Abstract
Identifying the neural correlates of positive interactions between friendship dyads may provide insights into mechanisms associated with adolescent social development. Forty-eight 14- to 18-year old typically developing adolescents were video-recorded discussing a shared positive event with a close friend and subsequently viewed clips during an fMRI scan of that friend during the interaction and of an unfamiliar peer in a similar interaction. Adolescents also reported on their positive affect in daily life while with friends using ecological momentary assessment. We used multivariate repeated measures models to evaluate how positive affect with friends in the laboratory and in daily life was associated with neural response to friend and stranger positive and neutral clips. Adolescents who exhibited more positive affect when with friends in the laboratory showed less dorsolateral prefrontal cortex to friend positive clips. More positive affect when with friends in daily life was associated with less bilateral anterior insula response to friend positive clips, but greater left anterior insula response to stranger positive clips. Findings provide information on the role of lateral prefrontal cortex and anterior insula in enjoyment of friendships during adolescence.
Keywords: Adolescence, Positive Affect, Close Friends, Reward
1. Introduction
Adolescence is a protracted period of development, starting at the initiation of puberty and lasting until the assumption of adult independence and responsibilities (Steinberg, 2005). It is characterized by notable social and neural developmental changes (Burnett, Sebastian, Kadosh, & Blakemore, 2011; Somerville & Casey, 2010; Spear, 2000; Steinberg, 2005; Steinberg, 2008). In particular, adolescents spend increasing amounts of time with peers (friends, romantic interests/partners) during this developmental period (Nelson, Leibenluft, McClure, & Pine, 2005). Additionally, neurodevelopmental changes in regions involved in reward processing and social cognition also appear to heighten the value and salience of peers during this period (Nelson et al., 2005; Somerville & Casey, 2010). Perhaps as a function of these combined social and neural developmental changes, adolescents report closer and more intimate relationships with friends at this time relative to earlier in childhood (La Greca & Harrison, 2005). These deepening emotional friendships are important, as positive friendships during adolescence appear to protect against a variety of emotional and problem behaviors (Hiatt, Laursen, Mooney, & Rubin, 2015).
These social changes, which have been described as a developmental social re-orientation that occurs during adolescence, are thought to be guided via the co-occurring maturation of neural systems involved in reward processing, self-regulation, and mentalizing (Nelson et al., 2005; Steinberg, 2008). In particular, reward regions such as the striatum show greater activity, particularly in response to peer-related stimuli during adolescence relative to childhood and adulthood (Casey, Getz, & Galvan, 2008; Luna, Padmanabhan, & O’Hearn, 2010; Steinberg, 2008). Areas implicated in self-regulation, such as the prefrontal cortex and anterior cingulate cortex (ACC), also show heightened responding during this time period (Somerville, 2013). Adolescence also involves changes in function in regions of the brain, such as the medial prefrontal cortex (mPFC), and portions of the temporal and parietal cortices, most notably the temporo-parietal junction (TPJ) and the precuneus, that are implicated in improving abilities in understanding others and accurately assessing emotional contexts (Burnett et al., 2011). These neural regions appear to aid in friendships perhaps via greater understanding of the mental states of others, or mentalization (TPJ, mPFC), and understanding how social events relate to the self (mPFC, precuneus; D’Argembeau et al., 2007; Johnson, Baxter, Wilder, Pipe, Heiserman, & Prigatano, 2002; Mitchell et al., 2005).
Although much work has focused on how peers influence maturing neural systems in regard to risk-taking and social evaluation (Albert, Chein, & Steinberg, 2013; O’Brien, Albert, Chein, & Steinberg, 2011; Burnett et al., 2011; Healey, Morgan, Musselman, Olino, & Forbes, 2014), less is known about the neural underpinnings of positive friendship and the brain-behavior associations relevant to positive affect in friendship contexts. The nature of friendships becomes more intimate during adolescence, relying on more sophisticated dyadic behavior, such as greater reciprocity during interactions (Youniss & Smollar, 1985). Although the mere presence of a peer may be associated with greater activation in regions implicated in reward processing, mentalizing, and perspective-taking, it is unclear how neural responding in these regions might be related to subjective pleasure experienced when interacting with a close friend in the natural environment. Along these lines, Perino, Miernicki, and, Telzer (2016) recently demonstrated that adolescents were more sensitive than children or adults to positive social visual stimuli (e.g., youths laughing together) relative to negative social stimuli (e.g., peer exclusion), as measured by more distraction (i.e., false alarms) during a cognitive task following positive peer stimuli. Moreover, this sensitivity was associated with activation in neural regions implicated in reward and social processing. These findings demonstrate greater reactivity to positive social stimuli in adolescents, but little work has evaluated neural and behavioral correlates of positive, close friendships, relative to peer relationships or to positive stimuli in general, during this developmental period of social re-orientation. Close friendships involve high levels of trust, affiliation, and attachment. Thus, neural regions related to these affiliative processes, such as the hypothalamus, striatum, and septum (Moll et al., 2012), may be especially important during adolescence when deeper, more intimate relationships are forming (La Greca & Harrison, 2005). These findings on adolescent neural response to peers, coupled with prior models highlighting social reorientation during adolescence, suggest the importance of positive friendships during this time period, and point to a greater need to understand the neural correlates associated with adolescent close friendships. Indeed, research using ecologically valid and personally relevant methods (such as viewing clips of one’s own close friend and reporting on daily affect using experience sampling methods) can be extended to research on adolescent friendships in order to obtain a more nuanced understanding of the neural bases of pleasant interactions with friends.
The current study used a novel, personally relevant fMRI task, laboratory behavioral interactions between friends, and ecological momentary assessment (EMA) to examine neural, behavioral, and subjective responses to a close friend during adolescence (Ambrosia et al., 2018). Whereas we previously evaluated how neural response to this novel task was associated with adolescent risky behavior (e.g., substance use, sexual behavior, deviancy etc.), the aim of the current study was to examine associations between neural response to this task and positive friendship affect and behavior (e.g., shared positive affect between friends in the laboratory and in daily life). We evaluated the association between adolescent neural, behavioral, and self-reported emotional responses to positive affect displayed by a close friend. We hypothesized that viewing positive clips of a friend, relative to positive clips of an unfamiliar peer or neutral clips of either friend or stranger, would be associated with greater engagement of brain regions implicated in reward and/or social processing (i.e., striatum, amygdala, TPJ, precuneus, mPFC), based on theory suggesting social re-orientation of these neural systems during adolescence (Nelson et al., 2005). Furthermore, we hypothesized that greater activation in regions implicated in reward processing and mentalizing and lower activation in regulatory regions (e.g., less down-regulation of PA) would be related to greater positive affect when interacting with friends in the laboratory (i.e., target an adolescent’s observed positive affect in response to his/her friend’s positive affect) and with friends in daily life (i.e., as reported via EMA).
2. Methods
2.1. Participants
Participants were 48 adolescents free of psychiatric illness (14–18 years old, Mage= 16.27, N=29 female) and their close friends (n=48) recruited from the community for a study on peer relationships in adolescence (Ambrosia et al., 2018). The absence of psychiatric diagnoses were determined by the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children (KSADS; Kaufman, Birmaher, Brent, & Rao, 1997), a semi-structured interview conducted by researchers trained in its delivery by licensed clinical interviewers. Participants were asked to bring in a close same-sex friend who was within 2 years of their age and participate in a video-recorded discussion task together. Participants also completed EMA in which they reported on their positive affect in daily life over the course of two extended weekends. Then, participants underwent an fMRI scan in which they viewed clips from the video-recorded interaction with their friend as well as clips of an unfamiliar peer in a similar interaction. Seventy participants initially participated in the study, but 22 were removed from analyses due to no longer qualifying after the initial visit due to a recent concussion (N=4), meeting criteria for a psychiatric illness (N=2), failure to complete the fMRI scan (N=8), not having usable fMRI data due to task error or signal loss in the VS (N=5), or not having coded observation data (N=3). All remaining 48 participants had >80% coverage in ventral regions (e.g., ventral striatum). Adolescents with usable data for the study (n=48) and those without did not differ on age, gender, or for those with coded data on levels of positive affect during the laboratory task (ps=.44-.59). The study was approved by the University of Pittsburgh Institutional Review Board and all participants, including the close friend, provided informed consent for participation in the study. Parental consent was also obtained for participants who were minors (including close friends).
2.2. Measures
2.2.1. Ecological Momentary Assessment
Similar to prior work from Silk and colleagues (see Silk et al., 2003, Silk et al., 2011), participants completed an EMA using cellular phone methodology to assess their real-world emotions during a social context. Youth were given answer-only cellular phones on which trained interviewers conducted EMA interviews. Prior to starting the EMA assessment period, youth were familiarized with the phone and interview questions. Youth received calls during two 5-day blocks starting Thursday night and ending Monday night. Two calls were made each weeknight (on Thursday, Friday, and Monday) and 4 calls were made on Saturday and Sunday, totaling 14 calls per week and 28 calls in total. Calls were placed within a set window (e.g., 4–8pm on a weeknight), but not at a pre-specified time so that participants were not expecting them. Adolescents were paid per call and were provided a monetary bonus for completing all calls.
Each call consisted of a brief 5-minute structured interview adapted from Silk et al. (2003, 2011). At each call, the youth were asked to rate their current emotion from a list of items on a 5-point scale ranging from (1) very slightly or not at all to (5) extremely from the Positive and Negative Affect Schedule for Children (PANAS-C; Laurent et al., 1999) at the moment of the call. Positive affect (PA) was measured as an aggregate of ratings on happy, cheerful, excited and interested. After assessing PA, youth were asked who they were interacting with to examine social context. For the current study, mean levels of PA were computed for calls in which the youth reported that a friend was physically present (friend, several friends, romantic interest/partner) (“Target PA with friends in daily life”).
2.2.2. Observed Positive Affect
Friend pairs were asked to discuss a recent, shared fun experience of their choosing (e.g., performing in a school play, attending a party) for 5 minutes and were video-recorded while discussing the experience. Affective behavior from this conversation was coded in two ways for different purposes, as a measure of participant positive affect using the LIFE coding system (Hops, Biglan, Tolman, Arthur, & Longoria, 1995) (1) and as means of creating task stimuli using the AFFEX coding system (Izard et al., 1983) (2) (see below and see Ambrosia et al., 2018).
Independent coders, using the LIFE coding system, coded instances of positive affect in both target participants and friends (ICC target = 0.82; friend = 0.71). The LIFE has an extensive validation history for assessment of affect in adolescent populations (e.g., Schwartz et al., 2011; Sheeber et al., 2009). Positive affect was defined as happy (e.g., laughter, smiling) or caring (e.g., soothing tone, displays of approval) affect as noted in voice tone, facial expression, or body position/movement. Conditional lagged probabilities were computed by considering target positive affect occurring 1 second after the onset of friend positive affect until 1 second after offset. From these values of joint probability, adjusted residuals were computed that reflected associations greater than expected (i.e., positive values) or less than expected (i.e., negative values) based on the sample mean. Thus, adjusted residuals were used as a measure of the target participant’s positive affect when a close friend in the laboratory (“Target Shared PA in Lab”).
2.2.3. fMRI Task
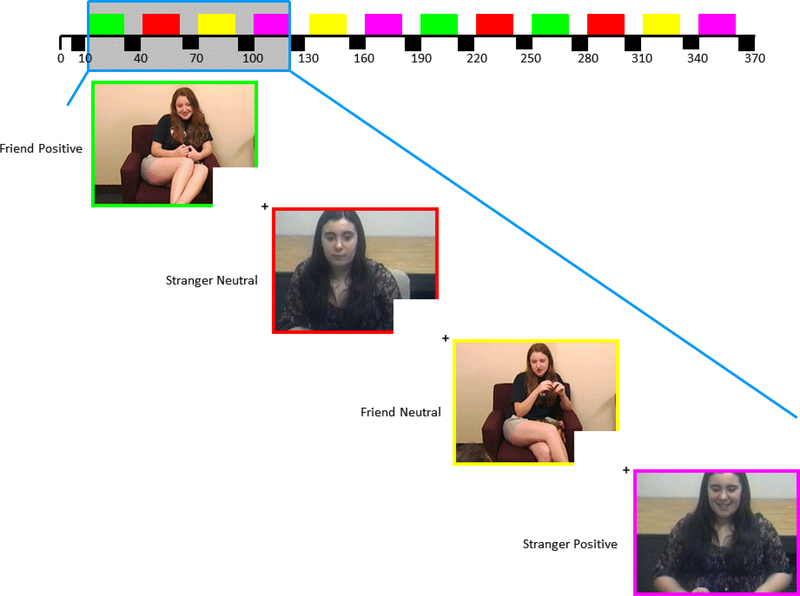
This novel, personally relevant task, “the Best Friend fMRI task (BFF task)”, contained 6 20-second video clips of the participant’s close friend from the lab discussion and 6 20-second video clips of an unfamiliar, same-sex “stranger” adolescent (3 positive affect, 3 neutral affect each) presented in a slow event-related design with 10-second fixation displays between clips. These fixation displays served as a baseline condition (130 seconds total). Clips were presented in a predetermined, pseudorandom order so that positive and neutral affect clips alternated and clips with the friend or unfamiliar peer “stranger” alternated (see Ambrosia et al., 2018). Participants were required to press a button when viewing each clip to ensure task engagement (80% response required). As previously mentioned, to create task stimuli, peer positive and neutral affect from the lab interaction videos were coded in 5-second epochs by a team of trained observers using an adaptation of the AFFEX coding system (Izard et al., 1983). Seventeen of the 70 (24.3%) videotapes were additionally coded by an extensively trained master coder for reliability (mean ICC for PA=.93, range=.86-.96; mean ICC for neutral affect=.92; range=.89-.94). The master coder selected 20-s clips based on codes, and personalized digital files were created for each participant. Stimulus clips included the head and shoulders of the identified best friend and audio of both adolescents. We made efforts to ensure that video clips were equivalent in lighting, camera angle, zoom, and intensity of affect. Using similar methods, video clips of unfamiliar peers were created for control stimuli using adolescent dyads−−1 male dyad, 1 female dyads. All female participants viewed the same female control dyad and all male participants viewed the same male control dyad. Control dyads were two trained adolescent actors interacting with one another in the same manner as actual participants (i.e., discussing a recent fun event) as described above. Control dyads were filmed in a city distant from the study assessment site and were thus unknown to them.
fMRI Acquisition and Preprocessing
Each participant was scanned using a Siemens 3T TIM Trio scanner. Structural images were acquired for co-registration with functional images using a T1-weighted high-resolution MPRAGE sequence (160 axial slices, 1.2 mm thick, TR/TE = 2300/2.98 ms, FOV = 256×240, matrix 256×240, flip angle = 9 degrees). BOLD functional images for the best friend fMRI task were acquired in a single run, with a gradient echo planar imaging sequence and covered 39 axial slices, 3.1 mm thick, beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE = 2000/30 ms, FOV = 205 X 205, matrix = 64×64, flip angle = 90 degrees, repetitions=240). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices (i.e., 240) to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired and inspected a reference EPI scan to confirm the absence of artifacts and good signal across the entire volume of acquisition.
Preprocessing of fMRI data was completed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Preprocessing included segmentation of structural images by tissue type (grey matter vs. white matter), co-registration of each participant’s structural image to functional data, realignment to correct for head motion, spatial normalization into standard stereostatic space using a Montreal Neurological Institute template with a 12-parameter affine model and smoothing with a 6 mm full-width at half-maximum Gaussian filter. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. Voxels were resampled during preprocessing to 2 mm3.
Participants with excess motion (> 3 SD from the subject’s mean, > 0.5 mm scan-to-scan translation, or > 0.01 degrees of scan-to-scan rotation) were identified using Artifact Detection Toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) software and excluded from analyses. For remaining participants, motion parameters from ART were saved for inclusion as a covariate in Level 1 models.
Data Analyses
The following conditions were modeled in the first level general linear model: friend positive, friend neutral, stranger positive, stranger neutral, baseline fixation, and within-subject motion parameters. Friend positive, friend neutral, stranger positive, and stranger neutral conditions were modeled as the average signal across the three 20-second clips of that condition. The baseline condition was modeled as the average signal across the 13 10-second cross-hair fixations (130 seconds total). The motion parameters came from within-subject motion parameters from ART software. Within-subject contrast maps were created for the following contrasts: friend positive affect > baseline, friend neutral affect > baseline, stranger positive affect > baseline, and stranger neutral affect > baseline. We used these contrast maps in our second-level analyses in MRM.
Next, we conducted a 2×2 within-subject ANCOVA in MATLAB using the Multivariate Repeated Measures (MRM) toolbox (McFarquhar, McKie, Emsley, Suckling, Elliot, & Williams, 2016). MRM allows for evaluation of within-subject repeated measures, such as neural response to multiple conditions of a task. We examined the main effects of stimulus affect (positive vs. neutral condition) and person viewed (friend vs. unfamiliar peer condition) and the interactive effect of affect x person on neural response. We included age and gender as covariates, based on previous research suggesting that salience and intimacy within friendships may vary based on gender and age (McNelles & Connolly, 1999).
Next, we ran two 2×2 within-subjects ANCOVA models in MRM. For the first of these, we evaluated how the effects of stimulus affect, person, and stimulus affect x person was moderated by level of Target shared PA in Lab. For the second analysis, we evaluated how the effect of stimulus affect, person, and stimulus affect x person was moderated by level of Target PA with friends in daily life. We evaluated these two ANCOVA models separately due to data loss from missing EMA data. For all models, type I error was controlled by applying a threshold of p < 0.001 and family-wise error correction at cluster level of p < 0.05 (Eklund et al., 2016).
After identifying brain regions with a significant interaction effect, we extracted values from those regions to compare differences in the magnitude of associations between PA and neural activation by condition, which cannot be modeled in MRM. We extracted this data using the MRM toolbox which provides values of average activation for a selected voxel for the identified cluster. We then ran correlations to examine associations between PA and neural activation by condition.
3. Results
3.1. Preliminary Analyses
Four adolescents chose not to complete the EMA protocol and had zero calls. The remaining 44 participants, completed on average 18.66 of 28 calls (67%, Range = 6–27 calls). Further, of these participants, 26 reported being with a friend (non-romantic or romantic) for at least three calls (M # of calls with peers = 4.66 (16% of calls), Range = 1–12). Participants who had completed at least 25% of total calls (7 out of 28) and were with a friend (non-romantic or romantic) for at least three calls were included in the Target PA in Daily Life model (n=26). All 48 participants were included in the task effect model and Target Shared PA in Lab models. Adolescents’ reported a mean PA rating of 3.02 when with friends in daily life (SD=.70, Range= 1.50–4.56). Further, adolescents had an average adjusted probability of 14.47 of shared PA when with friends in the lab (SD= 8.61, Range= −2.95–35.13). There was no significant correlation between Target Shared PA in Lab and Target PA with friends in daily life from the EMA calls (r=.06, p=.72).
3.2. fMRI Task Effects
Findings from the 2×2 ANOVA revealed that adolescents showed greater response to their close friend relative to an unfamiliar peer in the caudate head, orbitofrontal cortex, ventrolateral prefrontal cortex and insula, ventromedial prefrontal cortex, dorsomedial prefrontal cortex, inferior parietal lobe, superior temporal gyrus, precuneus and posterior cingulate, and occipital lobe (bilateral) (see Table 1). There was also a main effect of stimulus affect on neural response. Adolescents showed greater response to positive clips relative to neutral clips in bilateral temporoparietal junction, posterior cingulate, and occipital lobe (see Table 1).
Table 1.
Within-subjects 2×2 ANOVA: Affect, Person, and Affect x Person on Neural Response
| Variable | Cluster # | Region (s) | Peak Coordinates | Voxels | pFWE (cluster) |
|---|---|---|---|---|---|
| Affect | 1 | Left Middle Temporal Gyrus, Inferior Parietal Lobule, Insula, Occipital Lobe | −42, −62, 2 | 3643 | .001 |
| 2 | Right Middle Temporal Gyrus, Superior Temporal Gyrus, Inferior Parietal Lobule, Occipital Lobe | 48, −64, 4 | 5582 | .001 | |
| Person | 1 | Caudate Head, Thalamus, Lentiform Nucleus | −8, −24, −20 | 2195 | .001 |
| 2 | Right Orbitofrontal Cortex, Brodmann Area (BA) 47, Insula, Superior Temporal Gyrus | 42, 24, −6 | 1389 | .003 | |
| 3 | Left Orbitofrontal Cortex, BA 47, Middle Temporal Gyrus | −42, 16, −14 | 1870 | .002 | |
| 4 | Superior and Middle Temporal Gyrus, BA 39 | −52, −60, 20 | 1326 | .003 | |
| 5 | Occipital Lobe, Cuneus | 24, −96, 4 | 266 | .026 | |
| 6 | Medial Frontal Gyrus/ BA 10, Anterior Cingulate/ BA 32 | −6, 54, −6 | 936 | .005 | |
| 7 | Inferior Parietal Lobule, Middle Temporal Gyrus | 50, −48, 22 | 844 | .005 | |
| 8 | Precuneus, Posterior Cingulate | −8, −64, 22 | 1177 | .004 | |
| 9 | Precentral Gyrus | −30, 6, 36 | 169 | .047 | |
| 10 | Superior Frontal Gyrus, BA 6, Medial Frontal Gyrus | 10, 20, 66 | 528 | .008 | |
| Affect x Person | 1 | Left Ventrolateral Prefrontal Cortex, Precentral Gyrus, Inferior Frontal Gyrus/BA 44, Postcentral Gyrus | −54, 10, 0 | 432 | .011 |
Note. Coordinates are peak coordinates from voxel with the maximum t-value. Anatomical region labels are derived from Talairach Daemon.
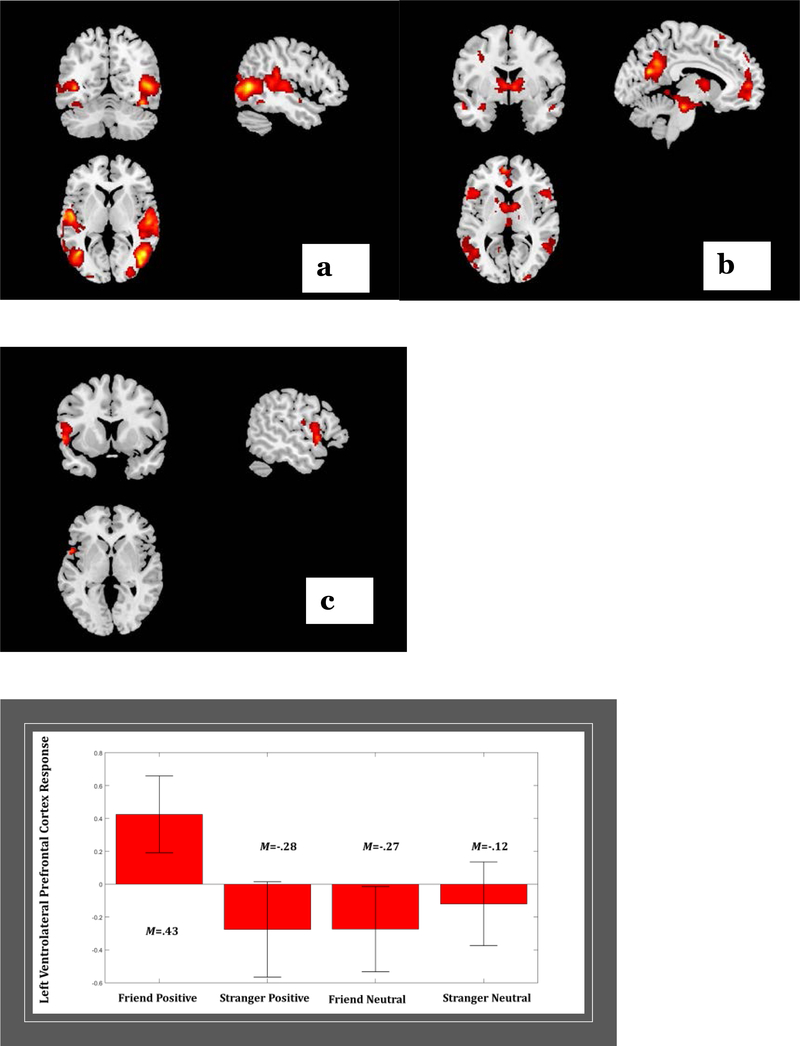
Similar to previous findings (Ambrosia et al., 2018), there was also a significant interactive effect of stimulus affect by person in the left ventrolateral prefrontal cortex (see Figure 2). Post-hoc least squares analysis of means (MFP = .43, MSP = −.28, MFN= −.27, and MSN = −.12) revealed that adolescents showed greater responding in the left vlPFC to friend positive clips relative to all other conditions.
Figure 2.
(a) Adolescents show greater response in temporo-parietal regions to the positive clips relative to neutral clips, regardless of the person.
(b) Adolescents show greater response in multiple neural regions, including caudate and medial prefrontal cortex to their own friend relative to a stranger, regardless of affective valence of clips.
(c) Adolescents show greater response in left ventrolateral prefrontal cortex to friend positive clips relative to stranger positive clips and both friend and stranger neutral clips.
3.3. Observed Positive Affect in Laboratory
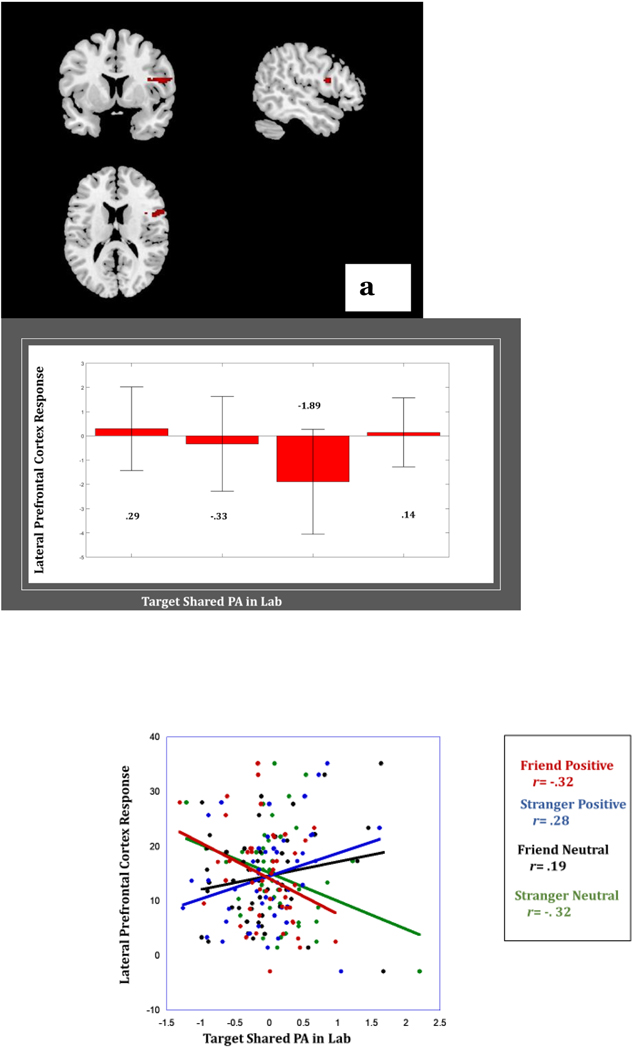
Findings in MRM did not reveal any main effect of Target Shared PA in Lab on neural response nor any 2-way interactive effects of Stimulus Affect x Target Shared PA in Lab or with Person x Target Shared PA in Lab. However, a significant 3-way Stimulus Affect x Person x Target Shared PA in Lab effect did emerge in the Inferior Frontal Gyrus/BA 44/Lateral Prefrontal Cortex (Table 2). Greater levels of Target Shared PA in Lab were related to lower response in this region to clips of friend positive affect (r= −.32) and stranger neutral affect (r= −.32) but were associated with greater response in this region in response to clips of an unfamiliar peer displaying positive affect (r=.28) or of the friend displaying neutral affect (r= .19) (Figure 3).
Table 2.
2×2 ANOVAs with Target Shared PA in Lab and Target PA with Friends in Daily Life
| Variable | Cluster | Region (s) | Peak Coordinates | Voxels | pFWE (cluster) |
|---|---|---|---|---|---|
| Target Shared PA in Lab | |||||
| Affect | No Significant Clusters | ||||
| Person | No Significant Clusters | ||||
| Affect x Person | No Significant Clusters | ||||
| Target Shared PA in Lab | No Significant Clusters | ||||
| Affect x Target Shared PA in Lab | No Significant Clusters | ||||
| Person x Target Shared PA in Lab | No Significant Clusters | ||||
| Affect x Person x PA in Lab | 1 | Inferior Frontal Gyrus/BA 44 | 62, 12, 18 | 198 | .046 |
| Target PA with Friends in Daily Life | |||||
| Affect | 1 | Superior Temporal Gyrus, Postcentral Gyrus | 58, −10, 0 | 388 | .016 |
| Person | 1 | Superior Temporal Gyrus, Inferior Frontal Gyrus | −38, 6, −14 | 908 | .002 |
| 2 | Precuneus, Posterior Cingulate, Caudate, Thalamus | 6, −24, −24 | 3818 | <.001 | |
| 3 | Inferior Frontal Gyrus, Orbitofrontal Cortex BA47 | 42, 24, −4 | 837 | .002 | |
| 4 | Middle Temporal Gyrus | 52, −8, −16 | 172 | .038 | |
| 5 | Inferior Parietal Lobule, Superior Temporal Gyrus | 60, −40, 24 | 798 | .003 | |
| 6 | Superior Temporal Gyrus, Inferior Parietal Lobule | −50, −62, 14 | 778 | .003 | |
| 7 | Middle Frontal Gyrus, Inferior Frontal Gyrus | 48, 18, 32 | 157 | .043 | |
| 8 | Superior Frontal Gyrus BA6 | 8, 20, 66 | 273 | .016 | |
| Affect x Person | No Significant Clusters | ||||
| Target PA with Friends in Daily Life | No Significant Clusters | ||||
| Affect X Target PA with Friends in Daily Life | No Significant Clusters | ||||
| Person x Target PA with Friends in Daily Life | No Significant Clusters | ||||
| Affect x Person x PA in Daily Life | 1 | Right Anterior Insula,Claustrum, Putamen | 50, −24, 22 | 877 | .012 |
| 2 | Left Anterior Insula,Postcentral Gyrus | −58, −16, 22 | 878 | .012 | |
| 3 | Precentral Gyrus/BA 6,Postcentral Gyrus,Superior Temporal Gyrus/BA 22 | 62, −14, 18 | 293 | .033 | |
Note. Coordinates are peak coordinates from voxel with the maximum t-value. Anatomical region labels are derived from Talairach Daemon.
Figure 3.
(a) Greater shared positive affect with a close friend in the laboratory is associated with lower lateral prefrontal cortex response to friend positive affect, but greater lateral prefrontal cortex response to stranger positive affect. Bar graph depicts average BOLD response in lateral prefrontal cortex per condition. Scatterplot depicts association between lateral prefrontal cortex response and Target Shared PA in Lab per condition.
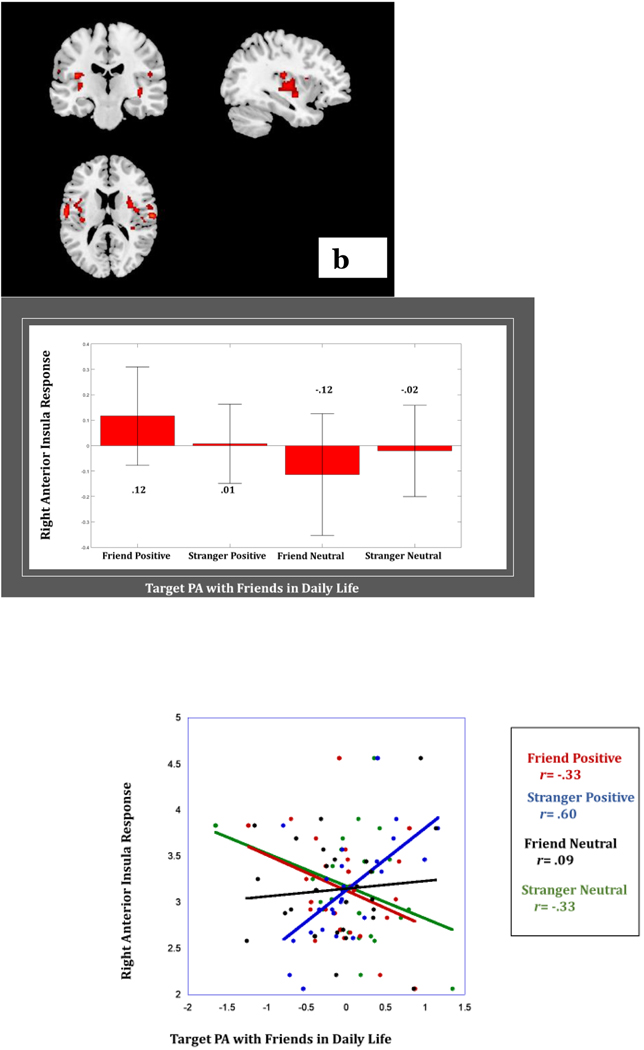
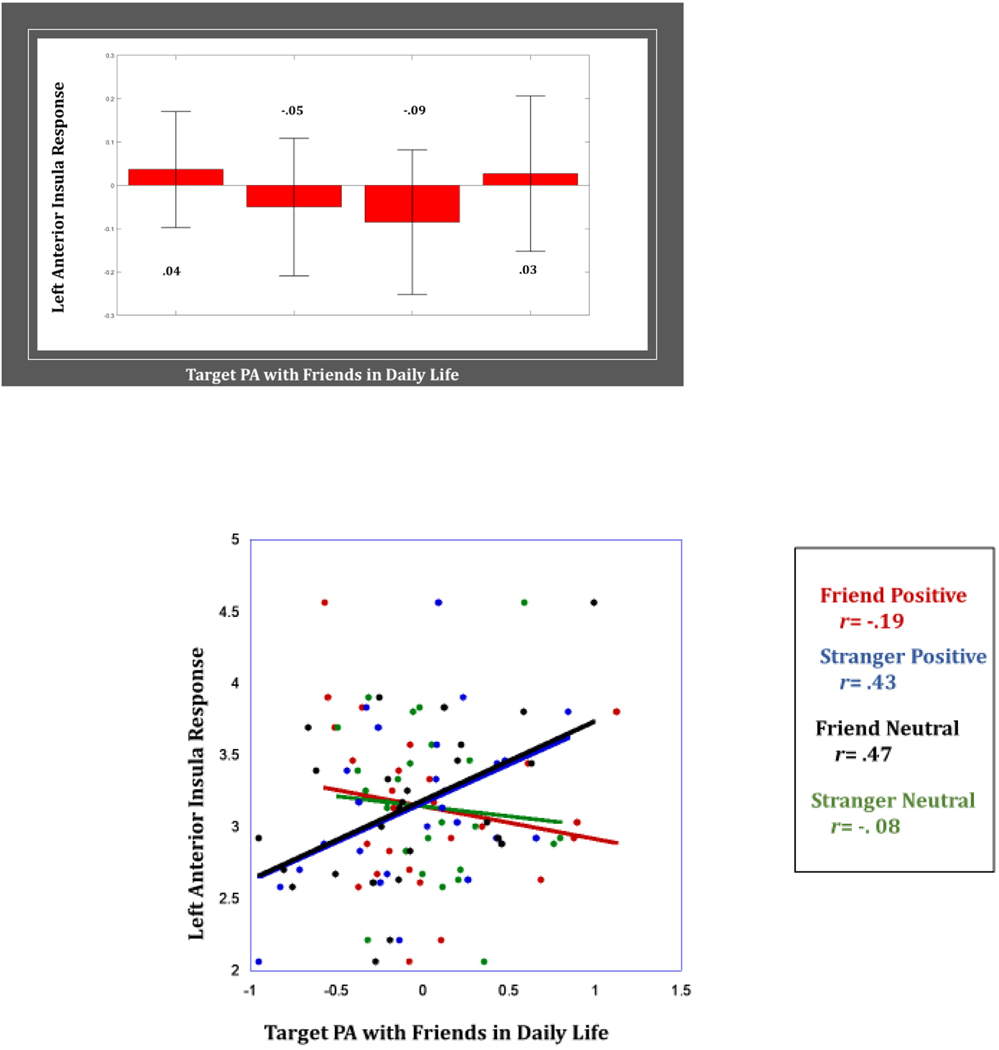
(b) Greater positive affect with friends is associated with greater bilateral insula response to stranger positive affect, but lower insula response to friend positive affect. Bar graphs depict average BOLD response in right and left anterior insula per condition. Scatterplot depicts association between right and left anterior insula and Target PA in daily life per condition.
3.4. Positive Affect in Daily Life
Findings revealed no significant main effect of Target PA with friends in daily life on neural response nor any significant 2-way interactive effects of Target PA with friends in daily life with Stimulus Affect or with Person. However, there was a significant 3-way interactive effect of Stimulus Affect x Person x Target PA with friends in daily life in the bilateral insula and the precentral gyrus/BA 6/lateral prefrontal cortex (Table 2). Greater Target PA with friends in daily life was associated with lower bilateral insula and lower precentral gyrus response to friend positive clips (rs= −.19, −.33, & −.43). Further, greater Target PA with friends in daily life was associated with lower bilateral insula and precentral gyrus response to stranger neutral clips (rs= −.08, −.33, & −.52). However, greater Target PA with friends in daily life was associated with higher bilateral insula and precentral gyrus response to stranger positive clips (rs=.43, .60, & .46) and higher bilateral insula and precentral gyrus response to friend neutral clips (rs= .47, .43, & .02) (Figure 4).
4. Discussion
This study demonstrated that experiencing positive affect when interacting with friends is associated with lower response in the lateral prefrontal cortex and the bilateral anterior insula. Our findings may suggest that inhibition of the lateral prefrontal cortex, a region involved in affect reappraisal, inhibitory control to emotional stimuli, and modulation of positive emotions (Ahmed, Bittencourt-Hewitt, & Sebastian, 2015; Mak, Hu, Zhang, Xiao, & Lee, 2009; Silvers, Wager, Weber, & Ochsner, 2010) may be involved in the promotion of positive affect in the context of positive friendships. In particular, lower activity in the lateral prefrontal cortex may be associated with decreases in inhibition and over-control of positive emotion, thereby aiding greater experience of positive affect. Likewise, our findings that greater PA with friends was associated with lower anterior insula response to friend positive clips may suggest that activity in the anterior insula may be involved in enjoyment of interactions with close friends, likely due to its putative functions in affective salience and social novelty (Singer, Critchley, & Preuschoff, 2009). In this case, adolescents may enjoy interactions with friends in part due to their being long-term, familiar, positive friendships.
We also found that positive affect when with friends in the laboratory and in daily life was associated with response in these same regions, lateral prefrontal cortex and anterior insula respectively, in response to unfamiliar peers in positive contexts but in the opposite direction. Specifically, greater PA in the lab and in daily life when with friends was associated with greater activity in the lateral prefrontal cortex and anterior insula, respectively, in response to unfamiliar peers engaging in positive interactions. Taken together, these findings may suggest that adolescents who are engaging in a mutual positive interaction with a close friend may be using less higher order processing of emotion and attention and more automatic processing of positive affect than when interacting with an unfamiliar peer (Wood & Grafman, 2003), and thereby may experience greater enjoyment of the moment. In contrast, adolescents may need to use more higher order processing (i.e., more appraisal and inhibitory control) to enjoy positive interactions with unfamiliar peers due to use of more complex actions with unfamiliar people and situations (see Wood & Grafman, 2003). However, adolescents who exhibit more anterior insula response to new peers may be more likely to enjoy positive interactions when with others because they are attuned to new peers and enjoy social novelty (i.e., higher anterior insula response). In contrast, adolescents who experience positive affect when with friends may enjoy these friendships due to their familiarity and comfort (i.e., lower anterior insula response). There are many factors in social behavior and social affect, and characteristics such as trait sociability, shyness, wish for emotional closeness, and sensation seeking could all come into play in the neural response to familiar and unfamiliar peers. Our findings provide greater evidence that the neural basis of enjoyment of interactions with close, familiar friends relative to unfamiliar peers may be related to less use of higher order emotional processing and cognitive control.
Beyond the associations with positive affect in the laboratory and daily life, we uncovered intriguing insights about neural mechanisms of social processing in general. Similar to prior findings (see Figure 1), adolescents showed greater responding in multiple regions, including reward-related regions, to positive social stimuli than to neutral stimuli. Indeed, we found that, overall, adolescents exhibited greater activation in the temporoparietal junction, posterior cingulate, and occipital lobe to positive clips relative to neutral clips, regardless of the person. This finding may suggest that adolescents engage in more visual and reflective processing to clips of a positive nature relative to neutral clips. The findings also extend prior work on adolescent neurobiological response to peers to show that adolescents showed greater responding in caudate head, OFC and vlPFC, and medial prefrontal cortex, to their own friend relative to a stranger peer, regardless of the affective valence of these clips. Intriguingly, neural response to their own close friend relative to an unfamiliar peer was related to greater activation in a cluster that encompassed both the caudate head and the septum, a region implicated in many affiliative processes such as unconditional trust (Krueger et al., 2007). Further, our affect x person findings demonstrated that adolescents responded more to clips of their own friend displaying positive affect in the ventrolateral prefrontal cortex, a region implicated in reward and reactivity, relative to stranger positive clips and to neutral clips from friends or strangers. Findings of greater activation in reward and affect region to positive, close friends, more so than to positive unfamiliar peers or to friends in a neutral condition, provide further neurobiological support for the salience and value of close friendships during adolescence. This finding is notable given that prior work has mostly utilized non-personalized peer stimuli (i.e., stranger peers) and has thus been unable to clearly identify neural response to friendship during adolescence. We should note that we also found that adolescents exhibited lower response to positive unfamiliar stimuli in the ventrolateral prefrontal cortex compared to all other stimuli (friend positive and stranger or friend neutral clips). This finding suggests that unfamiliar peers interacting in a positive context may elicit less reward-related activity and salience relative to close friends, regardless of whether their friends are displaying positive or neutral affect. This highlights the rewarding nature of close friendships, above and beyond peer relationships in general, during adolescence.
Figure 1.
Task image of Best Friends fMRI (BFF) task
It should be noted that our study focused on positive affect when with friends, either in the laboratory or in daily life. These findings highlight the unique role of reciprocal positive affect with friends during this developmental period of social re-orientation. However, our findings regarding adolescents’ responses to visual stimuli of unfamiliar peers may also shed light on how adolescents respond when engaging with unfamiliar peers in positive contexts. This is relevant as adolescents must navigate new social experiences during this developmental period for greater social and emotional growth. It is possible that audience effects may have influenced adolescent affect and behavior during the laboratory tasks (Zuberbühler, 2008). However, the content of friend conversations varied greatly from planning trips and playing games to risky sexual behavior and substance use. Of course, the extent to which viewing of video images generalizes to in person contexts also remains a question for future work. Future work should also examine other affective contexts in friendships (e.g., negative affect or conflict). For example, research on co-rumination, the experience of discussing negative events and experiences, has indicated that some aspects of co-rumination are associated with positive friendship adjustment (Rose, Schwartz-Mette, Glick, Smith, & Luebbe, 2014), and thus similar brain regions may also be activated in response to this shared affect experience. Future work should also explore how neural response to friends and to strangers in positive contexts are associated to adolescent positive affect when meeting new peers in the laboratory or in daily life. Furthermore, the current study used a psychiatrically healthy population, and future research could compare neural findings in healthy adolescents and psychiatrically ill adolescents (i.e., those with anxiety and/or depression) to see whether neural response to shared affect in peer relationships is related to mental health. Incorporating variables common to anxiety and depression into future research of friend relationships can add to the literature attempting to better understand the onset of these disorders in adolescence and how they affect neural and social development throughout adolescence. Finally, we note that the use of cluster-based inference has some limitations including difficulty localizing of sub-clusters that fall within different anatomical regions and bias against small anatomical brain regions.
It should be noted that the use of a novel, personally relevant task requires a trade-off between standardization and personalization; we made efforts to standardize lighting, camera angle, zoom, and intensity of affect, but personalized variables, such as topics discussed, could not be controlled. Adolescent PA in the laboratory and in daily life were not significantly related to one another in this study, which may have been due to their differing contexts (e.g., real world vs. laboratory). Further, adolescents may have been interacting with different peer(s) during the EMA assessment than during the laboratory interaction. Future work should evaluate this question with larger EMA samples. Further, we inferred that neural activity when passively viewing interactions with a close friend would reflect neural response when engaging in similar positive interactions in daily life. Future work could use mobile neuroimaging modalities (e.g., near infrared spectroscopy, EEG) to confirm our findings during dyadic interactions. Also, additional work is needed to evaluate our novel task in other samples to corroborate our findings.
Our study is unique in 1) linking neural findings from a new, personally-relevant task that assesses adolescent responding to mutual positive affect with a close friend as well as affective clips from unfamiliar peers with 2) independent observations and ecological momentary assessment of mutual positive affect during a developmental period in which neural and social changes heighten the salience and value of peer relationships. In doing so, our findings provide new ecologically valid information about how close, positive relationships during adolescence are associated with neural reward and social processing. Future work with this novel task can be used to identify how these types of peer relationships are associated with neural circuitry in adolescents with psychiatric disorders and may provide important information about common adolescent risk and vulnerability factors, such as the onset of mental disorders.
Acknowledgements.
This research was supported by Grant R21 DA033612 from the National Institutes of Health to Erika Forbes. We thank the staff and study participants of the Social Brain Network study.
5 References
- Ahmed SP, Bittencourt-Hewitt A, & Sebastian CL (2015). Neurocognitive bases of emotion regulation development in adolescence. Developmental Cognitive Neuroscience, 15, 11–25. doi: 10.1016/j.dcn.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert D, Chein J, & Steinberg L (2013). The Teenage Brain: Peer Influences on Adolescent Decision Making. Current Directions in Psychological Science, 22(2), 114–120. doi: 10.1177/0963721412471347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosia M Eckstrand KL, Morgan JK, Allen NB, Jones NP, Sheeber L, Silk JS, & Forbes EE (2018). Temptations of friends: Adolescents’ neural and behavioral responses to best friends predict risky behavior. Social Cognitive and Affective Neuroscience, 13, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, & Blakemore SJ (2011). The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews, 35(8), 1654–1664. doi: 10.1016/j.neubiorev.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, & Galvan A (2008). The adolescent brain. Developmental Review, 28, 62–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, … Salmon E (2007). Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience, 19(6), 935–944. doi: 10.1162/jocn.2007.19.6.935 [DOI] [PubMed] [Google Scholar]
- Hops H, Biglan A, Tolman A, Arthur J, Longoria N (1995). Living in Family Environments (LIFE) Coding System: Manual for Coders (Revised). Eugene, OR: Oregon Research Institute. [Google Scholar]
- Healey KL, Morgan J, Musselman SC, Olino TM, & Forbes EE (2014). Social anhedonia and medial prefrontal response to mutual liking in late adolescents. Brain and Cognition, 89, 39–50. doi: 10.1016/j.bandc.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt C, Lauresen B, Mooney KS, & Rubin KH (2015). Forms of friendship: A person-centered assessment of the quality, stability, and outcomes of different types of adolescent friends. Personality and Individual Differences, 77, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, & Prigatano GP (2002). Neural correlates of self-reflection. Brain : A Journal of Neurology, 125(Pt 8), 1808–1814. doi: 10.1093/brain/awf181 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, & Rao U (1997). Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Krueger F McCabe K, Moll J, Kriegeskorte N Zahn R, Strenziok M, Heinecke A, & Grafman J(2007). Neural correlates of trust. Proceedings of the National Academy of Sciences, 104 (50), 20084–20089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Greca M Annette, & Harrison HM (2005). Adolescent Peer Relations, Friendships, and Romantic Relationships: Do They Predict Social Anxiety and Depression? Journal of Clinical Child and Adolescent Psychology, 34(1), 49–61. doi: 10.1207/s15374424jccp3401 [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE Jr., Rudolph KD, Potter KI, Lambert S, Osborne L, & Gathright T (1999). A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment, 11, 326–388. [Google Scholar]
- Luna B, Padmanabhan A, & O’Hearn K (2010). What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain and Cognition, 72(1), 101–113. doi: 10.1016/j.bandc.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AKY, Hu ZG, Zhang JX, Xiao ZW, & Lee TMC (2009). Neural correlates of regulation of positive and negative emotions: an fmri study. Neuroscience Letters, 457(2), 101–106. doi. 10.1016/j.neulet.2009.03.094 [DOI] [PubMed] [Google Scholar]
- McFarquhar M, McKie S, Emsley R, Suckling J, Elliott R, & Williams S (2016). Multivariate and repeated measures (MRM): A new toolbox for dependent and multimodal group-level neuroimaging data. NeuroImage, 132, 373–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNelles LR & Connolly JA (1999). Intimacy between adolescent friends: Age and gender differences in intimate affect and intimate behaviors. Journal of Research on Adolescence, 9, 143–159. [Google Scholar]
- Mitchell JP, Banaji MR, & Macrae CN (2005). The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience, 17(8), 1306–1315. doi: 10.1162/0898929055002418 [DOI] [PubMed] [Google Scholar]
- Moll J, Bado P, de Oliveira-Souza R, Bramati IE, Lima DO, Paiva FF, Sato JR, Tovar-Moll F, & Zahn R (2012). A neural signature of affiliative emotion in the human septohypothalamic area. Journal of Neuroscience, 32 (36), 12499–12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB & Pine DS (2005). The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–174. doi: 10.1017/S0033291704003915 [DOI] [PubMed] [Google Scholar]
- O’Brien L, Albert D, Chein J, & Steinberg L (2011). Adolescents Prefer More Immediate Rewards When in the Presence of their Peers. Journal of Research on Adolescence, 21(4), 747–753. doi: 10.1111/j.1532-7795.2011.00738.x [DOI] [Google Scholar]
- Perino MT, Miernicki ME, & Telzer EH (2016). Letting the good times roll: Adolescence as a period of reduced inhibition to appetitive social cues. Social Cognitive and Affective Neuroscience, 1762–1771. doi: 10.1093/scan/nsw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Schwartz-Mette RA, Glick GC, Smith RL, & Luebbe AM (2014). An observational study of co-rumination in adolescent friendships. Developmental Psychology, 50(9), 2199–2209. doi: 10.1037/a0037465 [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, & Morris AS (2003). Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development, 74, 1869–1880. [DOI] [PubMed] [Google Scholar]
- Silk JS, Forbes EE, Whalen DJ, Jakubcak JL, Thompson WK, Ryan ND, Axelson DA, Birmaher B, & Dahl RE (2011). Daily emotional dynamics in depressed youth: A cell phone ecological momentary assessment study. Journal of Experimental Child Psychology, 110, 241–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Wager TD, Weber J, & Ochsner KN (2014). The neural bases of uninstructed negative emotion modulation. Social Cognitive and Affective Neuroscience, 10(1), 10–8. doi: 10.1093/scan/nsu016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz OS, Dudgeon P, Sheeber LB, Yap MB, Simmons JG, Allen NB (2011). Observed maternal responses to adolescent behavior predict the onset of major depression. Behavior Research and Therapy, 49, 331–338. doi: 10.1016/j.brat.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Sheeber LB, Allen NB, Leve C, Davis B, Shortt JW, & Katz LF (2009). Dynamics of affective experience and behavior in depressed adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines, 50(11), 1419–1427. doi: 10.1111/j.1469-7610.2009.02148.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley H.d., Preuschoff K (2009). A common role of insula in feelings, empathy, and uncertainty. Trends in Cognitive Science, 13, 334–340. [DOI] [PubMed] [Google Scholar]
- Somerville LH (2013). The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science, 22, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, & Casey B (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20(2), 236–241. doi: 10.1016/j.conb.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews (Vol. 24). doi: 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9(2), 69–74. doi: 10.1016/j.tics.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. doi: 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JN & Grafman J (2003). Human prefrontal cortex: Processing and representational perspectives. Nature Review Neuroscience, 4(2), 139. [DOI] [PubMed] [Google Scholar]
- Youniss J & Smollar J (1985). Adolescent relations with mothers, fathers, and friends. Chicago: University of Chicago Press. [Google Scholar]
- Zuberbühler K (2008). Audience effects. Current Biology, 18(5), R189–190. [DOI] [PubMed] [Google Scholar]