Abstract
The dynamic interplay between neoplastic cells and the immune microenvironment regulates every step of the metastatic process. Immune cells contribute to invasion by secreting a cornucopia of inflammatory factors that promote epithelial-to-mesenchymal transition and remodeling of the stroma. Cancer cells then intravasate to the circulatory system assisted by macrophages and use several pathways to avoid recognition by cytotoxtic lymphocytes and phagocytes. Circulating tumor cells that manage to adhere to the vasculature and encounter premetastic niches are able to use the associated myeloid cells to extravasate into ectopic organs and establish a dormant microscopic colony. If successful at avoiding repetitive immune attack, dormant cells can subsequently grow into overt, clinically detectable metastatic lesions, which ultimately account to most cancer-related deaths. Understanding how disseminated tumor cells evade and corrupt the immune system during the final stages of metastasis will be pivotal in developing new therapeutic modalities that combat metastasis.
Metastasis is a multistep process characterized by the spread of malignant cells to distant organs. This is evolutionary driven by genetic and/or epigenetic alteration within cancer cells but is also dependent on an intricate interaction with stromal cells at a local and systemic level (Fig. 1). Tumor cells must overcome several hurdles to establish macroscopic metastasis, collectively referred to as the invasion–metastatic cascade (Valastyan and Weinberg 2011). During this process, carcinoma cells deviate from their primary growth site (local invasion and intravasation), disseminate systemically via circulation through the lymphatic or vascular system (survival, arrest at distant site, extravasation), and survive and adjust to a new microenvironment (colonization and outgrowth) (Lambert et al. 2017). For cancer cells to migrate through tissue, they acquire several abilities that act in concert to promote invasion, including cytoskeletal reorganization, secretion of proteases, and altered adhesion receptor-ligand interactions (Kessenbrock et al. 2010; Quail and Joyce 2013). In addition, cancer cells can undergo a reversible program termed epithelial to mesenchymal transition (EMT) through which loss of epithelial polarization and intercellular adhesion allows for motility and invasiveness (Thiery et al. 2009). Neoplastic cells leaving the primary tumor and intravasating into circulation must then survive severe physical shear stress, attack by the immune system, and oxidative stress (Labelle et al. 2011; Le Gal et al. 2015). Once cells arrest at a distant site or are physically trapped in minute capillaries, they can proliferate within the intravascular space or extravasate by modifying the endothelium (Al-Mehdi et al. 2000; Deneve et al. 2013; Strilic and Offermanns 2017). Only a small fraction of cells that disseminate from the primary tumor will manage to invade and adapt to their new surrounding (Nagrath et al. 2007). Thus, the ability of neoplastic cells to co-opt immune processes to aid in these steps and evade immune recognition are necessary for successful metastasis (Sosa et al. 2014; Massagué and Obenauf 2016).
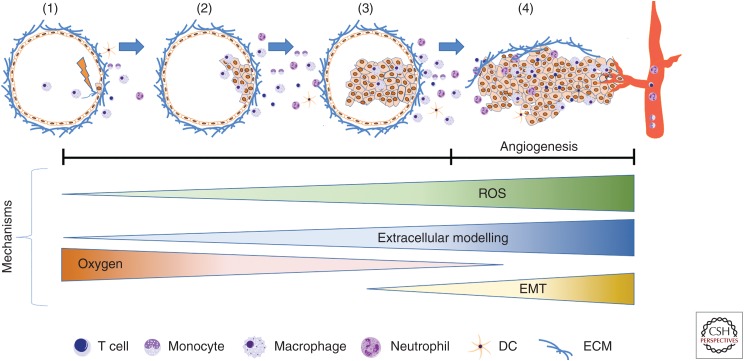
Figure 1.
Establishment of the tumor immune microenvironment: Environmental insults or spontaneous genetic alterations in epithelial cells can lead to immune surveillance (1) or the growth of premalignant cells that evade clearance by innate and adaptive immune cells (2). In addition to driving cell proliferation, certain oncogenic alterations are associated with the release of cytokines that recruit additional immune cells to aid neoplastic progression by providing mitogenic factors or producing reactive oxygen species that result in the accumulation of additional genetic mutations (3). Uncontrolled cellular growth at this stage causes environmental changes such as reduced oxygen levels (hypoxia), extracellular matrix (ECM) turnover, and stromal disruption (4). These signals of tissue damage result in a pathological healing response that includes the creation of a disordered and permeable vascular network (angiogenic switch), as well as morphological and transcriptional changes in epithelial cells that reflect a mesenchymal phenotype (EMT). Malignant cells are thus able to migrate toward and intravasate into the vascular lumen, becoming circulating tumor cells.
ESTABLISHMENT OF THE TUMOR IMMUNE MICROENVIRONMENT
The tumor immune microenvironment (TIME) promotes every aspect of carcinogenesis, including initiation, survival, growth, metastasis, and immune evasion. Depending on the tissue of origin, the TIME shows a unique immune repertoire, with different proportions and functional states of lymphocytes, granulocytes, monocytes/macrophages, and dendritic cells (DCs) (Gentles et al. 2015). Although several of these immune populations have the potential to eradicate malignant cells, numerous factors in the tumor act to blunt this activity or redirect the cells toward promoting tumorigenesis. In addition to relative proportions, the locations of immune cells are associated with disease progression. For example, sites of focal myoepithelial cell layer dysregulation in ductal carcinoma in situ (DCIS) display an enrichment of leukocytes, suggesting a role in driving or sustaining local invasion (Gil Del Alcazar et al. 2017), whereas in late stage disease the exclusion of T lymphocytes from tumor beds promotes resistance to immune checkpoint blockade (Jayaprakash et al. 2018; Jerby-Arnon et al. 2018). The degree to which parallel programs are used to establish the metastatic immune microenvironment (MIME) are largely unknown, with the exception of myeloid cell recruitment to establish the premetastatic niche (Liu and Cao 2016).
Tumor Intrinsic Signaling
In addition to the type of cancer, immune composition correlates with the underlying genetic alterations driving carcinogenesis (Rooney et al. 2015), as oncogenic signals in transformed cells are associated with the release of chemokines that attract immune cells to the tumor (Wellenstein and de Visser 2018). As one of the most frequently mutated genes in cancer, loss of Trp53 activates the NF-κB pathway, stimulates cytokines release from cancer cells, which through paracrine interactions modify the immune landscape of cancer (Meylan et al. 2009; Kastenhuber and Lowe 2017). Similarly, the activation of the transcription factor MYC endows cancer cells with the ability to overproduce potent proinflammatory cytokines that facilitate tumor angiogenesis and recruitment of protumoral mast cells, macrophages, and neutrophils (Soucek et al. 2007), whereas activation of the oncogenic EGFR family of receptor tyrosine kinases results in secretion of CSF-1, a chemotactic and survival factor for macrophages. Interestingly, oncogenic KrasG12D drives unique programs in different cancer types, although each cumulates in the recruitment of an immunosuppressive myeloid population. This includes expression of GM-CSF and CXCL3 in pancreatic cancer and colon carcinoma, respectively (Pylayeva-Gupta et al. 2012; Liao et al. 2019), as well as CCL9 expression in lung cancer in the context of Myc activation (Kortlever et al. 2017). Oncogenic Kras and Myc also cooperate to induce interleukin (IL)-23-dependent exclusion of lymphocytes (Kortlever et al. 2017). Collectively, these data establish an important role for tumor genotype in driving the immune repertoire in cancer.
There are also several studies showing that cells undergoing EMT modify the TIME through chemokine secretion (Dongre and Weinberg 2019). The EMT master regulator SNAIL promotes expression of CCL2 and CCL5, leading to macrophage recruitment (Hsu et al. 2014), and expression of CXCR2 ligands leading to recruitment of suppressive myeloid cells (Taki et al. 2018). Other mediators of epithelial mesenchymal transition, such as SRC-1, promote macrophage recruitment via expression of CSF-1 (Qin et al. 2009; Wang et al. 2009), and mesenchymal tumors display prominent macrophage infiltration (Dongre et al. 2017). The ability of EMT to promote an immunosuppressive environment is likely further augmented by high production of transforming growth factor (TGF)-β, which can lead to the exclusion of lymphocytes from tumor beds (Mariathasan et al. 2018; Tauriello et al. 2018) and regulate the activation state of most immune cells. This has been shown in one study with overexpression of SNAIL, which leads to production of TGF-β and thrombospondin-1, and increased conversion of regulatory T cells (Tregs) (Kudo-Saito et al. 2009). Given the critical role of EMT in the metastatic process it will be important to determine if these pathways are also involved in establishing the MIME.
Homeostatic Imbalance
Solid tumors show large regions with low oxygen levels (hypoxia) and nutrient insufficiency (Gatenby and Gillies 2004). Hypoxia has the ability to modulate immune functions directly by activating HIF-1α-dependent transcriptional changes within the immune cells, or indirectly by rewiring tumor cell secreted metabolites and cytokine repertoire (Cairns et al. 2007). For example, hypoxia was shown to trigger HIF-1α-dependent immunosuppressive activity in macrophages (Doedens et al. 2010). Through indirect effects on the tumor and the stroma, hypoxia also causes the overproduction of monocyte recruitment factors including CCL2, CCL5, CXC-chemokine ligand 12 (CXCL12), colony-stimulating factor (CSF-1), and vascular endothelial growth factor (VEGF) (Murdoch et al. 2004). As an indirect consequence of hypoxia, tumor cells up-regulate glycolysis and secrete lactic acid, which induces phenotypic changes and immunosuppressive function in macrophages (Colegio et al. 2014). Lactate-activated macrophages in turn enhance the glycolytic phenotype of breast cancer cells through the release of extracellular vesicles containing a HIF-1α-stabilizing long noncoding RNA (Chen et al. 2019). The acidic microenvironment of tumors also directly regulates macrophage phenotype, as seen following the genetic ablation of the macrophage pH sensor, Gpr132, or the buffering of tumor secreted acids, which reduces the spontaneous lung metastasis of breast cancer cells and prostate cancer invasiveness, respectively (Chen et al. 2017a; El-Kenawi et al. 2019). Finally, areas of hypoxia frequently coincide with regions of cell death, leading to recognition of apoptotic cells by phagocytes and/or the release of damage-associated molecular patterns (DAMPs) during secondary necrosis. Phosphatidylserine recognition receptors thus promote myeloid-dependent immune suppression in the TIME (Cook et al. 2013; Crittenden et al. 2016; Ubil et al. 2018); whereas several DAMPs have been shown to activate DCs and elicit a cytotoxic T-cell response (Kroemer et al. 2013).
Immune-Stroma Crosstalk
In addition to regulating the immune compartment directly, tumor cells drive phenotypic changes in the nonimmune stromal cells, including fibroblasts, endothelial cells, and pericytes. This “reactive” stroma possesses a unique gene/protein expression profile that differs from the signature of the corresponding normal tissue (Finak et al. 2008; Nonn et al. 2009) and is created, in part, by the influence of inflammatory mediators and cytokines. For example, TGF-β cooperates with SDF-1 (CXCL12) to generate myofibroblasts with enhanced tumor-promoting capability through autocrine signaling loops (Kojima et al. 2010). Cancer-associated fibroblasts can suppress a T-cell response through a myriad of pathways, including direct suppression via PD-L2 and FAS ligand expression (Lakins et al. 2018), expression of cytokines (Kato et al. 2018), and recruitment of immunosuppressive myeloid cells (Kumar et al. 2017). Cancer cells can even create gap junctions with astrocytes to transfer the second messenger cGAMP to astrocytes, activating the STING pathway and production of type I interferons (Chen et al. 2016). It should also be noted that changes in the physical properties of the stroma further modulate the TIME (DeNardo and Ruffell 2019). For example, higher levels of collagen (Wesley et al. 1998; Stahl et al. 2013; Pickup et al. 2014) and collagen cross-linking (Van Goethem et al. 2010; McWhorter et al. 2015) enhance the protumoral phenotype of macrophages, and inhibiting the hyperactivation of focal adhesion kinase (FAK) is sufficient to alter macrophage polarization and increase response to immune checkpoint blockade (Jiang et al. 2016).
There is also an elaborate interplay between immune cells in the tumor that controls the phenotype and functional role of the cells. Most well established is the ability of macrophages, monocytes, neutrophils, and other myeloid populations to suppress the cytotoxic function of lymphocytes, through mechanisms that have been reviewed elsewhere (Veglia et al. 2018; DeNardo and Ruffell 2019). As will be discussed, myeloid cells are a critical component of the immunosuppressive TIME and MIME, and depleting these cells can reduce metastasis by unleashing T and natural killer (NK) cell cytotoxicity. Similarly, Tregs promote pulmonary metastasis by blocking NK cell function (Olkhanud et al. 2009), with Treg conversion occurring through the activity of Bregs (Olkhanud et al. 2011). T and B lymphocytes can also promote the protumorigenic properties of macrophages (Gunderson and Coussens 2013), including IL-4-producing CD4+ T cells enhancing the ability of macrophages to foster invasion (DeNardo et al. 2009).
INVASION AND INTRAVASATION
Cancer cells must acquire phenotypic changes that endow them with ability to invade surrounding stroma as a precursor to intravasation. These invasive characteristics include alterations in expression of adhesion molecules and expression of proteases to degrade the extracellular matrix (ECM). Each of these characteristics is fuelled by the presence of immune cells and can potentially cross-regulate each other. For example, EMT leads to the recruitment of macrophages, which can secrete and activate TGF-β, further supporting the induction of EMT (Dongre and Weinberg 2019). Similar feedback loops are required for intravasation, with tumor cells stimulating the chemotactic and angiogenic properties of macrophages (Lewis et al. 2016). Interestingly, recent studies have shown that cells egressing the primary site early during cancer progression are more metastasis-efficient than cells leaving the tumor at later stages (Hüsemann et al. 2008; Ghajar and Bissell 2016; Harper et al. 2016; Hosseini et al. 2016). The metastatic competency of early disseminated cells—which would harbor fewer mutations—reiterates that microenvironmental factors contributes to the acquisition of metastatic traits (Hendry et al. 2017; Linde et al. 2018). In support of this, although the molecular profiles of invasive breast cancer and preinvasive was indistinguishable, aggressive lesions displayed high infiltration of macrophages and Tregs (Abba et al. 2015; Nelson et al. 2018).
Epithelial-to-Mesenchymal Transition
An established TIME provides several cytokines and chemokines with the potential to activate latent EMT programming in epithelial cells, causing decreased E-cadherin expression, loss of cell–cell junction and, morphological changes that enable cells to invade and migrate to distant sites (Dongre and Weinberg 2019). This can be mediated through cooperation with the TGF-β signaling pathway, one of the primary induces of EMT (Lu et al. 2014; Su et al. 2014; Chockley and Keshamouni 2016), although independent mechanisms have also been described. For example, in a model of gastric cancer, macrophage production of CXCL1 and CXCL5 increase Snail transcription through activation of a CXCR2/STAT3 feed-forward loop (Zhou et al. 2019), whereas in a model of Her2+ breast cancer Tie2+ macrophages initiate EMT through expression of Wnt (Linde et al. 2018). This association in breast cancer drives dissemination in the absence of a palpable lesion (Linde et al. 2018), and thus may also explain the impact of macrophage ablation on the invasive phenotype in a model of Trp53 null breast cancer (Carron et al. 2017). Intriguingly, macrophage expression of IL-35 at the site of metastasis has recently been shown to promote mesenchymal-to-epithelial transition (Lee et al. 2018), highlighting the critical role of macrophages at multiple stages of the metastatic process.
Immature monocytes and neutrophils have also been shown to contribute to EMT through the production of TGF-β, epidermal growth factor (EGF), and hepatocyte growth factor (HGF), and these cells are found around the invasive edge of tumors in which markers of EMT are most prevalent (Toh et al. 2011; Sangaletti et al. 2016; Ouzounova et al. 2017; Wang et al. 2019), with neutrophil levels at the invasive margin of gastric cancer being an independent, negative predictor of disease-free survival (Li et al. 2019). Neutrophils have also been shown to contribute to EMT by creating abnormal vasculature in lung tumors, resulting in hypoxia and induction of a Snail-dependent EMT program (Faget et al. 2017). Given an analogous role for macrophages in breast cancer (Stockmann et al. 2008), it may be that macrophages promote EMT through the same mechanism.
Although it has not been extensively evaluated, there are also a few reports suggesting that T cells can activate EMT. In vitro, coculturing premalignant cells with CD4+ T cells decreased E-cadherin expression and elevated expression of mesenchymal proteins (Goebel et al. 2015; Chen et al. 2017b). Whether this is mediated by TGF-β production by Tregs is not clear (Li et al. 2007; Worthington et al. 2012). One study also observed an in vivo role for CD8+ T cells involving selection of Her2/neu escape variants (Santisteban et al. 2009). However, this may be an artifact of this particular model system, and a role for T cells in vessel normalization (Motz et al. 2014; Schmittnaegel et al. 2017; Tian et al. 2017) suggests these cells could actually reduce hypoxia and EMT in other settings.
Extracellular Matrix Remodeling
The ECM is a dynamic, acellular, three-dimensional structure that serves as scaffold, on-demand reservoir for growth factors, and ligand for cellular adhesion. The ECM undergoes a continuous process of remodeling that is tightly regulated by the MMP and cathepsin family of proteases (Bonnans et al. 2014). Not surprisingly, ECM remodeling is highly abnormal in tumors, with increased deposition of key components such as collagen and fibronectin, increased degradation and turnover, and an abnormal structure marked by higher density and tensile force (Northey et al. 2017). Navigating the ECM is thus critical for cancer cells to invade tissue and gain access to the vasculature.
Studies documenting a role for immune cells in ECM deposition are limited, but production of SPARC (secreted protein, acidic rich in cysteine) by macrophages leads to increases in collagen and fibronectin, along with higher levels of metastasis (Sangaletti et al. 2008). Inflammatory monocytes have also been shown to elicit FXIIIA-mediated fibrin cross-linking, which provides a scaffold for local tumor cell invasion (Porrello et al. 2018). Conversely, production of interferon (IFN)-γ by NK cells restricts metastasis by inducing expression of fibronectin (Glasner et al. 2018), highlighting the complex association of ECM location and structure with the invasive process. A number of stromal proteases are also known to promote local tumor invasion, including urokinase/plasminogen activator (uPA), MMP-9 and cathepsin B, S, or Z (Almholt et al. 2005; Vasiljeva et al. 2006; Yang et al. 2008; Gocheva et al. 2010; Yan et al. 2010; Bekes et al. 2011; Akkari et al. 2014). Most of these proteases are expressed primarily by tumor macrophages, with the exception of MMP-9, which is also expressed by neutrophils and mast cells (Coussens et al. 2000; Bekes et al. 2011). However, it is currently unclear whether these proteases act by clearing out the ECM to permit invasion, release factors that promote an invasive phenotype (e.g., active TGF-β), alter the structure of the ECM to facilitate the creation of “tracks,” or act through some combination thereof (Condeelis and Segall 2003).
Intravasation
Angiogenesis is a hallmark of cancer, and angiogenic factors such as VEGF, fibroblast growth factor (FGF), and placental-derived growth factor (PlGF) are produced by immune cells in the tumor, especially macrophages (Rivera and Bergers 2015), which are critical for the angiogenic switch during tumor development (Lin et al. 2006). Macrophage production of these factors does not necessarily regulate the extent of angiogenesis in late stage tumors, as VEGF overexpression can bypass a requirement for macrophages (Lin et al. 2007). However, macrophages retain their importance as regulators of vascular structure and permeability (Stockmann et al. 2008). Thus ablating macrophages production of VEGF increases pericyte coverage, improves tissue perfusion, and increases tumor growth (Stockmann et al. 2008), while simultaneously increasing susceptibility to chemotherapy (Stockmann et al. 2008; Hughes et al. 2015) and reducing intravsation and metastasis (Harney et al. 2015). This phenotype is driven largely by a population of Tie2+CXCR4+ macrophages that reside in the perivascular space around vessels (De Palma et al. 2005; Welford et al. 2011), allowing for local production of VEGF to increase vessel permeability and ease transendothelial migration (Harney et al. 2015). Targeting these cells by blocking the ligand for Tie2, angiopoietin (Ang)-2, thus also results in reduced metastasis (Mazzieri et al. 2011). Although not necessarily related to the metastatic cascade, it is worth noting that vessel normalization promotes T-cell infiltration, which then maintains vessel normalization by producing IFN-γ (Motz et al. 2014; Schmittnaegel et al. 2017; Tian et al. 2017).
Macrophages are also responsible for recruiting tumor cells to these sites of vascular permeability through a well-described paracrine loop involving epidermal growth factor (EGF). As mentioned, tumor cells are an important source of CSF-1, and this induces the production of EGF in macrophages, resulting in tumor cell chemotaxis toward the vasculature (Wyckoff et al. 2004, 2007; Wang et al. 2009; Ishihara et al. 2013). This process is not mediated by Tie2+ perivascular macrophages, but rather the recently recruited population of monocyte-derived macrophages that forms the dominant population within tumors (Arwert et al. 2018). After entering the tumor parenchyma, these macrophages up-regulate expression of CXCR4 and are slowly recruited back to the vasculature via CXCL12-expressing fibroblasts, creating an EGF chemotactic gradient and pathway of altered ECM that directs cancer cell migration to the vasculature (Arwert et al. 2018). Finally, endothelial cell interactions promote differentiation into the Tie2+CXCR4+ perivascular macrophage population that facilitates transendothelial migration. Thus, interfering with monocyte recruitment (CCR2-CCL2), survival and differentiation (CSF1R-CSF1), migration to the vasculature (CXCR4-CXCL12), or maturation to perivascular cells (Tie2-Ang2) functions to severely limit the ability of tumor cells to intravasate into the blood stream (Qian and Pollard 2010; Kitamura et al. 2015). As with vascular normalization, the ability of macrophages to promote tumor cell chemotaxis is controlled by infiltrating T cells, with IL-4-expressing CD4+ T cells augmenting EGF expression by macrophages (DeNardo et al. 2009).
SURVIVAL AND EXTRAVASATION
Most primary tumors release millions of cells into the blood stream, but only a small number of metastatic lesions usually develop, indicating the inefficiency of tumor cell dissemination (Nagrath et al. 2007). In particular, circulating tumor cells (CTCs) have to resist physical shear stress caused by blood flow, death by anoikis, and avoid recognition by cytotoxic lymphocytes and phagocytic macrophages (Fig. 2) (Mohme et al. 2017). With this limited capacity to survive within the vasculature, the capacity of CTCs to adhere to the vasculature and migrate into the extravascular space is also central to their metastatic potential (Reymond et al. 2013), processes that are linked to the recruitment of neutrophils and monocytes/macrophages.
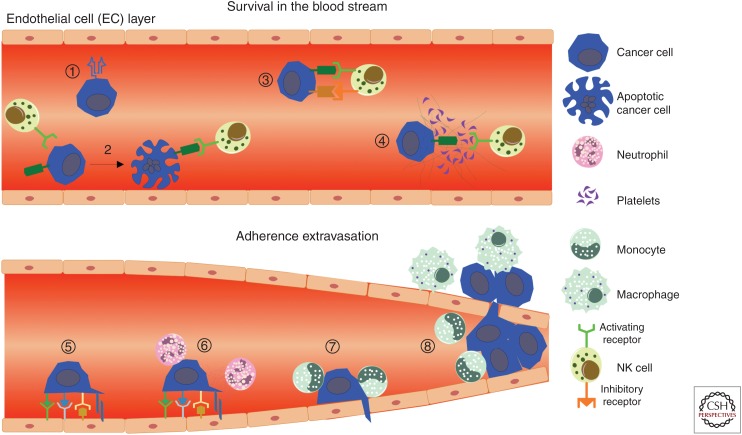
Figure 2.
Immune evasion and assistance during cancer cell dissemination. During circulation within the intravascular space, major histocompatibility complex (MHC) I expression protects by circulating tumor cells (CTCs) from natural killer (NK) cell recognition (1), whereas down-regulation of MHC I and up-regulation of NK cell activating ligands can lead to cytotoxicity (2). Conversely, recognition can be antagonized via up-regulation of NK cell inhibitory ligands (3) or physical shielding using platelet/fibrin coagulates (4). To extravasate into the tissue, CTCs interact with endothelial cells via selectins, cadherins, integrins, CD44, junctional adhesion molecules (5). Neutrophils are able to assist this process via ICAM-1 and VCAM-1 interactions, as well as through the release of NETs that trap CTCs and hide the cells from lympohcytes (6). Recruitment of CCR2+Ly6C+ monocytes (7) results in local production of VEGF, thereby increasing vascular permeability to facilitate CTC extravasation (8).
Avoiding Cytotoxic Cells
Increased NK cell cytotoxicity or enhanced expression of NK cell activation receptors are associated with good prognosis for patients at risk of metastatic disease (López-Soto et al. 2017). For example, the infiltration of NK cells expressing high levels of the activating receptors NCR1/NKp46 (natural cytotoxicity triggering receptor 1) and NKG2D (killer cell lectin-like receptor K1) was positively associated with recurrence-free survival in prostate cancer following surgical resection (Pasero et al. 2016). Several studies have shown that NKp46 expression in particular is critical for NK cell recognition of metastatic cells (Halfteck et al. 2009; Lakshmikanth et al. 2009; Elboim et al. 2010; Glasner et al. 2012) and loss of NK cells increases metastasis (Smyth et al. 1999; Bidwell et al. 2012). Although it is difficult to distinguish between a role for NK cells within the primary tumor, circulation, or metastatic sites, at least some experimental evidence exists for the direct killing of CTCs (Palumbo et al. 2005; Hanna et al. 2015), and poor infiltration by NK cells in the absence of treatment likely limits their role in most primary tumors (Böttcher et al. 2018).
Not surprisingly, tumors use multiple mechanisms to avoid recognition by NK cells (Box 1). First and foremost, down-regulated protein or surface expression of NK activating ligands, especially MHC I polypeptide-related sequence A (MICA), and MICB, can reduce NK cell recognition and killing of tumor cells (Nausch and Cerwenka 2008; Wang et al. 2014). Conversely, tumor cells can secrete soluble versions of these same molecules to suppress NK cell immunosurveillance (Schlecker et al. 2014; Zhang et al. 2015). This occurs within the primary tumor, but is sufficient to drive a systemic reduction in the cognate receptors on NK cells (Pasero et al. 2016), and high levels of these soluble ligands in the circulation correlate with disease progression (Paschen et al. 2009; Yamaguchi et al. 2012). That said, a high-affinity NKG2D ligand (MULT1) has also been shown to stimulate NK cell-mediated killing of tumor cells after shedding (Deng et al. 2015), so there may be a cost to this approach in some instances. Second, although HLA down-regulation is a common mechanism of evading T-cell recognition (Campoli and Ferrone 2008), HLA expression is never completely lost, likely because of the selective pressure of NK cells. Instead, HLA loss of heterozygosity is commonly observed as an alternative to loss of neoantigen expression, allowing tumors to limit detection by both of the cytotoxic lymphocyte populations simultaneously (McGranahan et al. 2017; Rosenthal et al. 2019).
BOX 1. NONCLASSICAL MHC SIGNALING.
Histocompatibility antigen class I G (HLA-G) is a human leukocyte antigen that belongs to the “nonclassical” HLA-class Ib molecules (Choo 2007). HLA-Ib molecules have minimal polymorphisms compared with the HLA-Ia molecules and are involved in immune modulation rather than antigen presentation (Kievits et al. 1987; Braud et al. 1997). HLA-G has been described to contribute to immune evasion in cancer (Lin and Yan 2015) and expression correlates with poor patient survival in melanoma, colorectal, hepatocellular, breast, glioma, ovarian, and lung cancers (Cai et al. 2009; de Kruijf et al. 2010; He et al. 2010; Guo et al. 2015). Moreover, soluble HLA-G is secreted by the primary tumor were identified and negatively correlate with patient outcome (Contini et al. 2003; Zheng et al. 2014). HLA-G can bind to a variety of receptors, such as CD8, leukocyte immunoglobulin-like receptor subfamily B member 1 (LIR-1), and killer cell immunoglobulin-like receptor (KIR), all of which protect cancer cells from CD8 T cell- or NK cell-induced killing (Wiendl et al. 2002; Kochan et al. 2013; Loumagne et al. 2014; Lin and Yan 2015). HLA-G polymorphisms also impact graft-versus-tumor responses in renal cell carcinoma (Crocchiolo et al. 2018) and correlate with outcome in epithelial ovarian carcinoma (Schwich et al. 2019).
There is also a complex interaction between tumor cells, platelets, and NK cells that regulates cytoxicity in the blood. Tumor cells become coated with platelets on entering the circulation, and this can promote survival, EMT, and adhesion to the endothelium (Gay and Felding-Habermann 2011; Labelle et al. 2011). This also has the effect of physically masking CTCs from NK cells (Camerer et al. 2004; Palumbo et al. 2005) and/or transferring platelet-derived membrane vesicles containing MHC I molecules that act to “normalize” the tumor cells (Placke et al. 2012). Additionally, platelet-derived TGF-β has been shown to induce down-regulation of NKG2D to prevent NK cell reactivity (Kopp et al. 2009). Cumulatively, platelets and their associated factors promote metastatic spread, a phenotype that is dependent on the presence of NK cells in most models (Camerer et al. 2004; Palumbo et al. 2005; Palumbo 2008; Turpin et al. 2014; Adams et al. 2015).
Avoiding Phagocytosis
CD47 is a surface protein belonging to the immunoglobulin superfamily that regulates a broad range of cellular functions, including adhesion, migration, proliferation, apoptosis and—most relevant for metastasis—recognition by phagocytes via signal regulatory protein α (SIRPα) (Reinhold et al. 1995; Jiang et al. 1999). SIRPα is expressed on monocytes, macrophages, and CD11b+ classical dendritic cells (cDCs), and on binding to CD47 the phagocytic activity of these cells is blocked, thereby acting as a “don't eat me” signal (Jaiswal et al. 2009). CD47 is ubiquitously expressed on mouse and human myeloid leukemias, thereby allowing them to evade clearance and populate organs with an abundance of macrophages (Jaiswal et al. 2009) and hinting that the same process may allow for CTCs to colonize ectopic organs.
Certainly, CD47 is overexpressed in many solid tumors (Liu et al. 2017), and it can protect cancer stem cells from elimination during conventional antitumor therapies, which in turn increases the probability of recurrence (Soltanian and Matin 2011). Gene expression profiling of primary tumors and CTCs from patients with colorectal cancers also found pronounced expression of CD47 (Steinert et al. 2014), whereas the presence of CD47+MET+CTCs in breast cancer patients was associated with increased metastasis and decreased survival (Baccelli et al. 2013, 2014). Taken together, the evidence favors CD47 expression reducing clearance by monocytes/macrophages. The question remains whether this clearance is relevant within the intravascular or extravascular space. It seems unlikely that monocytes and CTCs are interacting while they are both in circulation. However, nonclassical Ly6C− monocytes actively patrol the endothelial lumen (Auffray et al. 2007; Carlin et al. 2013) and rapidly take up cellular debris (Headley et al. 2016), which induces chemokine expression and recruitment of NK cells to limit metastasis (Hanna et al. 2015). Whether these patrolling monocytes also phagocytose live cells is unclear, and it may be that phagocytosis in the extravascular space or tissue parenchyma plays a more critical role in restricting metastasis.
Adhesion to the Endothelium
Circulating neutrophils correlate with poor prognosis (Teramukai et al. 2009; Lee et al. 2012; Gondo et al. 2013), and although this may partially reflect altered hematopoiesis and the establishment of an immunosuppressive microenvironment (Coffelt et al. 2015), it has also become increasingly clear that circulating neutrophils promote the conversion of CTCs to disseminated tumor cells (DTCs) through two distinct mechanisms. The first involves binding between integrins expressed by neutrophils (i.e., CD11b/CD18, VLA-4) and immunoglobulin superfamily ligands expressed by endothelial cells and CTCs (ICAM-1, VCAM-1). This allows neutrophils to form a cellular bridge for CTCs to adhere to the endothelium (Box 2) (Huh et al. 2010; Spicer et al. 2012). These interactions also induce “outside-in” signaling that promotes the proliferative capacity of CTCs, enhancing their metastatic potential (Szczerba et al. 2019). As the majority of CTCs are associated with neutrophils—at least in breast cancer patients—this appears to be an important mechanism underlying metastatic spread (Szczerba et al. 2019).
BOX 2. TUMOR–ENDOTHELIAL CELL INTERACTIONS.
CTCs becoming trapped in the capillaries is thought to be one of the major mechanisms of arrest in the circulation, but the process of adhesion to the endothelial layer and migration into the tissue is an active process (Chambers et al. 2002; Wirz et al. 2008; Strilic and Offermanns 2017). The vascular endothelium differs structurally depending on the organ, and as a result, leukocyte extravasation in lung and liver occurs in the microvasculature, whereas it occurs in the postcapillary venules in the skin, muscle, and mesentery (Aird 2007; Strell and Entschladen 2008). However, although the processes of adhesion and transmigration (diapedesis) are at least partially shared between leukocytes and tumor cells (Miles et al. 2008), the molecules described for tumor cell transmigration vary and are often tumor-specific (Bendas and Borsig 2012; Strilic and Offermanns 2017). However, it is still not entirely clear the degree to which all of these molecules are involved in tumor cell adhesion/transmigration in vivo, or whether different tumor subclones may use unique strategies to colonize different tissues (Caswell and Swanton 2017).
The other major mechanism by which neutrophils promote metastasis is through the release of neutrophil extracellular traps (NETs) containing their nuclear DNA (Park et al. 2016). Originally described to trap CTCs during injury or infection (Tohme et al. 2016), it has also been shown that release of NETs can be induced by the presence of CTCs in the lungs, leading to an increase in micrometastasis (Cools-Lartigue et al. 2013; Najmeh et al. 2017). Beyond simply sequestering CTCs, NETs inhibit NK cell-mediated cytotoxicity, and the release of factors such as IL-1B, MMP-9, and HMGB1 promotes extravasation (Spiegel et al. 2016; Tohme et al. 2016). Importantly, the formation of NETs in postoperative patients was linked to a reduction in survival, highlighting the potential clinical relevance of this mechanism (Tohme et al. 2016). Given the prevalence of CTC-neutrophil clusters in circulation (Szczerba et al. 2019) and the ability of tumor cells to induce the release of NETs109, it will be interesting to determine if this alters the dynamics of CTC retention and/or allows CTCs to create their own protective shell.
Extravasation
Ly6C+CCR2HI monocytes are recruited to the site of CTC arrest via a classical multistep adhesion cascade involving selectins, chemokines, and integrin ligands (Qian et al. 2011; Ferjančič et al. 2013; Häuselmann et al. 2016). In addition to CCL2 emanating from the tissue, cancer cell production of CCL2 is necessary for this process (Qian et al. 2011; Häuselmann et al. 2016), suggesting that it initiates the inflammatory cascade. Reminiscent of the role of macrophages in tumor cell intravasation, monocytes recruited to the endothelial lumen via CCL2 secrete VEGF and increase endothelial permeability, thereby enhancing transmigration of tumor cells (Qian et al. 2011). Interfering with the CCR2/CCL2 chemokine axis thereby reduces liver and lung metastasis in multiple murine model systems (Qian et al. 2011; Sanford et al. 2013), provided that the mice are left on treatment for the duration of the study (Bonapace et al. 2014). Platelet-bound CTCs can also recruit monocytes to promote metastasis, but the degree to which this process involves CCL2 and/or VEGF is unknown (Gil-Bernabe et al. 2012).
ECTOPIC GROWTH
Outgrowth of metastatic colonies represents the final stage of disease and is responsible for the majority of cancer-related deaths, and unfortunately, there is evidence that most patients have DTCs at the time of diagnosis (Friberg and Nystrom 2015; Massagué and Obenauf 2016). These DTCs are largely in a dormant state and thus resistant to conventional cytotoxic chemotherapies, resulting in metastatic recurrence for many patients long after the primary tumor has been removed. Addressing the issue of DTCs, as well as the treatment of overt metastasis, are therefore the two most critical areas of cancer research (Sosa et al. 2014; Massagué and Obenauf 2016). Thankfully, it is becoming clear that modulating the immune response has the potential to address both of these issues, with the approval of immune checkpoint blockade agents for metastatic disease being the most significant advancement.
Formation of the Premetastatic Niche
The premetastatic niche was originally described as VEGFR1-expressing myeloid cells accumulating at sites of distant metastasis before the arrival of tumor cells (Kaplan et al. 2005), and is usually defined by the recruitment of macrophages and neutrophils (Fig. 3) (Liu and Cao 2016). The formation of the niche is driven by a range of tumor and stroma-derived soluble factors (e.g., G-CSF, VEGF, CCL2, TNF-α, TGF-β, S100A8/A9) in addition to the release of tumor-derived extracellular vesicles (usually containing microRNAs [mRNAs]) (Liu and Cao 2016). Regardless of the molecules identified or the mechanism proposed, each appears to act primarily by inducing the recruitment of myeloid cells. For example, S100A8/A9 promotes the release of serum amyloid A3, which activates an inflammatory program in macrophages via toll-like receptor (TLR)4 (Hiratsuka et al. 2008). These cells then promote the ability of DTCs to become established by generating an immunosuppressive and/or permissive microenvironment, as we will discuss below. It is unclear why so many divergent approaches are used to achieve a similar goal, but presumably there are subtleties in the activation state of the recruited myeloid cells, as well as alternations in the functions of other stromal cells that play a complementary and supportive role in establishing the niche (McAllister and Weinberg 2014; Peinado et al. 2017).
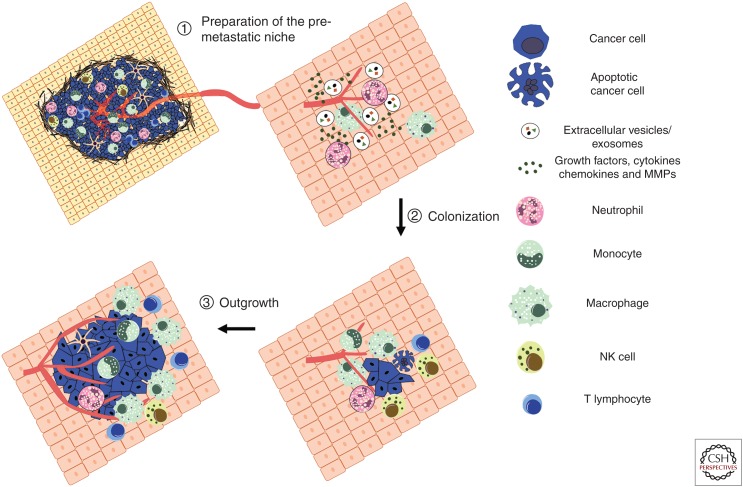
Figure 3.
Establishment of the metastatic immune microenvironment: The primary tumor can actively prime organs by forming a metastasis-permissive microenvironment before the arrival of disseminated tumor cells (DTCs) (1). This premetastatic niche includes recruited monocytes, macrophages, and neutrophils, as well as tumor-secreted factors such as cytokines, chemokines, growth factors, and proteases. Enhanced vascular leakiness, reorganization of the stroma and extracellular matrix, and the establishment of an immunosuppressive environment are hallmarks of the metastatic microenvironment (2). These allow DTCs to evade natural killer (NK) cell immunity and colonize the ectopic organ as single cells or micrometastatic lesions. Micrometastatic lesions often enter a period of dormancy, as they are ill-adapted to the new microenvironment, encounter growth suppressive factors such as TGF-β, and are subjected to NK cell-mediated immune surveillance. Outgrowth involves tumor-intrinsic changes alongside the induction of angiogenesis and the creation of a mature immunosuppressive microenvironment to evade recognition by cytotoxic lymphocytes (3).
Escaping Dormancy
There is only limited evidence that immune cells can promote the survival and growth of DTCs (Qian and Pollard 2010), with macrophages shown to provide survival signals via binding to VCAM-1 (Chen et al. 2011) and neutrophils enhancing tumorigenicity via leukotriene production (Wculek and Malanchi 2015). However, macrophages in primary tumors promote therapeutic resistance by producing factors such as TNF-α and IL-6 (Ruffell and Coussens 2015), which activate signaling pathways known to promote the proliferation of dormant cells (Massagué and Obenauf 2016). Thus, it is likely that these and other pathways are important at the metastatic site, and that our limited knowledge in this area reflects experimental difficulties evaluating the early stages of colonization. This includes the use of murine cancer cell lines that do not display dormancy or extended periods of latency, and evaluation of metastasis primarily to the lung even though there is evidence of a tissue-specific role for critical molecules such as TGF-β (Massagué and Obenauf 2016). Indeed, using a model of metastatic dormancy, it was recently shown that NETs promote metastasis via release of neutrophil elastase and MMP9, resulting in cleavage of the ECM component laminin (Albrengues et al. 2018). Rather than activating myeloid cells via TLRs, as has been described for versican in the premetastatic niche (Kim et al. 2009), cleaved laminin triggered tumor cell proliferation via integrin activation and FAK signaling (Albrengues et al. 2018).
Other studies that have addressed these experimental issues have noted a clear role for lymphocytes in maintaining tumor dormancy. CD8+ T-cell-depletion dramatically increased the rate of metastasis in a model of spontaneous uveal melanoma (Eyles et al. 2010) and following the surgical resection of implanted fibrosarcoma (Romero et al. 2014). Interestingly, although CD8+ T-cell-depletion resulted in a 100% rate of metastasis, NK cell-depletion led to a rate of 87%, and CD4+ T-cell-depletion resulted in a rate of 23% (Romero et al. 2014). This may reflect a complex interplay between these cell types in maintaining dormancy, or simply the production of a common set of cytokines such as IFN-γ and TNF-α. Certainly, the incidence of primary tumors is suppressed by these cytokines (Müller-Hermelink et al. 2008), either through cytostatic/cytotoxic effects on carcinoma cells or via suppression of angiogenic pathways (Müller-Hermelink et al. 2008; Andreu et al. 2010). Critically, a functional immune system prevents disease recurrence in humans, as seen from the unfortunate development of metastasis following kidney transplantations from donors with a previous history of melanoma or lung cancer (MacKie et al. 2003; Xiao et al. 2013).
Evading Immunity
Leaving the immunosuppressive microenvironment of the primary tumor exposes metastatic cells to recognition by cytotoxic lymphocytes, as seen from the ability of NK cells to protect against experimental and spontaneous metastasis but not primary tumor growth (Smyth et al. 1999, 2001; Takeda et al. 2001; Olkhanud et al. 2009; Milsom et al. 2013; Paolino et al. 2014). Thus, it is not surprising that successful metastasis is linked to the formation of a myeloid-rich environment (de Mingo Pulido and Ruffell 2016). What remains largely unexplored is the degree to which immunosuppressive mechanisms used by metastatic cells recapitulate those found in the primary tumor, represent the unique biology of the ectopic organ, and/or reflect an incipient microenvironment. For example, in a model of lobular breast cancer, primary tumors drive neutrophil expansion and an immunosuppressive phenotype via initiation of an IL-1β/IL-17/G-CSF cascade, which acts to blunt CD8+ T-cell-dependent rejection of early metastatic lesions (Coffelt et al. 2015). Interfering with any component of this pathway reversed this immune suppression, but had no impact on any measured parameter within primary tumors. This discrepancy may relate to the size of the lesion, as the complexity of an advanced microenvironment would make it resistant to perturbations in a single pathway and late stage metastatic lesions were not impacted by neutrophil depletion (Coffelt et al. 2015). However, it has also been described that macrophages in primary tumors indirectly suppress a CD8+ T-cell-response through production of IL-10, whereas their ability to limit T-cell activity in metastatic tumors occurs through a different mechanism of action (Ruffell et al. 2014), such as the production of reactive oxygen species or expression of CTLA4 ligands (Kitamura et al. 2018). Conversely, the clinical success of immune checkpoint blockade in metastatic and primary tumors, including in relatively immune privileged sites, highlights the importance of tumor immunogenicity (Hodi et al. 2014; Topalian et al. 2014; Overman et al. 2018). Thus, intrinsic, extrinsic, and temporal factors appear to regulate the vulnerability of metastatic lesions to immune surveillance, and understanding these factors should assist in the development of immunotherapies for patients with metastatic disease.
SUMMARY
The mechanisms underlying early steps of the metastatic process have been relatively well studied compared with colonization and immune evasion, despite early dissemination of tumor cells making these later steps more clinically relevant. The critical role for T cell- and NK cell-mediated surveillance in restraining the growth of metastatic lesions represents a unique therapeutic opportunity as agents that promote the activity of these cells become available. In addition to approved antagonist antibodies against CTLA-4 and PD-1/PD-L1, agents blocking LAG-3, TIGIT, and TIM-3 are in early phase clinical trials (Anderson et al. 2016), whereas several new targets have been identified, including those that unleash NK cell activity (André et al. 2018). Combining these agents with those that block the inhibitory function of myeloid cells, or activate their immunostimulatory potential, have already shown potential in murine models of primary tumors (DeNardo and Ruffell 2019).
Beyond the treatment of overt metastatic lesions, the role of lymphocytes in maintaining dormancy suggests the possibility of developing immunotherapies in an adjuvant setting. Although long-term treatment is difficult and expensive, the potential benefit of preventing recurrence would be immense, and the use of a minimal effective dose could improve the viability of this approach. Alternatively, understanding how the immune system promotes escape from dormancy could allow for the development of therapies targeting systemic inflammation. Recent technological developments including CRISPR/Cas9 screens, single cell genomics, and mass cytometry will allow progress to be made in this area, and dissecting how life events such as inflammatory diseases, infections, and injury relate to recurrence-free survival should allow findings in murine models to be correlated with patient data.
ACKNOWLEDGMENTS
K.H. was supported by a Postdoctoral Fellowship from the Swiss National Science Foundation (P400PM_183881). B.R. was supported by the Florida Breast Cancer Foundation, the Florida Department of Health Bankhead-Coley Cancer Research Program (8BC02), and sponsored research agreements with Tesaro, Inc.
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Abba MC, Gong T, Lu Y, Lee J, Zhong Y, Lacunza E, Butti M, Takata Y, Gaddis S, Shen J, et al. 2015. A molecular portrait of high-grade ductal carcinoma in situ. Cancer Res 75: 3980–3990. 10.1158/0008-5472.CAN-15-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GN, Rosenfeldt L, Frederick M, Miller W, Waltz D, Kombrinck K, McElhinney KE, Flick MJ, Monia BP, Revenko AS, et al. 2015. Colon cancer growth and dissemination relies upon thrombin, stromal PAR-1, and fibrinogen. Cancer Res 75: 4235–4243. 10.1158/0008-5472.CAN-15-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird WC. 2007. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173. 10.1161/01.RES.0000255691.76142.4a [DOI] [PubMed] [Google Scholar]
- Akkari L, Gocheva V, Kester JC, Hunter KE, Quick ML, Sevenich L, Wang HW, Peters C, Tang LH, Klimstra DS, et al. 2014. Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev 28: 2134–2150. 10.1101/gad.249599.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Kuttner V, et al. 2018. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361: eaao4227 10.1126/science.aao4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. 2000. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat Med 6: 100–102. 10.1038/71429 [DOI] [PubMed] [Google Scholar]
- Almholt K, Lund LR, Rygaard J, Nielsen BS, Danø K, Rømer J, Johnsen M. 2005. Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice. Int J Cancer 113: 525–532. 10.1002/ijc.20631 [DOI] [PubMed] [Google Scholar]
- Anderson AC, Joller N, Kuchroo VK. 2016. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 44: 989–1004. 10.1016/j.immuni.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A, et al. 2018. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175: 1731–1743.e13. 10.1016/j.cell.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. 2010. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17: 121–134. 10.1016/j.ccr.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, Condeelis JS. 2018. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep 23: 1239–1248. 10.1016/j.celrep.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670. 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T, Wallwiener M, et al. 2013. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 31: 539–544. 10.1038/nbt.2576 [DOI] [PubMed] [Google Scholar]
- Baccelli I, Stenzinger A, Vogel V, Pfitzner BM, Klein C, Wallwiener M, Scharpff M, Saini M, Holland-Letz T, Sinn HP, et al. 2014. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget 5: 8147–8160. 10.18632/oncotarget.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. 2011. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 179: 1455–1470. 10.1016/j.ajpath.2011.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendas G, Borsig L. 2012. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol 2012: 676731 10.1155/2012/676731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, et al. 2012. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med 18: 1224–1231. 10.1038/nm.2830 [DOI] [PubMed] [Google Scholar]
- Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. 2014. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515: 130–133. 10.1038/nature13862 [DOI] [PubMed] [Google Scholar]
- Bonnans C, Chou J, Werb Z. 2014. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786–801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C. 2018. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172: 1022–1037.e14. 10.1016/j.cell.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braud V, Yvonne Jones E, McMichael A. 1997. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol 27: 1164–1169. 10.1002/eji.1830270517 [DOI] [PubMed] [Google Scholar]
- Cai MY, Xu YF, Qiu SJ, Ju MJ, Gao Q, Li YW, Zhang BH, Zhou J, Fan J. 2009. Human leukocyte antigen-G protein expression is an unfavorable prognostic predictor of hepatocellular carcinoma following curative resection. Clin Cancer Res 15: 4686–4693. 10.1158/1078-0432.CCR-09-0463 [DOI] [PubMed] [Google Scholar]
- Cairns RA, Papandreou I, Sutphin PD, Denko NC. 2007. Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci 104: 9445–9450. 10.1073/pnas.0611662104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. 2004. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104: 397–401. 10.1182/blood-2004-02-0434 [DOI] [PubMed] [Google Scholar]
- Campoli M, Ferrone S. 2008. HLA antigen changes in malignant cells: Epigenetic mechanisms and biologic significance. Oncogene 27: 5869–5885. 10.1038/onc.2008.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. 2013. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell 153: 362–375. 10.1016/j.cell.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carron EC, Homra S, Rosenberg J, Coffelt SB, Kittrell F, Zhang Y, Creighton CJ, Fuqua SA, Medina D, Machado HL. 2017. Macrophages promote the progression of premalignant mammary lesions to invasive cancer. Oncotarget 8: 50731–50746. 10.18632/oncotarget.14913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell DR, Swanton C. 2017. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med 15: 133 10.1186/s12916-017-0900-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. 2002. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2: 563–572. 10.1038/nrc865 [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang XH, Massagué J. 2011. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20: 538–549. 10.1016/j.ccr.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K, et al. 2016. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533: 493–498. 10.1038/nature18268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, Siegwart DJ, Wan Y. 2017a. Gpr132 sensing of lactate mediates tumor–macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci 114: 580–585. 10.1073/pnas.1614035114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yang D, Zong H, Zhu L, Wang L, Wang X, Zhu X, Song X, Wang J. 2017b. Growth-induced stress enhances epithelial-mesenchymal transition induced by IL-6 in clear cell renal cell carcinoma via the Akt/GSK-3β/β-catenin signaling pathway. Oncogenesis 6: e375 10.1038/oncsis.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, Tu Q, Yin D, Lin D, Wong PP, et al. 2019. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol 21: 498–510. 10.1038/s41556-019-0299-0 [DOI] [PubMed] [Google Scholar]
- Chockley PJ, Keshamouni VG. 2016. Immunological consequences of epithelial–mesenchymal transition in tumor progression. J Immunol 197: 691–698. 10.4049/jimmunol.1600458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo SY. 2007. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med J 48: 11–23. 10.3349/ymj.2007.48.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C-S, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, et al. 2015. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522: 345–348. 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. 2014. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513: 559–563. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Segall JE. 2003. Intravital imaging of cell movement in tumours. Nat Rev Cancer 3: 921–930. 10.1038/nrc1231 [DOI] [PubMed] [Google Scholar]
- Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S, Puppo F. 2003. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol 33: 125–134. 10.1002/immu.200390015 [DOI] [PubMed] [Google Scholar]
- Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL, Redente E, Sandahl M, Hunter DM, Strunk KE, et al. 2013. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J Clin Invest 123: 3231–3242. 10.1172/JCI67655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. 2013. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 123: 3446–3458. 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. 2000. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103: 481–490. 10.1016/S0092-8674(00)00139-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden MR, Baird J, Friedman D, Savage T, Uhde L, Alice A, Cottam B, Young K, Newell P, Nguyen C, et al. 2016. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget 7: 78653–78666. 10.18632/oncotarget.11823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocchiolo R, Ringden O, Bay JO, Blaise D, Omasic B, Mazzi B, Picard C, Trinca S, Barkholt L, Peccatori J, et al. 2018. Impact of HLA-G polymorphism on the outcome of allogeneic hematopoietic stem cell transplantation for metastatic renal cell carcinoma. Bone Marrow Transplant 53: 213–218. 10.1038/bmt.2017.243 [DOI] [PubMed] [Google Scholar]
- de Kruijf EM, Sajet A, van Nes JGH, Natanov R, Putter H, Smit VTHBM, Liefers GJ, van den Elsen PJ, van de Velde CJH, Kuppen PJK. 2010. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol 185: 7452–7459. 10.4049/jimmunol.1002629 [DOI] [PubMed] [Google Scholar]
- de Mingo Pulido A, Ruffell B. 2016. Immune regulation of the metastatic process: Implications for therapy. Adv Cancer Res 132: 139–163. 10.1016/bs.acr.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Ruffell B. 2019. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol 19: 369–382. 10.1038/s41577-019-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. 2009. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16: 91–102. 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneve E, Riethdorf S, Ramos J, Nocca D, Coffy A, Daures JP, Maudelonde T, Fabre JM, Pantel K, Alix-Panabieres C. 2013. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem 59: 1384–1392. 10.1373/clinchem.2013.202846 [DOI] [PubMed] [Google Scholar]
- Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH. 2015. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 348: 136–139. 10.1126/science.1258867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. 2005. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8: 211–226. 10.1016/j.ccr.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. 2010. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res 70: 7465–7475. 10.1158/0008-5472.CAN-10-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongre A, Weinberg RA. 2019. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20: 69–84. 10.1038/s41580-018-0080-4 [DOI] [PubMed] [Google Scholar]
- Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, Weinberg RA. 2017. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res 77: 3982–3989. 10.1158/0008-5472.CAN-16-3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elboim M, Gazit R, Gur C, Ghadially H, Betser-Cohen G, Mandelboim O. 2010. Tumor immunoediting by NKp46. J Immunol 184: 5637–5644. 10.4049/jimmunol.0901644 [DOI] [PubMed] [Google Scholar]
- El-Kenawi A, Gatenbee C, Robertson-Tessi M, Bravo R, Dhillon J, Balagurunathan Y, Berglund A, Visvakarma N, Ibrahim-Hashim A, Choi J, et al. 2019. Acidity promotes tumor progression by altering macrophage phenotype in prostate cancer. Br J Cancer. doi:10.1038/s41416-019-0542-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y, et al. 2010. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest 120: 2030–2039. 10.1172/JCI42002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget J, Groeneveld S, Boivin G, Sankar M, Zangger N, Garcia M, Guex N, Zlobec I, Steiner L, Piersigilli A, et al. 2017. Neutrophils and Snail orchestrate the establishment of a pro-tumor microenvironment in lung cancer. Cell Rep 21: 3190–3204. 10.1016/j.celrep.2017.11.052 [DOI] [PubMed] [Google Scholar]
- Ferjančič Š, Gil-Bernabé AM, Hill SA, Allen PD, Richardson P, Sparey T, Savory E, McGuffog J, Muschel RJ. 2013. VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood 121: 3289–3297. 10.1182/blood-2012-08-449819 [DOI] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, et al. 2008. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14: 518–527. 10.1038/nm1764 [DOI] [PubMed] [Google Scholar]
- Friberg S, Nystrom A. 2015. Cancer metastases: Early dissemination and late recurrences. Cancer Growth Metastasis 8: 43–49. 10.4137/CGM.S31244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. 2004. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4: 891–899. 10.1038/nrc1478 [DOI] [PubMed] [Google Scholar]
- Gay LJ, Felding-Habermann B. 2011. Contribution of platelets to tumour metastasis. Nat Rev Cancer 11: 123–134. 10.1038/nrc3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. 2015. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21: 938–945. 10.1038/nm.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Bissell MJ. 2016. Pathways of parallel progression. Nature 540: 528–529. 10.1038/nature21104 [DOI] [PubMed] [Google Scholar]
- Gil-Bernabe AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis JL, et al. 2012. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 119: 3164–3175. 10.1182/blood-2011-08-376426 [DOI] [PubMed] [Google Scholar]
- Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F, Zi X, Kwak M, Bergholtz H, Su Y, et al. 2017. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov 7: 1098–1115. 10.1158/2159-8290.CD-17-0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner A, Ghadially H, Gur C, Stanietsky N, Tsukerman P, Enk J, Mandelboim O. 2012. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J Immunol 188: 2509–2515. 10.4049/jimmunol.1102461 [DOI] [PubMed] [Google Scholar]
- Glasner A, Levi A, Enk J, Isaacson B, Viukov S, Orlanski S, Scope A, Neuman T, Enk CD, Hanna JH, et al. 2018. NKp46 receptor-mediated interferon-γ production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity 48: 107–119.e4. 10.1016/j.immuni.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. 2010. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 24: 241–255. 10.1101/gad.1874010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel L, Grage-Griebenow E, Gorys A, Helm O, Genrich G, Lenk L, Wesch D, Ungefroren H, Freitag-Wolf S, Sipos B, et al. 2015. CD4+ T cells potently induce epithelial-mesenchymal-transition in premalignant and malignant pancreatic ductal epithelial cells-novel implications of CD4+ T cells in pancreatic cancer development. Oncoimmunology 4: e1000083 10.1080/2162402X.2014.1000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo T, Nakashima J, Ohno Y, Hashimoto T, Takizawa I, Sakamoto N, Horiguchi Y, Aoyagi T, Ohori M, Tachibana M. 2013. Preoperative prediction of malignant involvement of resected ureters in patients undergoing radical cystectomy for bladder cancer. Int J Urol 20: 501–506. 10.1111/j.1442-2042.2012.03203.x [DOI] [PubMed] [Google Scholar]
- Gunderson AJ, Coussens LM. 2013. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res 319: 1644–1649. 10.1016/j.yexcr.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZY, Lv YG, Wang L, Shi SJ, Yang F, Zheng GX, Wen WH, Yang AG. 2015. Predictive value of HLA-G and HLA-E in the prognosis of colorectal cancer patients. Cell Immunol 293: 10–16. 10.1016/j.cellimm.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Halfteck GG, Elboim M, Gur C, Achdout H, Ghadially H, Mandelboim O. 2009. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J Immunol 182: 2221–2230. 10.4049/jimmunol.0801878 [DOI] [PubMed] [Google Scholar]
- Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, et al. 2015. Patrolling monocytes control tumor metastasis to the lung. Science 350: 985–990. 10.1126/science.aac9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS. 2015. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by Tie2Hi macrophage-derived VEGFA. Cancer Discov 5: 932–943. 10.1158/2159-8290.CD-15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis RJ, et al. 2016. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540: 588–592. 10.1038/nature20609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grässle S, Bauer AT, Schneider SW, Borsig L. 2016. Monocyte induction of E-selectin-mediated endothelial activation releases VE–cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res 76: 5302–5312. 10.1158/0008-5472.CAN-16-0784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Dong DD, Yie SM, Yang H, Cao M, Ye SR, Li K, Liu J, Chen J. 2010. HLA-G expression in human breast cancer: Implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann Surg Oncol 17: 1459–1469. 10.1245/s10434-009-0891-9 [DOI] [PubMed] [Google Scholar]
- Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, Krummel MF. 2016. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 531: 513–517. 10.1038/nature16985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S, Pang JB, Byrne DJ, Lakhani SR, Cummings MC, Campbell IG, Mann GB, Gorringe KL, Fox SB. 2017. Relationship of the breast ductal carcinoma in situ immune microenvironment with clinicopathological and genetic features. Clin Cancer Res 23: 5210–5217. 10.1158/1078-0432.CCR-17-0743 [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. 2008. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol 10: 1349–1355. 10.1038/ncb1794 [DOI] [PubMed] [Google Scholar]
- Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM. 2014. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 312: 1744–1753. 10.1001/jama.2014.13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini H, Obradović MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, et al. 2016. Early dissemination seeds metastasis in breast cancer. Nature 540: 552–558. 10.1038/nature20785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DS, Wang HJ, Tai SK, Chou CH, Hsieh CH, Chiu PH, Chen NJ, Yang MH. 2014. Acetylation of Snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell 26: 534–548. 10.1016/j.ccell.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M, et al. 2015. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res 75: 3479–3491. 10.1158/0008-5472.CAN-14-3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. 2010. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 70: 6071–6082. 10.1158/0008-5472.CAN-09-4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, et al. 2008. Systemic spread is an early step in breast cancer. Cancer Cell 13: 58–68. 10.1016/j.ccr.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Ishihara D, Dovas A, Hernandez L, Pozzuto M, Wyckoff J, Segall JE, Condeelis JS, Bresnick AR, Cox D. 2013. Wiskott-Aldrich syndrome protein regulates leukocyte-dependent breast cancer metastasis. Cell Rep 4: 429–436. 10.1016/j.celrep.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, Van Rooijen N, Weissman IL. 2009. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138: 271–285. 10.1016/j.cell.2009.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, Ager C, Nicholas C, Jaiswal AR, Sun Y, et al. 2018. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest 128: 5137–5149. 10.1172/JCI96268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su MJ, Melms JC, Leeson R, Kanodia A, Mei S, Lin JR, et al. 2018. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175: 984–997.e24. 10.1016/j.cell.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Lagenaur CF, Narayanan V. 1999. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 274: 559–562. 10.1074/jbc.274.2.559 [DOI] [PubMed] [Google Scholar]
- Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, et al. 2016. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 22: 851–860. 10.1038/nm.4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827. 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber ER, Lowe SW. 2017. Putting p53 in context. Cell 170: 1062–1078. 10.1016/j.cell.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, Komoto S, Katsube R, Ninomiya T, Tazawa H, et al. 2018. Cancer-associated fibroblasts affect intratumoral CD8+ and FoxP3+ T cells via IL6 in the tumor microenvironment. Clin Cancer Res 24: 4820–4833. 10.1158/1078-0432.CCR-18-0205 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. 2010. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 141: 52–67. 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievits F, Ivanyi P, Krimpenfort P, Berns A, Ploegh HL. 1987. HLA-restricted recognition of viral antigens in HLA transgenic mice. Nature 329: 447–449. 10.1038/329447a0 [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. 2009. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457: 102–106. 10.1038/nature07623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Pollard JW. 2015. Immune cell promotion of metastasis. Nat Rev Immunol 15: 73–86. 10.1038/nri3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, Carragher N, Pollard JW. 2018. Monocytes differentiate to immune suppressive precursors of metastasis-associated macrophages in mouse models of metastatic breast cancer. Front Immunol 8: 2004 10.3389/fimmu.2017.02004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan G, Escors D, Breckpot K, Guerrero-Setas D. 2013. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunology 2: e26491 10.4161/onci.26491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, et al. 2010. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci 107: 20009–20014. 10.1073/pnas.1013805107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Placke T, Salih HR. 2009. Platelet-derived transforming growth factor-β down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69: 7775–7783. 10.1158/0008-5472.CAN-09-2123 [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, Littlewood TD, Evan GI. 2017. Myc cooperates with Ras by programming inflammation and immune suppression. Cell 171: 1301–1315.e14. 10.1016/j.cell.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. 2013. Immunogenic cell death in cancer therapy. Annu Rev Immunol 31: 51–72. 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. 2009. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15: 195–206. 10.1016/j.ccr.2009.01.023 [DOI] [PubMed] [Google Scholar]
- Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, et al. 2017. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32: 654–668.e5. 10.1016/j.ccell.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. 2011. Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590. 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. 2018. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+ T cells to protect tumour cells. Nat Commun 9: 948 10.1038/s41467-018-03347-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker A, et al. 2009. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 119: 1251–1263. 10.1172/JCI36022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AW, Pattabiraman DR, Weinberg RA. 2017. Emerging biological principles of metastasis. Cell 168: 670–691. 10.1016/j.cell.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, Bae DS, Kim BG. 2012. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 32: 1555–1561. [PubMed] [Google Scholar]
- Lee CC, Lin JC, Hwang WL, Kuo YJ, Chen HK, Tai SK, Lin CC, Yang MH. 2018. Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat Commun 9: 3763 10.1038/s41467-018-06268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P, Nilsson J. 2015. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7: 308re308 10.1126/scitranslmed.aad3740 [DOI] [PubMed] [Google Scholar]
- Lewis CE, Harney AS, Pollard JW. 2016. The multifaceted role of perivascular macrophages in tumors. Cancer Cell 30: 18–25. 10.1016/j.ccell.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. 2007. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26: 579–591. 10.1016/j.immuni.2007.03.014 [DOI] [PubMed] [Google Scholar]
- Li S, Cong X, Gao H, Lan X, Li Z, Wang W, Song S, Wang Y, Li C, Zhang H, et al. 2019. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res 38: 6 10.1186/s13046-018-1003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, Li J, Wang G, Lan Z, Li J, et al. 2019. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35: 559–572.e7. 10.1016/j.ccell.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Yan WH. 2015. Human leukocyte antigen-G (HLA-G) expression in cancers: Roles in immune evasion, metastasis and target for therapy. Mol Med 21: 782–791. 10.2119/molmed.2015.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. 2006. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66: 11238–11246. 10.1158/0008-5472.CAN-06-1278 [DOI] [PubMed] [Google Scholar]
- Lin EY, Li JF, Bricard G, Wang W, Deng Y, Sellers R, Porcelli SA, Pollard JW. 2007. Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol Oncol 1: 288–302. 10.1016/j.molonc.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I, Jones J, et al. 2018. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun 9: 21 10.1038/s41467-017-02481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cao X. 2016. Characteristics and significance of the pre-metastatic niche. Cancer Cell 30: 668–681. 10.1016/j.ccell.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Liu X, Kwon H, Li Z, Fu YX. 2017. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol 10: 12 10.1186/s13045-016-0381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. 2017. Control of metastasis by NK cells. Cancer Cell 32: 135–154. 10.1016/j.ccell.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Loumagne L, Baudhuin J, Favier B, Montespan F, Carosella ED, Rouas-Freiss N. 2014. In vivo evidence that secretion of HLA-G by immunogenic tumor cells allows their evasion from immunosurveillance. Int J Cancer 135: 2107–2117. 10.1002/ijc.28845 [DOI] [PubMed] [Google Scholar]
- Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, et al. 2014. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol 16: 1105–1117. 10.1038/ncb3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKie RM, Reid R, Junor B. 2003. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N Engl J Med 348: 567–568. 10.1056/NEJM200302063480620 [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, et al. 2018. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554: 544–548. 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Obenauf AC. 2016. Metastatic colonization by circulating tumour cells. Nature 529: 298–306. 10.1038/nature17038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, et al. 2011. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19: 512–526. 10.1016/j.ccr.2011.02.005 [DOI] [PubMed] [Google Scholar]
- McAllister SS, Weinberg RA. 2014. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16: 717–727. 10.1038/ncb3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P, Herrero J, et al. 2017. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 171: 1259–1271.e11. 10.1016/j.cell.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhorter FY, Davis CT, Liu WF. 2015. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci 72: 1303–1316. 10.1007/s00018-014-1796-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T. 2009. Requirement for NF-κB signalling in a mouse model of lung adenocarcinoma. Nature 462: 104–107. 10.1038/nature08462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FL, Pruitt FL, van Golen KL, Cooper CR. 2008. Stepping out of the flow: Capillary extravasation in cancer metastasis. Clin Exp Metastasis 25: 305–324. 10.1007/s10585-007-9098-2 [DOI] [PubMed] [Google Scholar]
- Milsom CC, Lee CR, Hackl C, Man S, Kerbel RS. 2013. Differential post-surgical metastasis and survival in SCID, NOD-SCID and NOD-SCID-IL-2Rγnull mice with parental and subline variants of human breast cancer: Implications for host defense mechanisms regulating metastasis. PLoS ONE 8: e71270 10.1371/journal.pone.0071270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohme M, Riethdorf S, Pantel K. 2017. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat Rev Clin Oncol 14: 155–167. 10.1038/nrclinonc.2016.144 [DOI] [PubMed] [Google Scholar]
- Motz GT, Santoro SP, Wang L-P, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. 2014. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 20: 607–615. 10.1038/nm.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hermelink N, Braumüller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, et al. 2008. TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13: 507–518. 10.1016/j.ccr.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Murdoch C, Giannoudis A, Lewis CE. 2004. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104: 2224–2234. 10.1182/blood-2004-03-1109 [DOI] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. 2007. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450: 1235–1239. 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau S, Ferri LE, et al. 2017. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer 140: 2321–2330. 10.1002/ijc.30635 [DOI] [PubMed] [Google Scholar]
- Nausch N, Cerwenka A. 2008. NKG2D ligands in tumor immunity. Oncogene 27: 5944–5958. 10.1038/onc.2008.272 [DOI] [PubMed] [Google Scholar]
- Nelson AC, Machado HL, Schwertfeger KL. 2018. Breaking through to the other side: Microenvironment contributions to DCIS initiation and progression. J Mammary Gland Biol Neoplasia 23: 207–221. 10.1007/s10911-018-9409-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonn L, Ananthanarayanan V, Gann PH. 2009. Evidence for field cancerization of the prostate. Prostate 69: 1470–1479. 10.1002/pros.20983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey JJ, Przybyla L, Weaver VM. 2017. Tissue force programs cell fate and tumor aggression. Cancer Discov 7: 1224–1237. 10.1158/2159-8290.CD-16-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. 2009. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res 69: 5996–6004. 10.1158/0008-5472.CAN-08-4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. 2011. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res 71: 3505–3515. 10.1158/0008-5472.CAN-10-4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzounova M, Lee E, Piranlioglu R, El Andaloussi A, Kolhe R, Demirci MF, Marasco D, Asm I, Chadli A, Hassan KA, et al. 2017. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun 8: 14979 10.1038/ncomms14979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman MJ, Lonardi S, Wong KYM, Lenz H-J, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A. 2018. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 36: 773–779. 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- Palumbo JS. 2008. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin Thromb Hemost 34: 154–160. 10.1055/s-2008-1079255 [DOI] [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. 2005. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105: 178–185. 10.1182/blood-2004-06-2272 [DOI] [PubMed] [Google Scholar]
- Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, Jamieson AM, Langdon WY, Ikeda F, Fededa JP, et al. 2014. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507: 508–512. 10.1038/nature12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, et al. 2016. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 8: 361ra138 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, et al. 2009. Differential clinical significance of individual NKG2D ligands in melanoma: Soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res 15: 5208–5215. 10.1158/1078-0432.CCR-09-0886 [DOI] [PubMed] [Google Scholar]
- Pasero C, Gravis G, Guerin M, Granjeaud S, Thomassin-Piana J, Rocchi P, Paciencia-Gros M, Poizat F, Bentobji M, Azario-Cheillan F, et al. 2016. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res 76: 2153–2165. 10.1158/0008-5472.CAN-15-1965 [DOI] [PubMed] [Google Scholar]
- Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, et al. 2017. Pre-metastatic niches: Organ-specific homes for metastases. Nat Rev Cancer 17: 302–317. 10.1038/nrc.2017.6 [DOI] [PubMed] [Google Scholar]
- Pickup MW, Mouw JK, Weaver VM. 2014. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15: 1243–1253. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, Salih HR. 2012. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72: 440–448. 10.1158/0008-5472.CAN-11-1872 [DOI] [PubMed] [Google Scholar]
- Porrello A, Leslie PL, Harrison EB, Gorentla BK, Kattula S, Ghosh SK, Azam SH, Holtzhausen A, Chao YL, Hayward MC, et al. 2018. Factor XIIIA–expressing inflammatory monocytes promote lung squamous cancer through fibrin cross-linking. Nat Commun 9: 1988 10.1038/s41467-018-04355-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. 2012. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21: 836–847. 10.1016/j.ccr.2012.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. 2010. Macrophage diversity enhances tumor progression and metastasis. Cell 141: 39–51. 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. 2011. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475: 222–225. 10.1038/nature10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Z, Chen H, Xu J. 2009. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res 69: 3819–3827. 10.1158/0008-5472.CAN-08-4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. 2013. Microenvironmental regulation of tumor progression and metastasis. Nat Med 19: 1423–1437. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. 1995. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J Cell Sci 108: 3419–3425. [DOI] [PubMed] [Google Scholar]
- Reymond N, d’Água BB, Ridley AJ. 2013. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13: 858–870. 10.1038/nrc3628 [DOI] [PubMed] [Google Scholar]
- Rivera LB, Bergers G. 2015. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol 36: 240–249. 10.1016/j.it.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero I, Garrido C, Algarra I, Collado A, Garrido F, Garcia-Lora AM. 2014. T lymphocytes restrain spontaneous metastases in permanent dormancy. Cancer Res 74: 1958–1968. 10.1158/0008-5472.CAN-13-2084 [DOI] [PubMed] [Google Scholar]
- Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. 2015. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160: 48–61. 10.1016/j.cell.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, Lund T, Tanić M, Reading JL, Joshi K, et al. 2019. Neoantigen-directed immune escape in lung cancer evolution. Nature 567: 479–485. 10.1038/s41586-019-1032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Coussens LM. 2015. Macrophages and therapeutic resistance in cancer. Cancer Cell 27: 462–472. 10.1016/j.ccell.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. 2014. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26: 623–637. 10.1016/j.ccell.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, et al. 2013. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: A role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19: 3404–3415. 10.1158/1078-0432.CCR-13-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaletti S, Di Carlo E, Gariboldi S, Miotti S, Cappetti B, Parenza M, Rumio C, Brekken RA, Chiodoni C, Colombo MP. 2008. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Res 68: 9050–9059. 10.1158/0008-5472.CAN-08-1327 [DOI] [PubMed] [Google Scholar]
- Sangaletti S, Tripodo C, Santangelo A, Castioni N, Portararo P, Gulino A, Botti L, Parenza M, Cappetti B, Orlandi R, et al. 2016. Mesenchymal transition of high-grade breast carcinomas depends on extracellular matrix control of myeloid suppressor cell activity. Cell Rep 17: 233–248. 10.1016/j.celrep.2016.08.075 [DOI] [PubMed] [Google Scholar]
- Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, et al. 2009. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 69: 2887–2895. 10.1158/0008-5472.CAN-08-3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecker E, Fiegler N, Arnold A, Altevogt P, Rose-John S, Moldenhauer G, Sucker A, Paschen A, von Strandmann EP, Textor S, et al. 2014. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res 74: 3429–3440. 10.1158/0008-5472.CAN-13-3017 [DOI] [PubMed] [Google Scholar]
- Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH, Laoui D, et al. 2017. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med 9: eaak9670 10.1126/scitranslmed.aak9670 [DOI] [PubMed] [Google Scholar]
- Schwich E, Rebmann V, Michita RT, Rohn H, Voncken JW, Horn PA, Kimmig R, Kasimir-Bauer S, Buderath P. 2019. HLA-G 3′ untranslated region variants +3187G/G, +3196G/G and +3035T define diametrical clinical status and disease outcome in epithelial ovarian cancer. Sci Rep 9: 5407 10.1038/s41598-019-41900-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. 1999. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol 162: 6658–6662. [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. 2001. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon γ-dependent natural killer cell protection from tumor metastasis. J Exp Med 193: 661–670. 10.1084/jem.193.6.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltanian S, Matin MM. 2011. Cancer stem cells and cancer therapy. Tumor Biol 32: 425–440. 10.1007/s13277-011-0155-8 [DOI] [PubMed] [Google Scholar]
- Sosa MS, Bragado P, Aguirre-Ghiso JA. 2014. Mechanisms of disseminated cancer cell dormancy: An awakening field. Nat Rev Cancer 14: 611–622. 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. 2007. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med 13: 1211–1218. 10.1038/nm1649 [DOI] [PubMed] [Google Scholar]
- Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE. 2012. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 72: 3919–3927. 10.1158/0008-5472.CAN-11-2393 [DOI] [PubMed] [Google Scholar]
- Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, et al. 2016. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov 6: 630–649. 10.1158/2159-8290.CD-15-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Schupp J, Jäger B, Schmid M, Zissel G, Müller-Quernheim J, Prasse A. 2013. Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS ONE 8: e81382 10.1371/journal.pone.0081382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert G, Schölch S, Niemietz T, Iwata N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, et al. 2014. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res 74: 1694–1704. 10.1158/0008-5472.CAN-13-1885 [DOI] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. 2008. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456: 814–818. 10.1038/nature07445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strell C, Entschladen F. 2008. Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal 4: 10 10.1186/1478-811X-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B, Offermanns S. 2017. Intravascular survival and extravasation of tumor cells. Cancer Cell 32: 282–293. 10.1016/j.ccell.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, et al. 2014. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 25: 605–620. 10.1016/j.ccr.2014.03.021 [DOI] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. 2019. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566: 553–557. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. 2001. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 7: 94–100. 10.1038/83416 [DOI] [PubMed] [Google Scholar]
- Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, Yamanoi K, Horikawa N, Hosoe Y, Nakamura E, et al. 2018. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun 9: 1685 10.1038/s41467-018-03966-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, et al. 2018. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554: 538–543. 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al. 2009. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 45: 1950–1958. 10.1016/j.ejca.2009.01.023 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RYJ, Nieto MA. 2009. Epithelial–mesenchymal transitions in development and disease. Cell 139: 871–890. 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, et al. 2017. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544: 250–254. 10.1038/nature21724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. 2011. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 9: e1001162 10.1371/journal.pbio.1001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A. 2016. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 76: 1367–1380. 10.1158/0008-5472.CAN-15-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. 2014. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32: 1020–1030. 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin B, Miller W, Rosenfeldt L, Kombrinck K, Flick MJ, Steinbrecher KA, Harmel-Laws E, Mullins ES, Shaw M, Witte DP, et al. 2014. Thrombin drives tumorigenesis in colitis-associated colon cancer. Cancer Res 74: 3020–3030. 10.1158/0008-5472.CAN-13-3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubil E, Caskey L, Holtzhausen A, Hunter D, Story C, Earp HS. 2018. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J Clin Invest 128: 2356–2369. 10.1172/JCI97354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. 2011. Tumor metastasis: Molecular insights and evolving paradigms. Cell 147: 275–292. 10.1016/j.cell.2011.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V. 2010. Matrix architecture dictates three-dimensional migration modes of human macrophages: Differential involvement of proteases and podosome-like structures. J Immunol 184: 1049–1061. 10.4049/jimmunol.0902223 [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Papazoglou A, Krüger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, et al. 2006. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res 66: 5242–5250. 10.1158/0008-5472.CAN-05-4463 [DOI] [PubMed] [Google Scholar]
- Veglia F, Perego M, Gabrilovich D. 2018. Myeloid-derived suppressor cells coming of age. Nat Immunol 19: 108–119. 10.1038/s41590-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, Xu J. 2009. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci 106: 151–156. 10.1073/pnas.0808703105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, Yang J, Xu SL, Ye XZ, Xu C, et al. 2014. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res 74: 5746–5757. 10.1158/0008-5472.CAN-13-2563 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen J, Yang L, Li J, Wu W, Huang M, Lin L, Su S. 2019. Tumor-contacted neutrophils promote metastasis by a CD90-TIMP-1 juxtacrine–paracrine loop. Clin Cancer Res 25: 1957–1969. 10.1158/1078-0432.CCR-18-2544 [DOI] [PubMed] [Google Scholar]
- Wculek SK, Malanchi I. 2015. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528: 413–417. 10.1038/nature16140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM. 2011. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest 121: 1969–1973. 10.1172/JCI44562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenstein MD, de Visser KE. 2018. Cancer-cell–intrinsic mechanisms shaping the tumor immune landscape. Immunity 48: 399–416. 10.1016/j.immuni.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Wesley RB II, Meng X, Godin D, Galis ZS. 1998. Extracellular matrix modulates macrophage functions characteristic to atheroma: Collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler Thromb Vasc Biol 18: 432–440. 10.1161/01.ATV.18.3.432 [DOI] [PubMed] [Google Scholar]
- Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M. 2002. A functional role of HLA-G expression in human gliomas: An alternative strategy of immune escape. J Immunol 168: 4772–4780. 10.4049/jimmunol.168.9.4772 [DOI] [PubMed] [Google Scholar]
- Wirz W, Antoine M, Tag CG, Gressner AM, Korff T, Hellerbrand C, Kiefer P. 2008. Hepatic stellate cells display a functional vascular smooth muscle cell phenotype in a three-dimensional co-culture model with endothelial cells. Differentiation 76: 784–794. 10.1111/j.1432-0436.2007.00260.x [DOI] [PubMed] [Google Scholar]
- Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA. 2012. Regulation of TGFβ in the immune system: An emerging role for integrins and dendritic cells. Immunobiology 217: 1259–1265. 10.1016/j.imbio.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. 2004. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64: 7022–7029. 10.1158/0008-5472.CAN-04-1449 [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. 2007. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67: 2649–2656. 10.1158/0008-5472.CAN-06-1823 [DOI] [PubMed] [Google Scholar]
- Xiao D, Craig JC, Chapman JR, Dominguez-Gil B, Tong A, Wong G. 2013. Donor cancer transmission in kidney transplantation: A systematic review. Am J Transplant 13: 2645–2652. 10.1111/ajt.12430 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Chikumi H, Shimizu A, Takata M, Kinoshita N, Hashimoto K, Nakamoto M, Matsunaga S, Kurai J, Miyake N, et al. 2012. Diagnostic and prognostic impact of serum-soluble UL16-binding protein 2 in lung cancer patients. Cancer Sci 103: 1405–1413. 10.1111/j.1349-7006.2012.02330.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. 2010. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 70: 6139–6149. 10.1158/0008-5472.CAN-10-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, et al. 2008. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13: 23–35. 10.1016/j.ccr.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Basher F, Wu JD. 2015. NKG2D ligands in tumor immunity: Two sides of a coin. Front Immunol 6: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Xu C, Chu D, Zhang X, Li J, Ji G, Hong L, Feng Q, Li X, Wu G, et al. 2014. Human leukocyte antigen G is associated with esophageal squamous cell carcinoma progression and poor prognosis. Immunol Lett 161: 13–19. 10.1016/j.imlet.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Xia G, Xiang Z, Liu M, Wei Z, Yan J, Chen W, Zhu J, Awasthi N, Sun X, et al. 2019. A C-X-C chemokine receptor type 2–dominated cross-talk between tumor cells and macrophages drives gastric cancer metastasis. Clin Cancer Res 25: 3317–3328. 10.1158/1078-0432.CCR-18-3567 [DOI] [PMC free article] [PubMed] [Google Scholar]