedical Literature, China National Knowledge Infrastructure (CNKI), and Wanfang databases. Each eligible study was extracted, and a meta-analysis was performed using hazard ratios (HRs) and 95% confidence intervals (95% CIs) to assess the prognostic value of NLR. Evaluation of the clinicopathological significance of NLR in SCLC used odds ratios (ORs) and 95% confidence intervals (95% CIs). We included a total of 20 studies with 21 outcomes (5141 patients) in this meta-analysis. The results showed that high pretreatment NLR was closely related to poorer progression free survival (PFS) and overall survival (OS) (PFS, HR = 1.55, 95% CI = 1.27–1.88, P < 0.0001; I2 = 0%; OS, HR = 1.40, 95% CI = 1.26–1.55, P < 0.00001; I2 = 64%). In addition, pretreatment NLR was significantly associated with clinical stage of SCLC (OR = 2.14, 95% CI = 1.35–3.39, P = 0.001). Our meta-analysis showed that high levels of pretreatment NLR were significantly associated with a more serious clinical stage and poorer PFS and OS in SCLC.
Introduction
Small cell lung cancer (SCLC) is a highly malignant neuroendocrine tumor, accounting for 15–20% of lung cancer cases [1]. Compared with non-small cell lung cancer, SCLC exhibits more aggressive invasiveness, earlier distant metastasis, and poorer prognosis. Despite the continuous development of medical technology, the prognosis of SCLC is still not optimistic, and the median overall survival is often less than 6 months[2]. Thus, the identification of novel biomarkers for predicting prognosis is essential for improving long-term outcomes. Although some new biomarkers have been shown to be independent prognostic factors for SCLC[3, 4], most of these biomarkers are expensive and time-consuming to detect. Therefore, identification of inexpensive and simple biomarkers for SCLC may have important clinical implications.
In recent years, the association between systemic inflammation and tumors has become an essential research hotspot. Many studies have shown that inflammation is involved in all aspects of tumor development[5, 6]. The neutrophil-to-lymphocyte ratio (NLR) is a simple, widely available clinical indicator of inflammation and has been shown to be associated with the prognosis of a variety of malignant tumors[7–9]. Recently, many scholars have evaluated the relationships between pretreatment NLR and prognosis in patients with SCLC; however, the conclusions have been inconsistent.
Therefore, we conducted this meta-analysis in order to assess the clinicopathological and prognostic value of the pretreatment NLR in SCLC.
Materials and methods
Search strategies
We searched the PubMed, Embase, Cochrane Library, Web of Science, Chinese Biomedical Literature, China National Knowledge Infrastructure (CNKI), and Wanfang databases. The search was performed using a combination of the following technical terms: (“Neutrophil-lymphocyte ratio” OR NLR OR “Neutrophil lymphocyte ratio” OR “Neutrophil to lymphocyte ratio”) and (“Lung Cancer” OR “Lung Carcinoma” OR “Small Cell Lung Cancer” OR “Oat Cell Lung Cancer” OR “Small Cell Cancer Of The Lung” OR “Carcinoma, Small Cell Lung” OR “Oat Cell Carcinoma of Lung” OR "Small Cell Lung Carcinoma"). The search time limit was from the time of establishment of the database to February 1, 2020. There was no geographical restriction on the literature search; however, only Chinese and English studies were considered. Additionally, the references included in the literature were retrieved to avoid missing detection.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the subject was a patient who had been diagnosed with SCLC; (2) the pretreatment NLR value was obtained; (3) the purpose of the study was to explore the relationships between NLR and OS or PFS in SCLC; (4) the hazard ratio (HR) and 95% confidence interval (95% CI) were reported in the literature; and (5) the language of the document was English or Chinese.
The exclusion criteria were as follows: (1) case reports, letters, reviews, meta-analyses, and conference reports; (2) duplicate publications; and (3) unable to obtain the full text or data from the text.
Data extraction and quality assessment
Two authors independently used the Newcastle-Ottawa Scale (NOS) [10] to assess the quality of the studies. If the authors disagreed, the disagreement was resolved through discussion. The following data were extracted: first author, publication year, country, ethnicity, age, sample size, follow-up (months), median OS (months), cut-off value, clinical stage (limited stage or/and extensive stage)[11], type of survival analysis, and outcome. The study was considered to be of high quality when the NOS score was greater than or equal to 6.
Statistical analysis
The HR and 95% CI were pooled to assess the prognostic value of NLR for patients with SCLC. Evaluation of the clinicopathological significance of NLR in SCLC used odds ratios (ORs) and 95% confidence intervals (95% CIs). We used Cochran’s Q statistic test and then analyzed the heterogeneity between studies based on I2 and P values [12]. According to the Cochrane Handbook [13], when I2 was less than or equal to 50% and the P-value was greater than 0.10, the heterogeneity was acceptable. When the different studies included were not heterogeneous, they were combined using a fixed-effects model; otherwise, random-effects models were used [14], and subgroup analyses [15] and meta-regression [16] were used to discuss heterogeneity sources. When the combined HR was greater than 1, the survival rate was poor. If the 95% CI did not contain 1, the result was considered statistically significant. Sensitivity analysis was also needed to assess whether the results were stable. At the same time, quantitative analysis of publication bias was performed using Begg’s tests [17] and Egger’s tests [18], and if necessary, the trim and fill method [19] was used to quantitatively analyze publication bias. Results with P values of less than 0.05 were considered statistically significant. Stata 12.0 statistical analysis software (Stata Corporation, College Station, TX, USA) and Review Manager software (version 5.3; Cochrane Collaboration, London, UK) were used for all studies.
Results
Study search
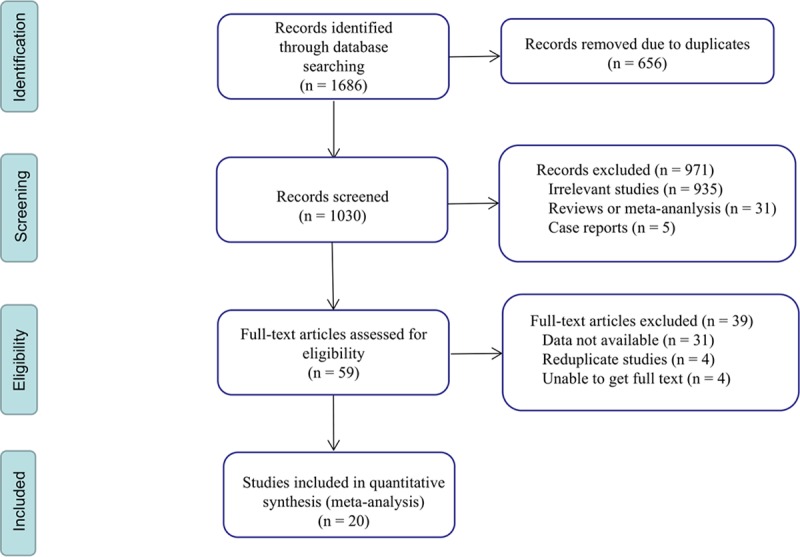
In total, 1686 studies were retrieved according to the search strategy. According to the inclusion and exclusion criteria, after reviewing duplicates and screening the titles or abstracts, 59 research articles were evaluated. Finally, 20 eligible studies were included [20–39], with 21 outcomes. The flow chart for the study is shown in Fig 1.
Fig 1. The flow chart of the study selection process.
Study characteristics
This meta-analysis included 5141 patients with SCLC (3461 men and 1680 women). The number of patients in each study ranged from 52 to 919, with a median of 172. The cut off value of NLRs ranged from 2.258 to 5.0, with a median of 3.70. The main characteristics of the incorporated literature are shown in Table 1.
Table 1. Characteristics of all included studies in the meta-analysis.
| First Author | Year | Country | Ethnicity | Age (range and median) |
Sample Size | Follow-up (months) | Median OS (months) |
Cut off | Clinical stage |
Survival analysis | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ju[20] | 2018 | China | Asian | NR | 154 | NR | NR | 3.70 | L+E | M | OS | 8 |
| Wang (1)[21] | 2017 | China | Asian | 31–83 | 172 | NR | 19.18 | 3.86 | L+E | M | OS | 7 |
| Huang[22] | 2016 | China | Asian | 31–88 | 112 | NR | NR | 4.50 | L+E | M | OS | 8 |
| Zhang[23] | 2017 | China | Asian | 59 (30–78) | 265 | NR | 16 | 4.0 | L+E | M | OS | 8 |
| Wang (2)[24] | 2016 | China | Asian | 62 (28–79) | 153 | NR | 23.3 | 3.20 | L+E | M | OS/PFS | 8 |
| Wang (3)[25] | 2017 | China | Asian | NR | 181 | NR | NR | 3.60 | L+E | M | OS | 8 |
| Bernhardt[26] | 2018 | Germany | Caucasian | 64 (37–93) | 350 | NR | 20 | 4.0 | L | U | OS | 7 |
| Murray[27] | 2014 | UK | Caucasian | 61.6 (38.3–77.4) |
52 | 26.1 | 21.1 | 5 | L | M | OS | 6 |
| Hong[28] | 2015 | China | Asian | 56 (16–84) | 919 | NR | 10.4 | 5 | L+E | M | OS | 8 |
| Sakin[29] | 2019 | Turkey | Caucasian | 61 (35–83) | 113 | 6 (1–33) | NR | 3 | E | M | OS | 8 |
| Suzuki {1}[30] | 2018 | USA | Caucasian | 63 | 252 | NR | 11.0 | 4.0 | E | M | OS | 8 |
| Wang (4)[31] | 2014 | China | Asian | NR | 114 | NR | 14 | 3 | L+E | M | OS | 8 |
| Xie ①[32] | 2015 | China | Asian | 68 (27–91) | 555 | 10.8 | NR | 5 | E | M | OS | 8 |
| Xie ②[32] | 2015 | China | Asian | 68 (27–91) | 383 | 10.8 | NR | 5 | L | M | OS | 8 |
| Suzuki {2}[33] | 2018 | USA | Caucasian | 65 | 122 | NR | 16.6 | 2.9 | L | M | OS | 7 |
| Käsmann[34] | 2017 | Germany | Caucasian | NR | 65 | NR | 20 | 4.0 | L | M | OS | 8 |
| Deng[35] | 2017 | China | Asian | 58 (24–81) | 320 | 39.1 | 13.8 | 2.65 | L+E | M | OS/PFS | 8 |
| Lohinai[36] | 2019 | Hungary | Caucasian | 58 | 155 | NR | NR | 2.258 | L+E | M | OS | 7 |
| Wang (5)[37] | 2019 | China | Asian | 58(39–71) | 228 | 46 | 20 | 2.3 | L+E | M | OS/PFS | 8 |
| Li[38] | 2019 | China | Asian | NR | 160 | NR | NR | 2.32 | L+E | M | OS | 7 |
| Liu[39] | 2019 | China | Asian | 59 | 316 | NR | 11 | 2.68 | L+E | M | OS | 8 |
(1)–(5): different authors and different studies; {1}–{2}: the same author but different studies; ①–②: the same author and the same study; L: limited stage; E: extensive stage; M: multivariate; U: univariate; NR: not reported; NOS: Newcastle-Ottawa scale
The prognostic value of the pretreatment NLR in SCLC
According to the included literature, 20 studies provided data on OS related to pretreatment NLR in patients with SCLC. As shown in Fig 2, a high pretreatment NLR was closely related to poor OS (HR = 1.40, 95% CI = 1.26–1.55, P < 0.00001; I2 = 64%; Fig 2). In the heterogeneity analysis, significant heterogeneity was observed among the included studies. Therefore, subgroup and meta-regression analyses were required to explore the source of heterogeneity.
Fig 2. Forest plot of HR for the association of pretreatment NLR in patients with SCLC.
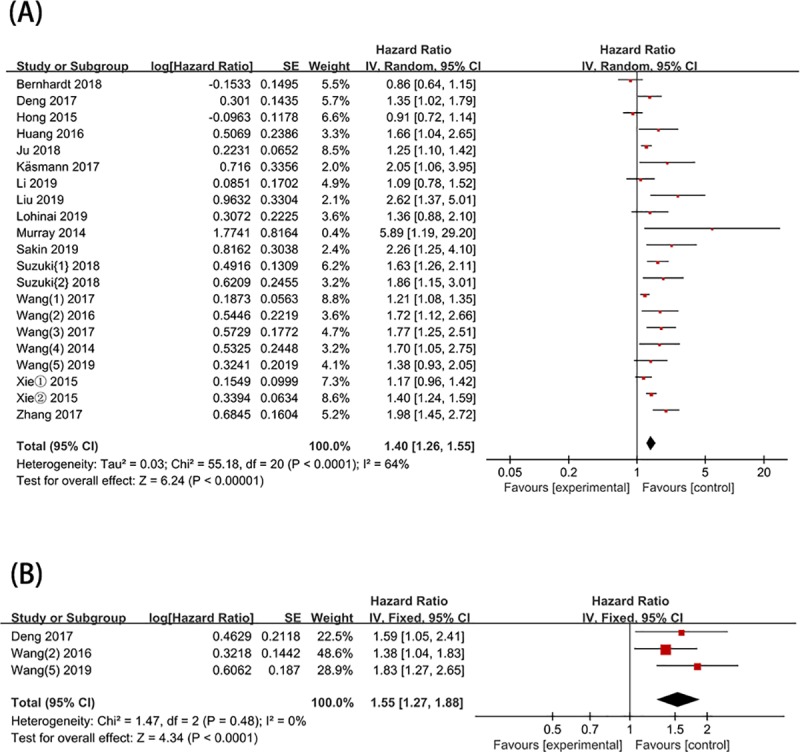
(A) OS; (B) PFS.
Data on PFS related to pretreatment NLR in patients with SCLC were provided by 3 studies. The pooled result showed that high pretreatment NLR was closely related to PFS (HR = 1.55, 95% CI = 1.27–1.88, P < 0.0001; I2 = 0%) (Fig 2).
The pretreatment NLR and clinicopathological features of SCLC
As shown in Table 2, the pretreatment NLR was significantly associated with clinical stage of SCLC (OR = 2.14, 95% CI = 1.35–3.39, P = 0.001). However, significant association between the pretreatment NLR and SCLC was not found in sex, age, and smoking.
Table 2. The association between NLR and clinicopathological features of SCLC.
| Variables | Studies | OR [95% CI] | P value | Heterogeneity | Model | |
|---|---|---|---|---|---|---|
| I2 (%) | P2 value | |||||
| (1) Sex (Male vs. Female) | 9 | 0.75 [0.50–1.15] | 0.19 | 63 | 0.006 | random |
| (2) Age (≥ 60 vs. < 60) | 4 | 0.81 [0.38–1.73] | 0.58 | 80 | 0.002 | random |
| (3) Clinical stage (E vs. L) | 7 | 2.14 [1.35–3.39] | 0.001 | 67 | 0.005 | random |
| (4) Smoking (Yes vs. No) | 6 | 0.98 [0.63–1.51] | 0.92 | 53 | 0.06 | random |
L:limited stage; E:extensive stage
Subgroup and meta-regression analyses
To elucidate the source of heterogeneity between studies, we performed subgroup analyses by clinical stage, ethnicity, cut-off values for NLR, and sample size (Table 3). Through subgroup analysis, the prognostic role of pretreated NLR in OS did not change significantly (Table 3), and significant heterogeneity remained between most subgroups. We used meta-regression analysis for quantitative analysis. The results of the univariate analysis revealed that sample size (p3 = 0.017) partly explained the source of heterogeneity (Table 3). Multivariate analysis (p3 = 0.031) also showed that the sample size may be the main source of heterogeneity (Table 3).
Table 3. Subgroup and meta-regression analyses between NLR and OS.
| Variables | Number of outcomes | HR [95% CI] | P value | Heterogeneity | Model | P3-value of Meta-regression | ||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P2 value | Univariate | Multivariate | |||||
| (1) Ethnicity | 0.329 | 0.762 | ||||||
| Asian | 14 | 1.35 [1.22–1.51] | < 0.00001 | 61 | 0.001 | random | ||
| Caucasian | 7 | 1.40 [1.16–2.21] | 0.005 | 71 | 0.002 | random | ||
| (2) Cut-off value | 0.659 | 0.476 | ||||||
| < 4.0 | 12 | 1.30 [1.21–1.39] | < 0.00001 | 40 | 0.08 | fixed | ||
| ≥ 4.0 | 9 | 1.36 [1.11–1.67] | 0.003 | 78 | < 0.0001 | random | ||
| (3) Sample size | 0.017 | 0.031 | ||||||
| N < 150 | 6 | 1.89 [1.50–2.38] | < 0.00001 | 0 | 0.73 | fixed | ||
| 150 < N ≤ 200 | 6 | 1.26 [1.16–1.36] | < 0.00001 | 30 | 0.21 | fixed | ||
| N > 200 | 9 | 1.34 [1.11–1.59] | 0.002 | 77 | < 0.0001 | random | ||
| (4) Clinical stage | 0.563 | 0.925 | ||||||
| L | 5 | 1.47 [1.03–2.10] | 0.04 | 75 | 0.003 | random | ||
| E | 3 | 1.51 [1.08–2.10] | 0.02 | 72 | 0.03 | random | ||
| L + E | 13 | 1.38 [1.22–1.57] | < 0.00001 | 61 | 0.002 | random | ||
L:limited stage; E:extensive stage
Sensitivity analysis
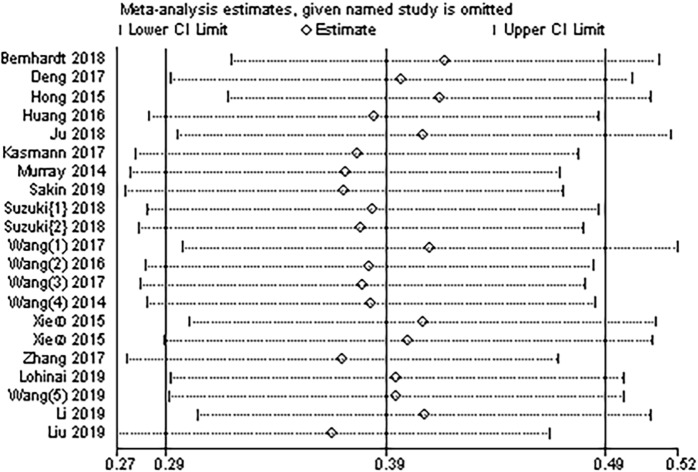
We conducted a sensitivity analysis to assess whether individual studies affected the overall analysis. Our results showed that any of the studies could be removed, and the remaining HRs of the combined studies remained within the 95% CI of the combined HR in the meta-analysis (Fig 3). These findings indicated that the meta-analysis had good stability.
Fig 3. Sensitivity analysis of the relationship between pretreatment NLR and OS in patients with SCLC.
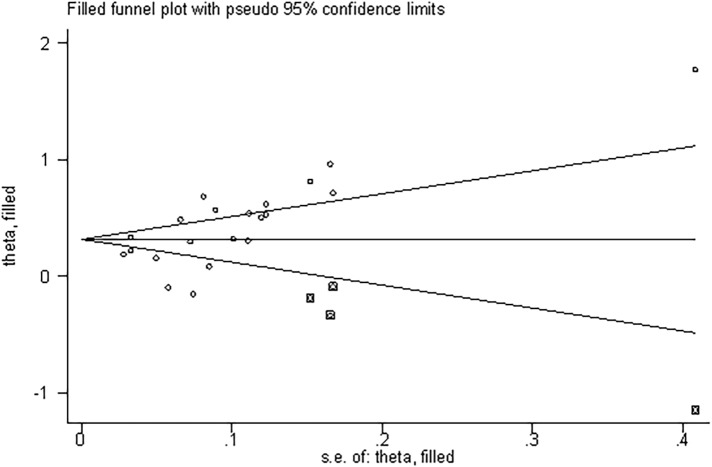
Publication bias and the trim and fill method
Quantitative analysis of publication bias was performed using the Begg method and the Egger method. The results showed the presence of a significant publication bias (Begg’s test: P = 0.015; Egger’s test: P = 0.015; Fig 4). Therefore, we used the trim and fill method to estimate the asymmetry in the funnel plot. After filling four unpublished studies by calculation, the funnel plot was symmetrical (S1 Text). No statistically significant change was observed in the results (Fig 5).
Fig 4. Funnel plot for analysis of publication bias.
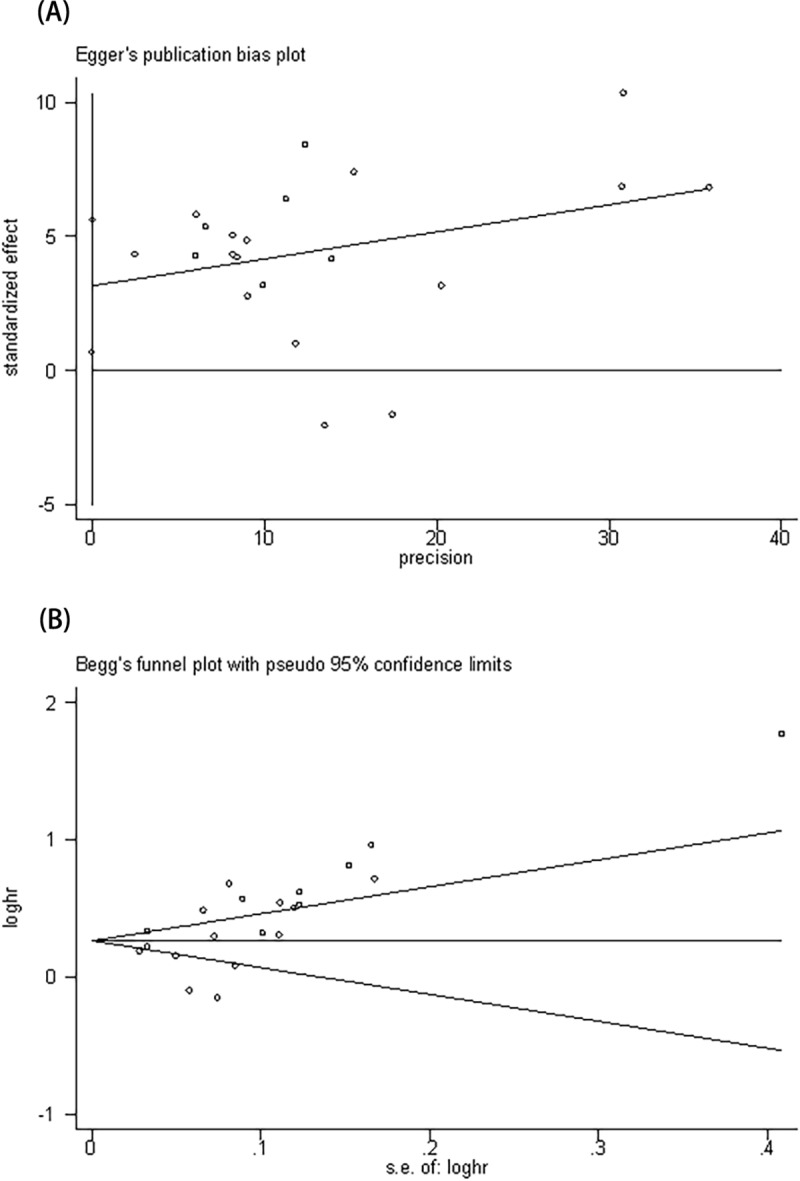
(A) Funnel plot developed using the Egger method; (B) funnel plot using the Begg method.
Fig 5. Funnel plot adjusted by the trim and fill method.
Discussion
The tumor-associated inflammatory response is a potential prognostic indicator prior to treatment, manifesting as peripheral blood neutrophilia and relative lymphopenia [40]. Pretreatment NLR is readily available to assess the prognosis of patients with SCLC without high costs, allowing patients and physicians to make informed decisions before clinical treatment.
By pathological typing, lung cancer can be divided into small cell lung cancer and non-small cell cancer. Small cell lung cancer is a separate category, which has special biological behavior and clinical characteristics. Previous meta-analyses[41, 42] have focused on the relationships between NLR and prognosis in patients with non-small cell lung cancer. Additionally, the prior meta-analyses of SCLC [43, 44] only included two studies, which have not explored the clinicopathological value of NLR in SCLC. In our current meta-analysis, the conclusions presented herein identified 16 studies with 17 outcomes, most of which were published in the last two years and showed pretreatment NLR could provide a clinical reference for predicting prognosis in patients with SCLC.
The meta-analysis assessed the clinicopathological and prognostic value of the pretreatment NLR in SCLC. The results suggested that pretreatment NLR levels were inversely proportional to PFS and OS (PFS, HR = 1.55, 95% CI = 1.27–1.88, P < 0.0001; I2 = 0%; OS, HR = 1.40, 95% CI = 1.26–1.55, P < 0.00001; I2 = 64%). However, although the pooled result of PFS with only includes two studies has no apparent heterogeneity, the result requires larger sample studies to validate. At the same time, the Pooling analysis found a significant association between pretreatment NLR and clinical stage. High pretreatment NLR is a risk factor for extensive-stage small cell lung cancer.
However, this meta-analysis also had some limitations. First, significant heterogeneity was observed. The main source of heterogeneity by meta-regression analysis was the sample size. Subgroup and sensitivity analyses were used to confirm that the results of this meta-analysis were stable. Second, the papers showed publication bias, and affirmative results are easier to publish. Importantly, however, after correction with the trim and fill method, there were no changes in the prognostic value of pretreatment NLR in patients with SCLC. Finally, the clinical data between pretreatment NLR and PFS with SCLC is relatively small. Therefore, more large-scale prospective studies are needed.
Conclusion
The meta-analysis showed that high pretreatment NLR was a risk factor for extensive-stage small cell lung cancer. Moreover, high levels of pretreatment NLR were significantly associated with reduced PFS and OS in patients with SCLC and that NLR was readily available and less costly, suggesting that NLR could be used as a biomarker for the prognosis of SCLC.
Supporting information
(DOC)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015; 10(9): 1240–2. 10.1097/jto.0000000000000663 . [DOI] [PubMed] [Google Scholar]
- 2.Oronsky B, Reid TR, Oronsky A, Carter CA. What's New in SCLC? A Review. Neoplasia (New York, NY). 2017; 19(10): 842–7. 10.1016/j.neo.2017.07.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sodja E, Rijavec M, Koren A, Sadikov A, Korosec P, Cufer T. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiology and oncology. 2016; 50(2): 188–96. 10.1515/raon-2015-0027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salgia R, Weaver RW, McCleod M, Stille JR, Yan SB, Roberson S, et al. Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: exploratory analysis of a phase II study. Investigational new drugs. 2017; 35(3): 334–44. 10.1007/s10637-017-0446-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010; 140(6): 883–99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England). 2001; 357(9255): 539–45. 10.1016/s0140-6736(00)04046-0 . [DOI] [PubMed] [Google Scholar]
- 7.Kiriu T, Yamamoto M. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PloS one. 2018; 13(2): e0193018 10.1371/journal.pone.0193018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirican A, Kucukzeybek BB, Alacacioglu A, Kucukzeybek Y, Erten C, Varol U, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? International journal of clinical oncology. 2015; 20(1): 70–81. 10.1007/s10147-014-0672-8 . [DOI] [PubMed] [Google Scholar]
- 9.Haruma T, Nakamura K, Nishida T, Ogawa C, Kusumoto T, Seki N, et al. Pre-treatment neutrophil to lymphocyte ratio is a predictor of prognosis in endometrial cancer. Anticancer research. 2015; 35(1): 337–43. . [PubMed] [Google Scholar]
- 10.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp2009.
- 11.Zhao H, Ren D, Liu H, Chen J. Comparison and discussion of the treatment guidelines for small cell lung cancer. 2018; 9(7): 769–74. 10.1111/1759-7714.12765 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genetic epidemiology. 2005; 28(2): 123–37. 10.1002/gepi.20048 . [DOI] [PubMed] [Google Scholar]
- 13.Higgins J, Green S. Cochrane Handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] London, United Kingdom: The Cochrane Collaboration; 2011. Available from: https://training.cochrane.org/handbook. [Google Scholar]
- 14.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research synthesis methods. 2010; 1(2): 97–111. 10.1002/jrsm.12 . [DOI] [PubMed] [Google Scholar]
- 15.Alosh M, Huque MF. Multiplicity considerations for subgroup analysis subject to consistency constraint. Biometrical journal Biometrische Zeitschrift. 2013; 55(3): 444–62. 10.1002/bimj.201200065 . [DOI] [PubMed] [Google Scholar]
- 16.Harbord RM, Higgins JP. Meta-regression in Stata. The Stata Journal. 2008; 8(4): 493–519. [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50(4): 1088–101. . [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997; 315(7109): 629–34. 10.1136/bmj.315.7109.629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56(2): 455–63. 10.1111/j.0006-341x.2000.00455.x . [DOI] [PubMed] [Google Scholar]
- 20.Ju S, Xu A. Prognostic impact of neutrophil lymphocyte ratio and platelet lymphocyte ratio on patients with small cell lung cancer Journal of Clinical Pulmonary Medicine. 2018; 23(12): 2251–5. [Google Scholar]
- 21.Wang L, Lv X. Evaluation of neutrophil lymphocyte ratio and platelet lymphocyte rate in peripheral blood to predict prognosis of small cell lung cancer patients. Journal of Clinical Pulmonary Medicine. 2017; 22(06): 983–7. [Google Scholar]
- 22.Huang Y, Liu D, Li W, Zhang L, Li L. Analysis of relation of neutrophil lymphocyte ratio and Prognosis of small cell lung cancer patients. Med J West China. 2016; 28(01): 109–12. [Google Scholar]
- 23.Zhang X. Analysis of Prognosis Factors in small cell lung cancer: North China University of Science and Technology; 2017. [Google Scholar]
- 24.Wang X. Pretreatment Neutrophil-to- lymphocyte Ratio as a survival predictor for small cell lung cancer: University of Jinan; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zhi X. The prognostic value of neutrophil/lymphocyte and platelet/lymphocyte ratios in small cell lung cancer patients. Chinese Journal of the Frontiers of Medical Science(Electronic Version) 2017; 9(05): 106–10. [Google Scholar]
- 26.Bernhardt D, Aufderstrasse S, Konig L, Adeberg S, Bozorgmehr F, Christopoulos P, et al. Impact of inflammatory markers on survival in patients with limited disease small-cell lung cancer undergoing chemoradiotherapy. Cancer management and research. 2018; 10: 6563–9. 10.2147/CMAR.S180990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray LJ, Young C, Owen LC, Clarke KL, Snee M, Turner RN, et al. Potential prognostic role for the neutrophil to lymphocyte ratio in patients receiving concurrent chemo-radiotherapy for small cell lung carcinoma. Lung Cancer. 2014; 83. [Google Scholar]
- 28.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. The Tohoku journal of experimental medicine. 2015; 236(4): 297–304. 10.1620/tjem.236.297 . [DOI] [PubMed] [Google Scholar]
- 29.Sakin A, Sahin S, Yasar N, Demir C, Arici S, Geredeli C, et al. The Relation between Hemogram Parameters and Survival in Extensive-Stage Small Cell Lung Cancer. Oncology research and treatment. 2019: 1–9. 10.1159/000502425 . [DOI] [PubMed] [Google Scholar]
- 30.Suzuki R, Lin SH, Wei X, Allen PK, Welsh JW, Byers LA, et al. Prognostic significance of pretreatment total lymphocyte count and neutrophil-to-lymphocyte ratio in extensive-stage small-cell lung cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2018; 126(3): 499–505. 10.1016/j.radonc.2017.12.030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Jiang R, Li K. Prognostic significance of pretreatment laboratory parameters in combined small-cell lung cancer. Cell biochemistry and biophysics. 2014; 69(3): 633–40. 10.1007/s12013-014-9845-3 . [DOI] [PubMed] [Google Scholar]
- 32.Xie D, Marks R, Zhang M, Jiang G, Jatoi A, Garces YI, et al. Nomograms Predict Overall Survival for Patients with Small-Cell Lung Cancer Incorporating Pretreatment Peripheral Blood Markers. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2015; 10(8): 1213–20. 10.1097/jto.0000000000000585 . [DOI] [PubMed] [Google Scholar]
- 33.Suzuki R, Wei X, Allen PK, Cox JD, Komaki R, Lin SH. Prognostic Significance of Total Lymphocyte Count, Neutrophil-to-lymphocyte Ratio, and Platelet-to-lymphocyte Ratio in Limited-stage Small-cell Lung Cancer. Clinical lung cancer. 2019; 20(2): 117–23. 10.1016/j.cllc.2018.11.013 . [DOI] [PubMed] [Google Scholar]
- 34.Kasmann L, Bolm L, Schild SE, Janssen S, Rades D. Neutrophil-to-Lymphocyte Ratio Predicts Outcome in Limited Disease Small-cell Lung Cancer. Lung. 2017; 195(2): 217–24. 10.1007/s00408-017-9976-6 . [DOI] [PubMed] [Google Scholar]
- 35.Deng M, Ma X, Liang X, Zhu C, Wang M. Are pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio useful in predicting the outcomes of patients with small-cell lung cancer? Oncotarget. 2017; 8(23): 37200–7. 10.18632/oncotarget.16553 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohinai Z, Bonanno L, Aksarin A, Pavan A, Megyesfalvi Z, Santa B, et al. Neutrophil-lymphocyte ratio is prognostic in early stage resected small-cell lung cancer. PeerJ. 2019; 7: e7232 10.7717/peerj.7232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D, Guo D, Shi F, Zhu Y, Li A, Kong L, et al. The predictive effect of the systemic immune-inflammation index for patients with small-cell lung cancer. Future oncology (London, England). 2019; 15(29): 3367–79. 10.2217/fon-2019-0288 . [DOI] [PubMed] [Google Scholar]
- 38.Li C. Correlations between neutrophil to lymphocyte ratio and prognosis in patients with small cell lung cancer: University of Nanchang; 2019. [Google Scholar]
- 39.Liu Q, Yang W. Relationship between systemic immune-inflammation index and prognosis of patients with brain metastases from small cell lung cancer. Tumor. 2019; 39(8): 649–57. 10.3781/j.issn.1000-7431.2019.33.831 [DOI] [Google Scholar]
- 40.Bonomi M, Patsias A, Posner M, Sikora A. The role of inflammation in head and neck cancer. Advances in experimental medicine and biology. 2014; 816: 107–27. 10.1007/978-3-0348-0837-8_5 . [DOI] [PubMed] [Google Scholar]
- 41.Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Scientific reports. 2015; 5: 12493 10.1038/srep12493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Zhan P, Lv Y, Shen K, Wei Y, Liu H, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Translational lung cancer research. 2019; 8(3): 214–26. 10.21037/tlcr.2019.06.10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: A meta-analysis of 7,219 patients. Molecular and clinical oncology. 2017; 7(3): 498–506. 10.3892/mco.2017.1342 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Y, Wang J, Wang X, Gu L, Pei H, Kuai S, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo, Brazil). 2015; 70(7): 524–30. 10.6061/clinics/2015(07)10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.