Abstract
Background:
The relationship between ventricular rate (VR) during atrial fibrillation (AF) and the skin sympathetic nerve activity (SKNA) remain unclear.
Objective:
To test the hypothesis that SKNA bursts accelerate VR during AF.
Methods:
We simultaneously recorded electrocardiogram and SKNA from 8 patients (median age 66.0 years [interquartile range (IQR) 59.0 – 77.0], 4 males) with 30 paroxysmal AF episodes (all >10 min long) and 12 patients (73.0 years [IQR 60.5 – 80.0], 6 males) with persistent AF. The average voltage of SKNA (aSKNA, μV) during AF was analyzed in one-min windows and binned, showing two Gaussian distributions. We used the mean + 3 standard deviation as the threshold that separates burst from baseline (non-burst) SKNA. All 1-min aSKNA above threshold were detected and the area between aSKNA and baseline of every 1-min was calculated and added together as burst area.
Results:
The VR was higher during SKNA bursts than during non-burst period (103 bpm [IQR 83 – 113] vs. 88 bpm [IQR 76 – 101], respectively, p = 0.003). In the highest quartile of the burst area during persistent AF, scatter plot of maximal aSKNA and VR during each SKNA burst shows higher aSKNA and VR. Overall estimates of correlation coefficient between maximal VR and aSKNA during bursts shows a positive correlation in the highest quartile of the burst area (0.64 [95% CI 0.54 – 0.74], p < 0.0001).
Conclusion:
SKNA bursts are associated with VR acceleration. These SKNA bursts may be new therapeutic targets for rate control during AF.
Keywords: Autonomic nervous system, Sympathetic nerve burst activity, Skin sympathetic nerve activity, atrial fibrillation, rate control
Introduction
Ventricular rate (VR) control is an important strategy in managing patients with atrial fibrillation (AF).1 The optimal VR targets for rate control are controversial.2 However, a resting VR of < 80 bpm is a target for rate control in AFFIRM trial.1 While the most commonly recommended drugs for rate control during AF are beta blockers,2 the relationship between sympathetic nerve activity (SNA) and VR during AF remains incompletely understood. Specifically, it remains unclear if any specific SNA patterns are associated with poor rate control. Direct recordings of the stellate ganglion (SG), vagal nerve and the inferior vena cava-inferior atrial ganglionated plexus (IVC-IAGP) suggest that sympathetic activation increases while parasympathetic activation reduces VR in ambulatory dogs with AF.3–5 Microneurography is the standard but challenging technique for direct SNA recording in humans.6 One study showed that single unit muscle SNA (MSNA) positively correlated with RR interval (i.e., negatively with VR).7 That finding is opposite to the conventional thinking that SNA accelerates VR in AF. However, the MSNA is sensitive to blood pressure, which may be highly variable due to irregular RR intervals. MSNA may not be a valid measure of cardiac sympathetic tone during AF. In contrast, skin SNA (generally abbreviated as SSNA in microneurography literature) is sensitive to emotion.8 SSNA but not MSNA is positively correlated with heart rate and the mean blood pressure during white coat hypertension.9 These data suggest that SSNA may be more useful than MSNA in estimating the cardiac sympathetic tone during AF. However, we were not able to find any published work supporting that relationship. This gap of knowledge is likely due to the technical difficulties of obtaining stable SSNA recordings over sufficient duration of time during AF for a robust analysis of the SNA and VR. The sympathetic postganglionic fibers from the SG are distributed in the skin area between the third cervical vertebra and the level of the thirteenth rib in dogs.10 The direct communication between the SG and the cervical/thoracic skin provides a physiological basis for the use of skin SNA to estimate the stellate ganglion nerve activity (SGNA) in canine models11, 12 and in humans.13–16 The purpose of the present study is to simultaneously record superficial skin SNA (SKNA) and electrocardiogram (ECG) using conventional patch electrodes (neuECG) to test the hypothesis that SKNA bursts are responsible for poor VR control during AF.
Methods
This research protocol was approved by the Institutional Review Board of the Indiana University School of Medicine. Written and informed consent was obtained from each patient. We recorded SKNA and ECG (neuECG) from a total of 56 hospitalized patients, including 39 with a history of paroxysmal AF and 17 with persistent AF. Among the patients with paroxysmal AF, 26 had no AF episodes during recording and 5 had the AF lasting less than 10 min. Five patients with persistent AF were excluded because of pacing artifacts during recording. The remaining 8 patients with paroxysmal AF and 12 patients with persistent AF were included in this study. The data from 5 of the 8 patients with paroxysmal AF were used in previous reports to study the relationship between SKNA and arrhythmia onset/termination.15,16
Detailed methods of data analyses are included in an online supplement.
Statistical analysis
Continuous variables were summarized by median (Interquartile range). Categorical variables were summarized by frequency and percentage. The Mann-Whitney U test, chi-square test, the Wilcoxon signed rank test, Pearson’s or Spearman’s correlation coefficient (r value) and multiple regression analysis with standard least squares were performed. Two-sided p values ≤ 0.05 were considered statistically significant. See online supplement for details.
Results
Patient characteristics
The patient characteristics are shown in Supplemental Table 1. We included 8 patients (median age 66.0 years [interquartile range (IQR) 59.0 – 77.0], 4 males) with paroxysmal and 12 patients (median age 73.0 years [IQR 60.5 – 80.0], 6 males) with persistent AF. There were no significant differences in body-mass index, left ventricular ejection fraction (LVEF), left atrial diameter, CHA2DS2-VASc score, diseases or medication between paroxysmal and persistent AF patients. Among the paroxysmal AF episodes, 30 (median 1.0 episodes [IQR 1.0 – 7.8]) had > 10-min duration and were included in the analyses. The median recording duration in paroxysmal and persistent AF were 24.3 hrs [IQR 21.3 – 39.2] and 13.5 hrs [IQR 3.5 – 23.6], respectively (p = 0.0339). The median percentage of paroxysmal AF during recording was 27.8 % (IQR 9.3 – 68.8). There were no significant differences in median study duration between paroxysmal and persistent AF (5.5 hrs [IQR 1.0 – 27.1] vs. 13.0 hrs [IQR 3.5 – 23.5], p = 0.298).
Correlation between average amplitude of SKNA with VR during AF
Figure 1 shows the actual recording of SKNA and ECG during sinus rhythm and AF, respectively over a 60-s window. The data during sinus rhythm (Figure 1A) is from patient B (Figure 2). In sinus rhythm, the SKNA discharges preceded the VR elevation with a short latency. On the other hand, the data during AF (Figure 1B and 1C) are from patient O (Figure 3). Figure 1B shows the average VR during the SKNA discharges (double headed arrows) increased from 96 bpm to 107 bpm. However, in Figure 1C, the VR elevation by the SKNA discharges was less apparent. Because of the VR variations during AF, the temporal relationship between SKNA and VR elevation was more difficult to appreciate during this short time period than during sinus rhythm. Figure 2 shows VR and average amplitude of SKNA (aSKNA, μV) correlation of all 8 patients with paroxysmal AF. Each dot shows the data over 1-min. Different episodes from the same patients were pooled together to form one graph for that patient. A significant positive correlation was found in 6 of 8 patients. The remaining two patients (A and E) had only limited duration of AF for this analysis. The median slopes of linear regression (red lines) in all patients studies were 18.7 (IQR 6.5 – 37.5). Figure 3 show the scatter plots between aSKNA and VR in patients with persistent AF. All but one patient had significant positive correlation between aSKNA and VR.
Figure 1.
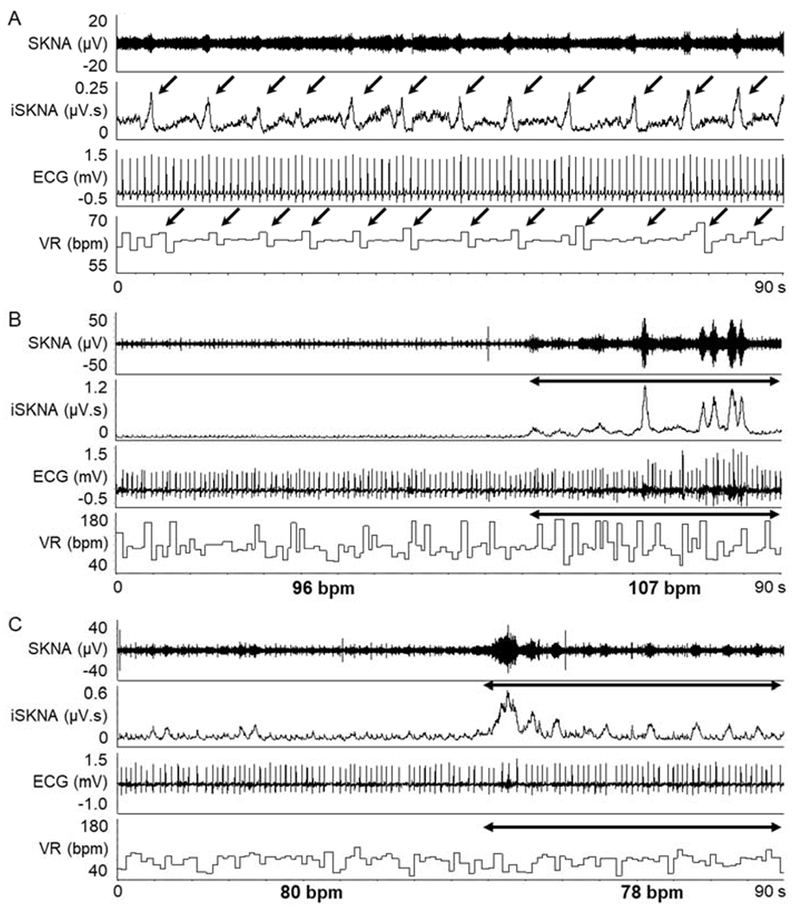
Representative neuECG recording from the patients with AF. A, recordings during sinus rhythm from patient B in Figure 2 (a 58 year-old male without other diseases). The SKNA discharges (down arrows) is associated with VR elevation. B and C, recording during AF from patient O in Figure 3 (a 73 year-old male with valvular disease). The SKNA discharges during AF (double headed arrow in B) increased the average VR from 96 bpm to 107 bpm. C shows that in a different time period, there was little VR changes during SKNA discharges (double headed arrow). SKNA = skin sympathetic nerve activity; iSKNA = integrated SKNA; AF = atrial fibrillation; ECG = electrocardiogram; VR = ventricular rate.
Figure 2.
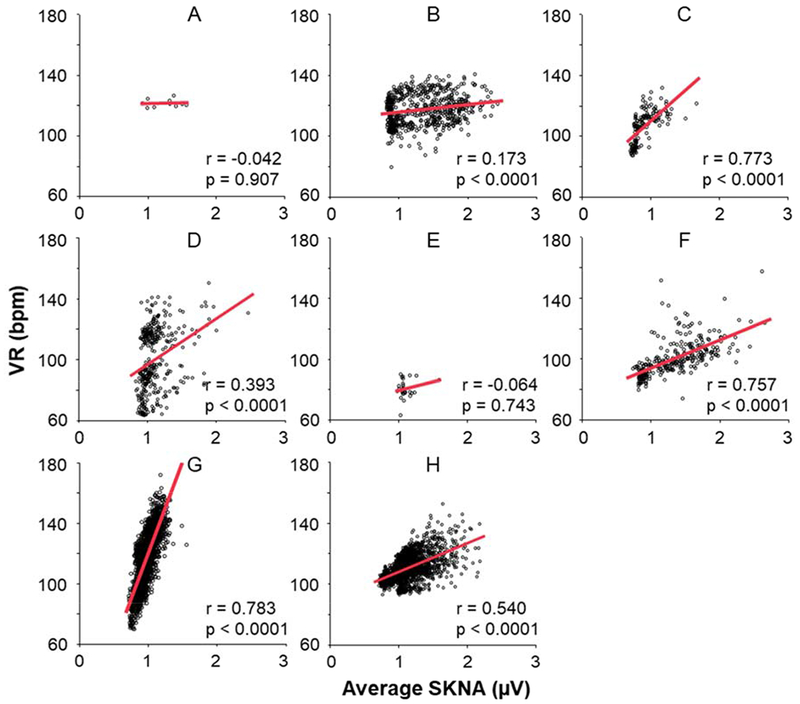
Correlation between aSKNA and VR during paroxysmal AF. A-H indicate patient ID in this study. The scatter plots show the correlation between aSKNA and VR every 60-s, and red lines mean the linear regression between aSKNA and VR. In a total of 6 patients, there were significant positive correlation between aSKNA and VR, and all the slopes of linear regression (red lines) indicated positive. aSKNA = average amplitude of SKNA.
Figure 3.
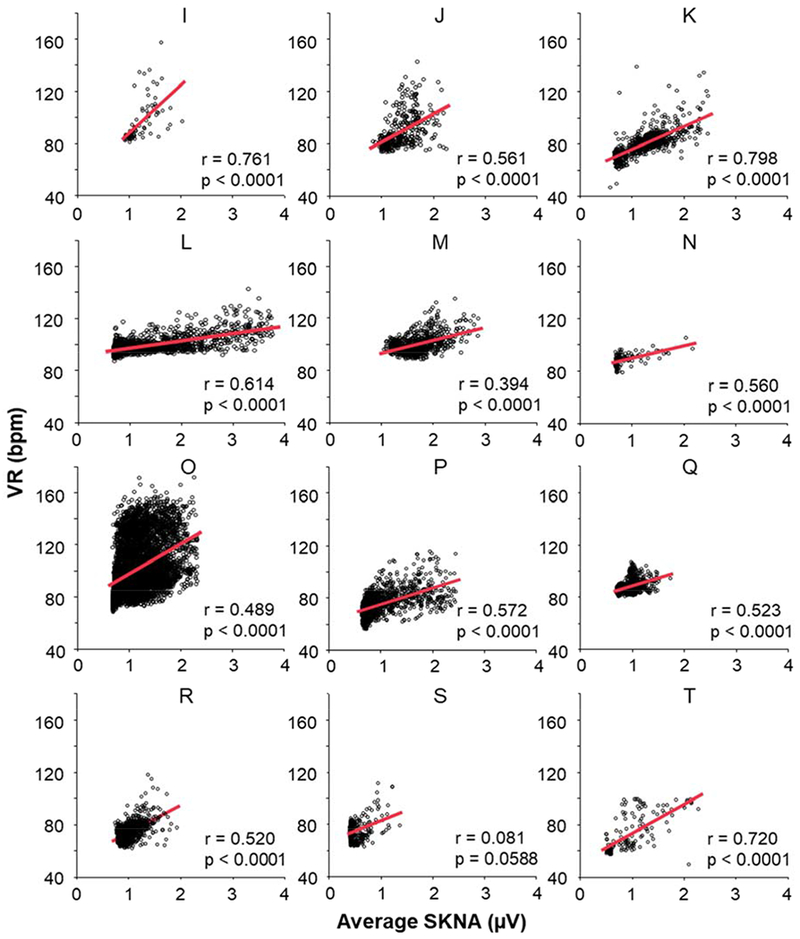
Correlation between aSKNA and VR during persistent AF in all 12 patients (I-T) with persistent AF. Each dot indicates the aSKNA and VR in a 60-s window. The red lines indicate the linear regression between aSKNA and VR. There were significant positive correlation between aSKNA and VR in 11 of the 12 patients.
The box-and-whiskers plots in Figure 4A–C compare various parameters between paroxysmal and persistent AF. There were no significant differences of aSKNA in paroxysmal and persistent AF (1.12 μV [IQR 1.02 – 1.30] vs. 1.09 μV [IQR 0.91 – 1.33], respectively, p = 0.729) (Figure 4A), but the VR was higher during paroxysmal AF than persistent AF (105 bpm [IQR 97 – 118] vs. 88 bpm [IQR 74 – 99], respectively, p = 0.008) (Figure 4B). The median slopes of linear regression (red lines in Figures 2 and 3) were 18.7 (IQR 6.5 – 37.5) in patients with paroxysmal AF and 16.7 (IQR 10.5 – 22.0) in patients with persistent AF (p=0.787) (Figure 4C). Supplemental Figures 1A and 1B indicate the overall estimates of correlation coefficients between aSKNA and VR during paroxysmal and persistent AF. In random effects model, there were no differences in overall estimate of the correlation coefficient between paroxysmal AF and persistent AF (0.51 [95% confidence interval; CI 0.36 – 0.66] vs. 0.55 [95% CI 0.47 – 0.64] respectively, p = 0.609).
Figure 4.
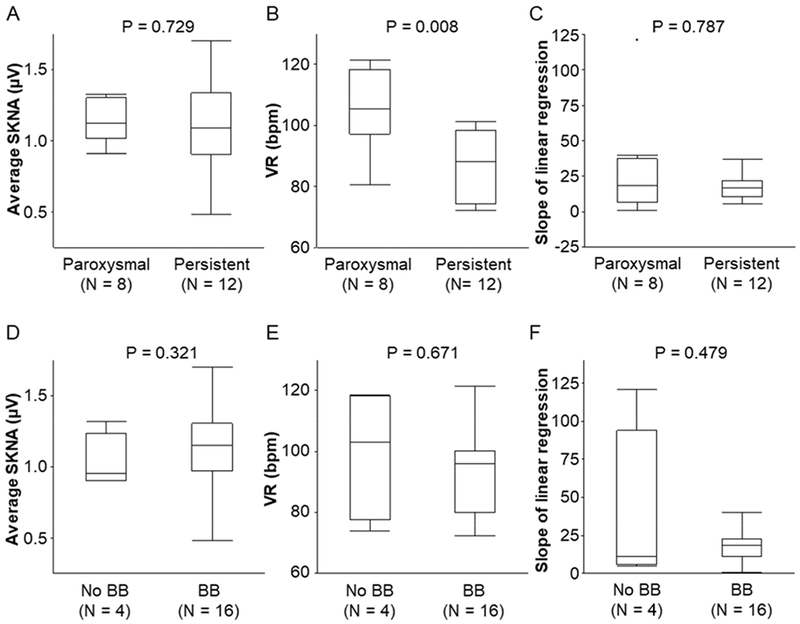
Relationship between AF types and beta-blocker therapy on aSKNA, VR and slopes of linear regression. The box-and-whiskers plots (A-C) show that there were no differences of aSKNA, VR and average slope of linear correlation, respectively, between paroxysmal and persistent AF. The box-and-whiskers plots (D-F) show that beta blocker (BB) therapy did not change the aSKNA, VR or the slopes of linear correlation, respectively. P values were determined by the Mann-Whitney U test.
Relationship between VR during AF and clinical parameters
Table 1 shows multiple regression analysis to determine the factors that contribute to VR control during AF. It shows that aSKNA and LVEF strongly and positively correlated with VR, with Beta of 0.646 (p = 0.011) and 0.683 (p = 0.013), respectively. Age is a significant negative contributor (Beta = −0.531, p = 0.018).
Table 1.
Multiple regression analysis between VR and clinical parameters
| Model | Unstandardized
coefficients |
Standardized
coefficients |
t | P-value | VIF | ||
|---|---|---|---|---|---|---|---|
| B | SE | Beta | |||||
| (Constant) | 74.752 | 28.620 | 2.61 | 0.022 | |||
| aSKNA (μV) | 38.443 | 12.869 | 0.646 | 2.99 | 0.011 | 1.695 | |
| LVEF (%) | 0.590 | 0.206 | 0.683 | 2.87 | 0.013 | 2.049 | |
| Age, years | −0.753 | 0.277 | −0.531 | −2.72 | 0.018 | 1.385 | |
| Beta-blocker | −8.506 | 6.419 | −0.227 | −1.33 | 0.208 | 1.060 | |
| CCB | 7.784 | 5.936 | 0.247 | 1.31 | 0.213 | 1.289 | |
| Male | 4.940 | 6.350 | 0.165 | 0.78 | 0.451 | 1.621 | |
Because there were no significant differences in clinical characteristics or demographics between patients with paroxysmal AF as compared with patients with persistent AF, all patients were analyzed together as a single group. R2 = 0.641, adjusted R2 = 0.475. VR = ventricular rate; SE = standard error; VIF = variance inflation factor; aSKNA = average amplitude of skin sympathetic nerve activity; LVEF = left ventricular ejection fraction; CCB = calcium channel blocker.
The effects of beta-blockers on the VR
Among the study subjects, 16 were treated with beta-blockers and 4 were not. Beta blocker therapy did not change aSKNA (Figure 4D, 1.15 μV [IQR 0.97 – 1.31] vs. 0.96 μV [IQR 0.91 – 1.24], p = 0.321, respectively), VR (Figure 4E, 96 bpm [IQR 80 – 100] vs. 103 bpm [IQR 78 – 118], p = 0.671, respectively) or the slopes of linear correlation (Figure 4F, 18.7 [IQR 11.5 – 22.9] vs. 10.9 [IQR 6.0 – 94.0], p = 0.479). Supplemental Figure 2 indicates the overall estimates of correlation coefficient with beta-blocker therapy. In random effects model, there were no differences in overall estimated correlation coefficients between aSKNA and VR during AF with or without beta-blockers (0.54 [95% CI 0.46 – 0.62] vs. 0.53 [95% CI 0.32 – 0.73], p = 0.999).
Effects of age on VR
Figure 5A and 5B show the effects of age on aSKNA and VR during AF. The aSKNA (blue dots) and VR (orange dots) were both negatively correlated with age (r = −0.542, p = 0.014, and r = −0.460, p = 0.041, respectively).
Figure 5.
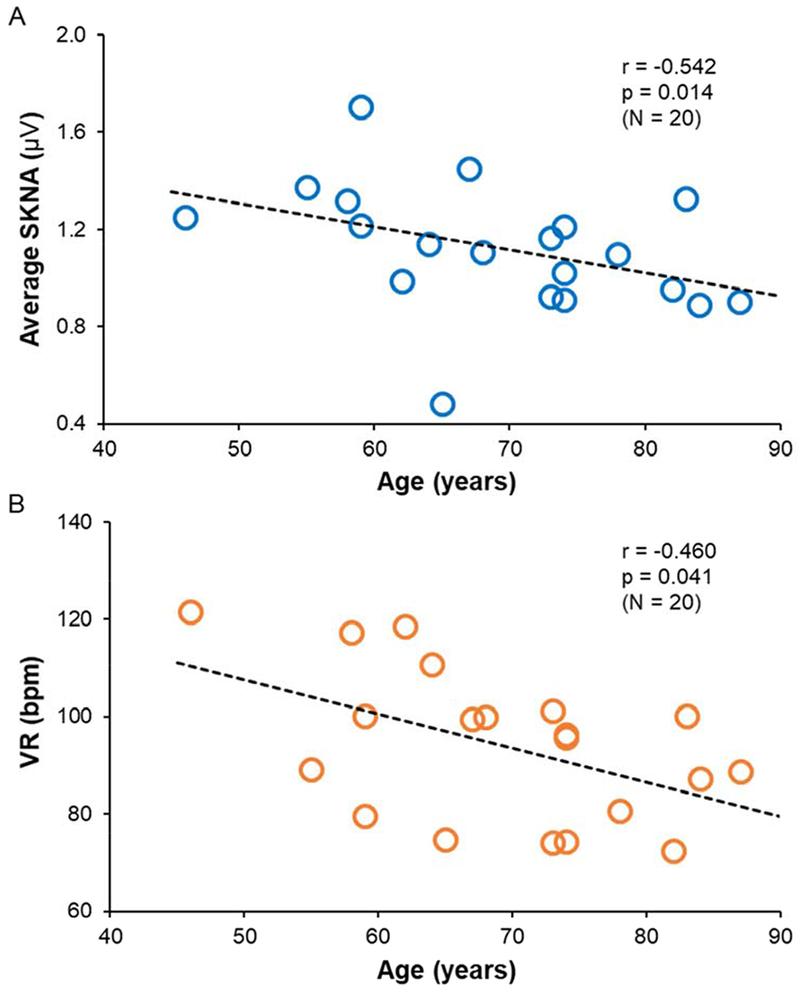
Effects of age on aSKNA and VR. Each dot indicates a patient with paroxysmal or persistent AF. Both the aSKNA (panel A) and VR (panel B) were negatively correlated with age (r = −0.542, p = 0.014, and r = −0.460, p = 0.041, respectively).
Trajectory of SKNA and VR during AF over 24-hr
Supplemental Figure 3 shows the trajectory of aSKNA and VR during AF over 24-hr. The aSKNA was the lowest at midnight and increased gradually during the day. It eventually peaked in the early evening (Supplemental Figure 3A). Meanwhile, the VR during AF had two peaks in the morning and early evening (Supplemental Figure 3B). There was a dip in VR but not aSKNA around noon. However, there were no circadian variations in slope of linear regression and correlation coefficient between aSKNA and VR during AF (p = 0.82, p = 0.08, respectively).
Effects of SKNA bursts on VR during AF
Figure 6A shows the values of aSKNA (blue line) and VR (black line) in each one-min window over a 24-hr period from patient O. Red dotted line indicates the threshold of SKNA bursts, associating with VR acceleration. Red and black arrows correspond to the Figures 1B and 1C, respectively. Supplemental Figure 4A shows our methods to separate burst from non-burst SKNA. These data were from patient K. We binned the aSKNA on the X axis and plotted their proportions on the Y axis. There are two groups of aSKNA; each can be fitted into a Gaussian distribution. The first (left) Gaussian distribution represents the baseline nerve discharges while the second (right) Gaussian distribution represents the burst activity. We used the mean plus 3 times SD of the first Gaussian distribution (0.817 μV) as the threshold to separate these two groups of activities. The aSKNA burst thresholds for paroxysmal and persistent AF were 1.21 μV (IQR 1.05 – 1.31) and 0.87 μV (IQR 0.77 – 1.19), respectively (p = 0.041). Using individualized threshold levels, we divided the SKNA recording of each patient into burst and non-burst. Supplemental Figure 4B shows a representative scatter plot of aSKNA and VR from the patient P (Figure 3). The slopes of linear regression were positive for both burst and non-burst activities, but was steeper in the latter. The aSKNA during burst was 1.40 μV (IQR 1.23 – 1.56) and during the non-burst was 0.92 μV (IQR 0.73 – 1.04, p < 0.0001) (Supplemental Figure 4C). VR was higher during SKNA bursts than during non-burst (103 bpm [IQR 83 – 113] vs. 88 bpm [IQR 76 – 101], respectively, p = 0.003) (Supplemental Figure 4D). The slope of linear regression during non-burst was significantly steeper than that during burst SKNA (29.9 [IQR 13.8 – 77.3] vs. 10.6 [IQR 7.9 – 14.9], respectively, p = 0.003). (Supplemental Figure 4E).
Figure 6.
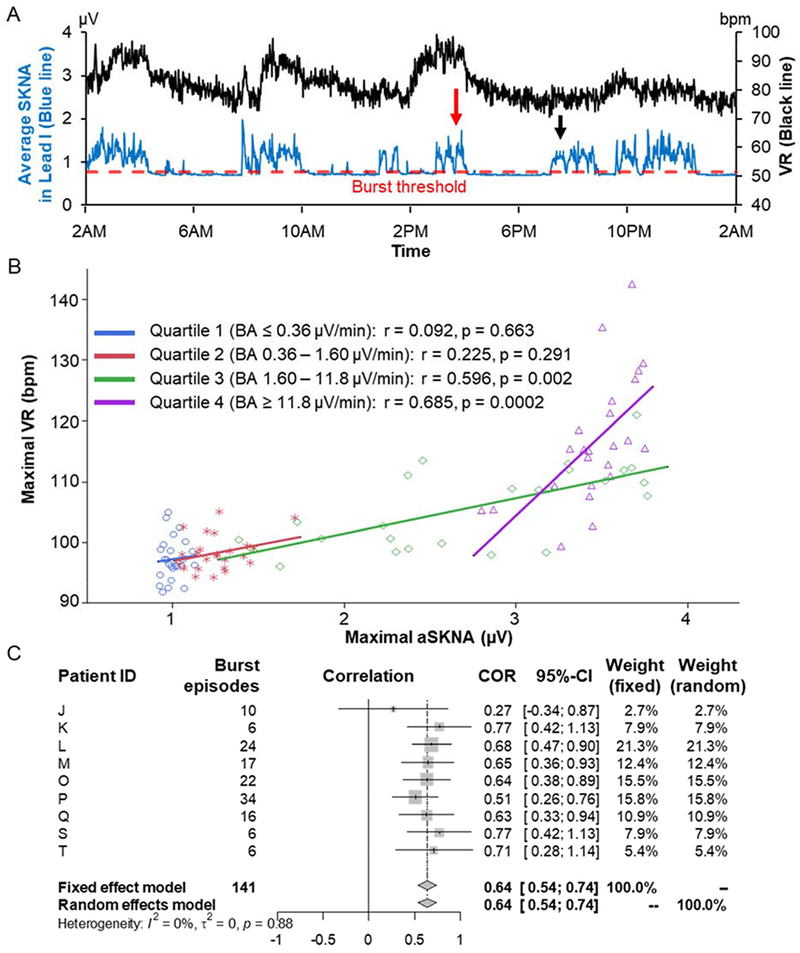
VR and SKNA bursts. A) Average SKNA (aSKNA) from Lead I (blue line) and VR (black) in one-min windows over 24-hr from patient O. Red dotted line indicates the threshold of SKNA bursts, associating with VR acceleration. Red and black arrows correspond to the Figures 1B and 1C, respectively. The total SKNA burst and non-burst periods were 11 hrs and 13 hrs, respectively. B) Representative scatter plots from the patient L (a 67 year-old male with reduced left ventricular ejection fraction and enlarged left atrium), showing maximal VR and aSKNA during SKNA bursts in each quartile of the burst area. Each line segment indicates the linear regression. The highest quartile of the burst area (purple) shows higher aSKNA and VR and steeper positive linear regression. C) Forest plots of correlation coefficients between maximal VR and aSKNA during SKNA bursts in the highest quartile of the burst area (quartile 4). Patient ID are same in Figure 3. In random effects model, there was a positive correlation between maximal aSKNA and VR during SKNA bursts in the highest quartile of the burst area (0.64 [95% CI 0.54 – 0.74], p < 0.0001). COR = correlation; CI = confidence interval; I2 = I-squared; τ2 = Tau-squared.
Supplemental Figure 4F shows enlarged view of aSKNA from 2:30 PM to 4:30 PM in Figure 6A. Pink region is the aSKNA area over baseline, or burst area. For the study of burst area, we excluded 3 patients with persistent AF and all the patients with paroxysmal AF whose data included only few SKNA bursts or were non-continuous. In the remaining 9 patients with persistent AF, the SKNA burst parameters were calculated as below: burst frequency (bursts/hr) 3.7 (IQR 2.8 – 5.4); burst duration (%) 58.4 (IQR 29.1 – 62.7); total burst area (μV*min) 198.0 (IQR 93.8– 485.3). Figure 6B shows that the representative scatter plot of maximal aSKNA and VR during each SKNA burst across quartile of the burst area from the patient L (Figure 3). The higher aSKNA and VR were shown in the quartile 4 of the burst area than in the lower ones. These features were consistently observed in the other patients. Figure 6C shows the overall estimates of correlation coefficient between maximal VR and aSKNA during SKNA bursts in the quartile 4 of the burst area. In random effects model, there was a positive correlation between maximal aSKNA and VR during SKNA bursts in the highest quartile of the burst area (0.64 [95% CI 0.54 – 0.74], p < 0.0001). On the other hand, Supplemental Figure 5 shows there were no strong relationships in overall estimate of correlation coefficients in the lower quartiles (quartile 1: 0.28 [95% CI −0.02 – 0.58], p = 0.068; quartile 2: 0.02 [95% CI −0.26 – 0.30], p = 0.887; quartile 3: 0.36 [95% CI 0.12 – 0.60], p 0.003). These results suggest that the higher and longer SKNA burst accelerate VR during AF.
Discussion
We found that the aSKNA positively correlates with VR during both paroxysmal and persistent AF. Large and long SKNA bursts are associated with VR acceleration. These findings indicate that large SKNA bursts are responsible for poor rate control during AF. These large SKNA bursts may be a new therapeutic target for rate control in AF.
Autonomic control of AV conduction
The VR during chronic AF demonstrates a prominent circadian variation.17 Provocative maneuvers that increase sympathetic or parasympathetic tone can respectively increase and reduce the VR during AF.18 Direct nerve recordings in ambulatory dogs showed that spontaneous vagal nerve activity and vagal nerve stimulation are associated with VR reduction.4, 5 These studies suggest that parasympathetic activation reduces VR during AF through the activation of IVC-IAGP. In addition to parasympathetic activation, SNA is also important in VR control during AF. Chinda et al19 recorded SGNA in ambulatory dogs during persistent AF. The results clearly indicate that increased SGNA is associated with an abrupt acceleration of the VR. The same study found that chronic vagal nerve stimulation damages the SG, reduces the SGNA, leading to reduced VR during AF. Taking together, these data indicate that SNA accelerates while parasympathetic nerve activity reduces VR during AF in ambulatory dogs. Neuromodulation methods that increases parasympathetic tone and reduces sympathetic tone may facilitate rate control during AF. The results of the present study is consistent with the results of those canine studies, showing SKNA is positively associated with VR during both paroxysmal and persistent AF in humans. However, a strong positive correlation was not always observed, suggesting that parasympathetic activity might also play a role in VR control during AF.
Quantitative analyses of SKNA
One advantage of neuECG recording, over the microelectrode recording, is that neuECG recording uses equipment calibrated for voltage recording rather than using artificial amplitude units. The availability of the absolute voltage allows us to compare the amplitudes of SKNA among different individuals. We used two different methods to quantitate SKNA. The first method is to integrate all SKNA signals over the entire recording window and divide the total voltage by the number of digitized samples in the same window to obtain the average voltage of SKNA per sample (aSKNA).13 The strength is that it is an unbiased measurement of nerve activity. However, because microneurography studies primarily use the number of bursts as a quantitative measure,20 it is difficult to compare aSKNA with the burst activity reported in the microneurography literature. We have therefore developed a second method of analyses that includes the identification of burst and non-burst SKNA. A prominent characteristic of the electrical activity in the nervous system is the prevalence of spontaneous activity, both at the level of the spatially summed potentials observed with a gross electrode and at the level of the action potentials of a single neuron.21 These spontaneous activities include both single-spike activity and the burst-firing mode.22 The single spike mode of nerve activity demonstrates variations of activation frequencies and amplitude with Gaussian distribution.21, 22 The burst mode increases the number of nerve spikes within a unit time, resulting in increased amplitudes and firing frequencies. Based on these characteristics, we designed a method to separate burst activity from non-burst activity of SKNA. The results show that SKNA bursts are the primary SKNA patterns responsible for VR accelerate during AF. The IQR 83 – 113 bpm during SKNA bursts indicate that these burst activities are responsible for poor VR control during AF according to the AFFIRM trial criteria.1
Effects of Age and LVEF
Previous studies using microneurography techniques showed that aging increased the resting MSNA 23 but impaired SSNA.24, 25 The latter effects contribute to age-related decrements in reflex cutaneous vasoconstriction. These aging-related changes of SSNA may therefore negatively affect thermoregulation in elderly individuals. Aging is known to be negatively associated with VR, a finding attributed to increasing prevalence of impaired atrioventricular (AV) conduction.26 We found a negative correlation between age and the magnitudes of the SKNA, which is known to correlate with the SGNA.11 In addition to reduced AV nodal function, the reduced cardiac sympathetic tone might also contribute to the development of bradycardia in the elderly during AF. In addition to SKNA and age, a third factor that significantly correlates with VR is the LVEF. Heart failure can induce AV nodal remodeling, including increased fibrosis.27 It is possible that the magnitude of remodeling increases with worsening heart failure, leading to reduced AV nodal conduction and VR.
Limitations of the study
A major limitation is that we did not measure the parasympathetic nerve activity, which may be as important as SNA in triggering AF.28 However, because the rhythm is AF, we were not able to use heart rate variability analyses to estimate the parasympathetic tone. Therefore, the importance of sympathetic/parasympathetic balance in AV conduction cannot be directly determined. A second limitation is the small sample size due in part to the various technical difficulties. For example, a subset of patients with a diagnosis of paroxysmal AF but did not have spontaneous AF episodes during the recording period. The data were obtained while 17 (85%) of the study subjects were being treated for hypertension. It is unclear if the hypertension has affected the SKNA patterns and rapid VR. However, the blood pressure of these patients in this study were clinically controlled.
Conclusion
We conclude that the SKNA bursts are associated with VR acceleration. These SKNA bursts may be new therapeutic targets for rate control during AF.
Supplementary Material
Acknowledgements:
We thank David Adams, BSEE for his assistance.
Sources of Funding:
This study was supported in part by NIH Grants R42DA043391 (Dr Everett), R56 HL71140, TR002208, R01 HL139829, 1OT2OD028190 (Dr. Chen), a Charles Fisch Cardiovascular Research Award endowed by Dr Suzanne B. Knoebel of the Krannert Institute of Cardiology (Drs. Kusayama and Everett), a Medtronic-Zipes Endowment, and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative (Dr. Chen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: The authors declare no competing financial interests. Indiana University has submitted patent application to protect the intellectual property related to this work.
References
- 1.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 3.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation 2008;118:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park HW, Shen MJ, Han S, et al. Neural control of ventricular rate in ambulatory dogs with pacing-induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Z, Zhao Y, Tsai WC, et al. Effects of Vagal Nerve Stimulation on Ganglionated Plexi Nerve Activity and Ventricular Rate in Ambulatory Dogs With Persistent Atrial Fibrillation. JACC Clinical electrophysiology 2018;4:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Victor RG, Leimbach WN Jr., Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 1987;9:429–436. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda T, Murai H, Kaneko S, et al. Augmented single-unit muscle sympathetic nerve activity in heart failure with chronic atrial fibrillation. The Journal of physiology 2012;590:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown R, Macefield VG. Skin sympathetic nerve activity in humans during exposure to emotionally-charged images: sex differences. Frontiers in physiology 2014;5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassi G, Seravalle G, Buzzi S, et al. Muscle and skin sympathetic nerve traffic during physician and nurse blood pressure measurement. Journal of hypertension 2013;31:1131–1135. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T, Morimoto M, Taniguchi Y, Takasaki M, Totoki T. Cutaneous distribution of sympathetic postganglionic fibers from stellate ganglion: A retrograde axonal tracing study using wheat germ agglutinin conjugated with horseradish peroxidase. J Anesth 1994;8:441–449. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Z, Zhao Y, Doytchinova A, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart rhythm 2015;12:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson EA, Rhee KS, Doytchinova A, et al. Estimating sympathetic tone by recording subcutaneous nerve activity in ambulatory dogs. Journal of cardiovascular electrophysiology 2015;26:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart rhythm 2017;14:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabir RA, Doytchinova A, Liu X, et al. Crescendo Skin Sympathetic Nerve Activity and Ventricular Arrhythmia. Journal of the American College of Cardiology 2017;70:3201–3202. [DOI] [PubMed] [Google Scholar]
- 15.Uradu A, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart rhythm 2017;14:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusayama T, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI insight 2019;4:e125853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raeder EA. Circadian fluctuations in ventricular response to atrial fibrillation. Am J Cardiol 1990;66:1013–1016. [DOI] [PubMed] [Google Scholar]
- 18.Nagayoshi H, Janota T, Hnatkova K, Camm AJ, Malik M. Autonomic modulation of ventricular rate in atrial fibrillation. The American journal of physiology 1997;272:H1643–1649. [DOI] [PubMed] [Google Scholar]
- 19.Chinda K, Tsai WC, Chan YH, et al. Intermittent Left Cervical Vagal Nerve Stimulation Damages the Stellate Ganglia and Reduces Ventricular Rate During Sustained Atrial Fibrillation in Ambulatory Dogs. Heart Rhythm 2016;13:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart EC, Head GA, Carter JR, et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. American journal of physiology Heart and circulatory physiology 2017;312:H1031–h1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerstein GL, Mandelbrot B. RANDOM WALK MODELS FOR THE SPIKE ACTIVITY OF A SINGLE NEURON. Biophysical journal 1964;4:41–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. The Journal of neuroscience : the official journal of the Society for Neuroscience 1999;19:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase S, Mano T, Watanabe T, Saito M, Kobayashi F. Age-related changes of sympathetic outflow to muscles in humans. Journal of gerontology 1991;46:M1–5. [DOI] [PubMed] [Google Scholar]
- 24.Smith CJ, Alexander LM, Kenney WL. Nonuniform, age-related decrements in regional sweating and skin blood flow. American journal of physiology Regulatory, integrative and comparative physiology 2013;305:R877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanhewicz AE, Greaney JL, Alexander LM, Kenney WL. Blunted increases in skin sympathetic nerve activity are related to attenuated reflex vasodilation in aged human skin. Journal of applied physiology (Bethesda, Md : 1985) 2016;121:1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hnatkova K, Waktare JE, Murgatroyd FD, et al. Age and gender influences on rate and duration of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 1998;21:2455–2458. [DOI] [PubMed] [Google Scholar]
- 27.Nisbet AM, Camelliti P, Walker NL, et al. Prolongation of atrio-ventricular node conduction in a rabbit model of ischaemic cardiomyopathy: Role of fibrosis and connexin remodelling. Journal of molecular and cellular cardiology 2016;94:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coumel P Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J 1994;15 Suppl A:9–16. [DOI] [PubMed] [Google Scholar]
- 29.Uradu A, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity precedes the onset and termination of paroxysmal atrial tachycardia and fibrillation. Heart rhythm 2017;14:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusayama T, Wan J, Doytchinova A, et al. Skin sympathetic nerve activity and the temporal clustering of cardiac arrhythmias. JCI insight 2019;4:e125853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Autonomic neuroscience : basic & clinical 2015;193:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart rhythm 2017;14:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


