Abstract
Although autonomic nervous system (ANS) functioning is “context-dependent,” few studies examined children’s normative sympathetic and parasympathetic autonomic responses to distinct challenges in early childhood years. Examining children’s ANS responsivity to distinct challenges is important for understanding normative autonomic responses towards everyday life stressors and identifying paradigms that effectively elicit a “stress response.” We examined children’s (N=278) sympathetic (preejection period [PEP]) and parasympathetic (respiratory sinus arrhythmia [RSA]) responses to cognitive (i.e., problem-solving & cognitive control) and negatively-valenced emotional (i.e., blocked-goal & unfairness) challenges in preschool, kindergarten, and grade 1. Children, on average, demonstrated parasympathetic inhibition (RSA withdrawal) in response to all challenges but the magnitude of these responses depended on the task. Children showed sympathetic activation (PEP shortening) towards the problem-solving task at each assessment and there was no sample-level change in the magnitude of this response over time. Children showed greater sympathetic responsivity towards the cognitive control task over time, with evidence for a sympathetic activation response only in grade 1. Children experienced sympathetic inhibition (PEP lengthening) towards the unfairness tasks; but did not experience significant sympathetic responsivity towards the blocked-goal tasks. Parasympathetic responsivity to most challenges were modestly stable but there was no stability in sympathetic responsivity across time.
Keywords: Autonomic nervous system, stress responsivity, respiratory sinus arrhythmia, preejection period, self-regulation, early childhood
Responding effectively to everyday challenges is critical for adaptive functioning. On a typical day, young children experience a variety of challenges, including those that are emotionally demanding such as experiencing interpersonal conflict, as well as those that are cognitively demanding such as solving difficult problems. As one of the fastest responding stress response systems, the autonomic nervous system (ANS) plays an important role in preparing and energizing individuals to deal effectively with such challenges. As such, most daily challenges lead to a change in autonomic nervous system activity from the basal level to a level that is considerably higher or lower. Both the direction and the magnitude of this autonomic response pattern are thought to be important indicators of individuals’ experiences during stressful situations. Given the context-dependent nature of autonomic nervous system functioning, it has been suggested that autonomic responses to distinct challenges may either differentially relate to adaptive functioning or moderate the influence of contextual factors on children’s adaptive functioning in differential ways (Obradović, Bush, & Boyce, 2011). For example, autonomic responses to socioemotional challenges that mimic the adversities children encounter in real-life has been shown to buffer against the negative family experiences (i.e., parental conflict), whereas autonomic responsivity to cognitive challenges has been shown to act as a biological sensitivity to context factor, leading to better outcomes in lower adversity and worse outcomes in greater familial adversity (Obradović et al., 2011). Given the importance of understanding children’s autonomic responsivity towards distinct challenges, the goals of the study were to examine children’s normative responses toward distinct cognitive and negatively-valenced emotional challenges, and examine the longitudinal stability and continuity/change towards these challenges from preschool to first grade.
The Functioning and Measurement of ANS Branches
The ANS responds to challenges via the orthogonal and synergistic coordination of its anatomically distinct branches, the sympathetic and parasympathetic systems. The sympathetic nervous system promotes increased metabolic output to effectively mobilize the individual to produce defense responses (i.e., fight, flight), whereas the parasympathetic nervous system down-regulates the body’s energy resources to promote the body’s growth and restoration. The two branches of the ANS are supported largely by distinct neural processes and anatomical structures; exert orthogonal influences on the body organs; and have distinct response timelines (Janig, 2008). Compared to the parasympathetic system, the sympathetic system has a slower timescale, characterized by a shorter latency of action, a slower rise time, and a lower frequency capacity (Berntson, Cacioppo, & Quigley, 1991). Specifically, the sympathetic nerves exert influence within a few seconds, whereas the parasympathetic nerves lead to changes within milliseconds (Nunan, Sandercock, & Brodie, 2010). Thus, it has been proposed that the moment-to-moment changes in autonomic responses that support flexible behavioral responses are largely supported by the parasympathetic system (Saul, 1990; Smith, Thayer, Khalsa, & Lane, 2017).
Sympathetic and parasympathetic ANS responsivity can be measured via a multitude of measurement strategies. Although sympathetic nervous system functioning can be indexed by measures such as salivary alpha-amylase or skin conductance (Nater & Rohleder, 2009), an index that offers a fine-tuned time resolution is a cardiac indicator: pre-ejection period (PEP). PEP refers to the time interval in milliseconds between the onset of ventricular depolarization (Q wave of the electrocardiogram [ECG]) and onset of left ventricular ejection. This measure is thought to reflect the force of myocardial contraction via beta-adrenergic influences and index the overall sympathetic influence on the heart. PEP shortening (i.e., decreases in PEP from baseline to task) reflects sympathetic activation, whereas PEP lengthening (i.e., increases in PEP from baseline to task) reflects sympathetic inhibition. Similarly, although there are alternative ways of measuring parasympathetic activity, Porges (1995) developed a method that quantifies the amplitude of respiratory sinus arrhythmia (RSA), which is a measure of high-frequency heart rate variability. RSA withdrawal (i.e., decreases in RSA from baseline to task) reflects parasympathetic inhibition, whereas RSA augmentation (i.e., increases in RSA from baseline to task) reflects parasympathetic augmentation.
The Context-Dependent Nature of ANS Functioning
Both the direction and amplitude of the ANS systems’ responsivity are likely impacted by the context or specific challenge experienced. Based on the Polyvagal Theory (Porges, 2007, 2011) individuals first respond to a challenge via the phylogenetically newer myelinated vagal system and only recruit the phylogenetically older sympathetic system if the parasympathetic system’s response is not sufficient to deal with the challenge. As such, during a challenge, the vagal system increases heart rate by disinhibiting vagal influence on the heart (i.e., vagal withdrawal or parasympathetic inhibition) to promote active coping. However, during more severe challenges, the vagal system also withdraws its inhibitory influence on the sympathetic system to facilitate active mobilization. Based on this approach, individuals would experience only parasympathetic inhibition during mildly challenging situations but experience both sympathetic activation and parasympathetic inhibition in challenging or threatening situations.
In regards to autonomic responses during cognitive challenges, there is evidence suggesting that exertion of cognitive effort leads to sympathetic activation and parasympathetic withdrawal. In a randomized experimental design, Richter, Friedrich, and Gendolla (2008) examined differences in adults’ sympathetic responses across four conditions of task difficulty: low, medium, high, and impossible. Findings showed that the intensity of sympathetic activation paralleled the task difficulty, such that more difficult tasks elicited greater sympathetic activation, as long as success was possible. Adults have been shown to experience sympathetic activation (PEP shortening) and parasympathetic inhibition (RSA withdrawal) during a mental arithmetic task (Berntson, Cacioppo, & Fieldstone, 1996) and during a selective attention task (Giuliano et al. 2017). These findings together suggest that challenges that require cognitive effort lead to sympathetic activation and parasympathetic withdrawal in adults.
On the other hand, it has been shown that young children did not experience change in sympathetic activity but experienced parasympathetic inhibition in response to a mental scale task (Buss, Goldsmith, & Davidson, 2005) and a reaction time task (Quigley & Stifter 2006). Moreover, 5–6-year-old children did not experience change in sympathetic activity and experienced parasympathetic activation towards a forward digit span task relative to a condition designed to control for psychomotor movement (Bush, Alkon, Obradović, Stamperdahl, & Boyce, 2011). These findings suggest that, unlike adults, children may not experience change in sympathetic activity during cognitive challenges and may experience parasympathetic withdrawal or augmentation towards such challenges. Given these mixed findings, it would be important to examine children’s sympathetic and parasympathetic responsivity in the context of different cognitive challenges across time.
In regards to autonomic responses towards negatively-valenced emotional challenges, children has been shown to experience RSA withdrawal in response to a range of emotionally challenging situations including a Stranger Approach task designed to elicit fear, and a Toy Removal tasks designed to elicit sadness and feelings of unfairness (Buss, Goldsmith, & Davidson, 2005), a stressful worksheet task during which children received negative feedback (Roos, Giuliano, Beauchamp, Gunnar, Amidon & Fisher, 2017) and an interview designed to elicit stress (Quigley & Stifter, 2006), suggesting that parasympathetic withdrawal may be a common response to challenges designed to elicit negative affect. Findings regarding sympathetic responses towards negative-emotion-eliciting challenges have been more mixed. Toddlers did not show a change in their sympathetic activity relative to baseline towards the Stranger Approach and Toy Removal tasks (Buss, Goldsmith, & Davidson, 2005) and 4-year-olds did not experience change in sympathetic activity relative to baseline (as assessed via skin conductance) toward psychological conflict and emotional film (Fowles, Kochanska, & Murray, 2000). On the other hand, 4–5 year old children were shown to experience sympathetic inhibition (PEP lengthening) during an emotionally evocative video and during an interview designed to elicit stress (Quigley & Stifter, 2006) and adults have been shown to experience sympathetic inhibition (PEP lengthening) during a film designed to elicit sadness (Kreibig, Wilhelm, Roth, & Gross, 2007). Finally, 5–6 year-olds showed sympathetic activation (PEP shortening) in response to a video designed to elicit fear relative to a paired control condition that accounted for psychomotor activity (Bush et al., 2011), and 4–6 year olds were shown to experience sympathetic activation (PEP shortening) towards a stressful task during which children received negative feedback about their performance (Roos et al., 2017). There is also evidence suggesting that PEP shortening during incentive tasks may reflect dopamine reactivity to reward (see Beauchaine, 2012). These findings may suggest that children’s sympathetic responses towards negatively-valenced emotional challenges may depend on the type of emotional challenge. Although these studies may suggest that autonomic responses depend on the laboratory task, there is need for empirical work aimed towards understanding children’s sympathetic and parasympathetic ANS responsivity in light of the psychological demands posed by the challenges.
Longitudinal Stability in ANS Functioning in Early Childhood
Examining longitudinal stability in children’s ANS responsivity to distinct challenges may be important for several reasons. First, greater stability in ANS responsivity may suggest that children’s ANS activity reflect trait-like characteristics (i.e. how they typically respond to stressors), and thus may be potential markers of resilience or vulnerability (Gatzke-Kopp & Ram, 2018). Second, ANS reactivity may not be stable at younger ages but may become more stable in later years, and therefore examining longitudinal stability in ANS responsivity can advance our understanding of when children start to show a trait-like way of dealing with certain types of challenges. Third, this line of investigation can determine whether longitudinal stability in ANS responsivity depends on the laboratory task, such that children’s responses may be relatively stable in one task but not in another task.
Previous studies have consistently demonstrated moderate levels of stability in children’s baseline RSA and PEP in childhood (e.g., Alkon, Boyce, Davis, & Eskenazi, 2011; Esposito et al., 2016; Gatzke-Kopp & Ram, 2018); however, results on the stability of responsivity scores have been more mixed. For example, Alkon et al. (2011) calculated composite scores for children’s RSA and PEP responsivity to multiple 1–2-minute-long challenges at 6−, 12−, 42− and 60-months of age and reported no stability in RSA and PEP responsivity scores over time, except for the modest stability in RSA responsivity from 42-months to 60-months. Perry et al. (2012) reported modest stability in aggregate scores of RSA responsivity to two 3–4-minute emotionally frustrating challenges from 3 to 4 years, and from 4 to 5 years of age. Calkins and Keane (2004) measured children’s RSA responsivity towards four tasks (attention, empathy, frustration, and problem-solving) at 2 and 4.5 years and found modest stability in RSA responsivity towards only certain tasks (e.g., problem-solving task), but not others (e.g., empathy). These findings together suggest that children’s RSA responsivity, at least towards certain challenges, may show modest stability during preschool years. Importantly, Hinnant, Philbrook, Erath, and El-Sheikh (2018) did not find stability in RSA responsivity towards a 3-minute long star-tracing challenge in middle childhood (from 8 to 10 years of age) but did find moderate-to-high levels of stability in RSA responsivity towards the same task in adolescence (from 16 to 18 years of age), suggesting that RSA responsivity towards at least certain challenges may not become stable until late adolescence. As such, these studies highlight the importance of understanding whether RSA responsivity towards certain challenges show stability in early childhood. Given the scarcity of longitudinal work on children’s PEP responses, it is also important to test whether individual differences in PEP responsivity are stable over time.
Sample-Level Continuity and Change in ANS Functioning in Early Childhood
In regards to the sample-level continuity and change in children’s ANS functioning, more work has examined change in baseline sympathetic and parasympathetic functioning (e.g., Esposito, Koss, Donzella, & Gunnar, 2016), but few studies examined longitudinal sample-level change in children’s ANS responsivity towards different tasks. Previous research conducted to answer this question frequently assessed ANS responses across different tasks over time (e.g., Perry et al., 2013) or created composite scores derived from children’s ANS responses to a range of laboratory tasks (e.g., Alkon et al., 2003; Boyce et al., 2001). Based on the assumption that ANS responses may be context specific, examining children’s ANS responsivity towards challenges that were repeated over time can inform us about the changes in children’s ANS responsivity towards specific challenges in early childhood.
Previous research reported increases in children’s baseline RSA during early childhood (Perry et al., 2013; Alkon et al., 2011). On the other hand, the few studies that examined continuity and change in RSA responsivity over time revealed mixed findings. Calkins and Keane (2004) found that the magnitude of RSA withdrawal decreased from 2 to 4.5 years of age, such that children tended to engage in lower levels of vagal withdrawal as they got older. Given that vagal withdrawal reflects coping responses to challenge (Porges, 2011), it is possible that children needed to rely less on physiological coping responses if the laboratory tasks became easier for them over time. In another longitudinal study, Perry et al. (2013) examined change in RSA withdrawal in response to emotion regulation tasks from 3 to 5 years of age and found that there was no change in the means of vagal withdrawal over time. Notably, this study used different emotion regulation tasks at different time points for the tasks to be novel and stressful for the children. Given that these tasks may not have been equivalent with respect to how frustrating or stressful they were, and that different children may find different types of tasks frustrating, the null findings may be a function of using different tasks over time. As such, it would be important to examine changes RSA responsivity towards the same tasks over time. For example, if tasks become less challenging for children, there may be decreases the magnitude of RSA withdrawal; however, certain tasks may not get easier across early childhood, in which case there may be continuity in children’s ANS responses.
The few studies that examined change in baseline sympathetic cardiac functioning, as indexed by baseline PEP, found increases during early childhood. For example, Alkon et al. (2010) reported increases in baseline PEP from 4 to 6 years of age, and Esposito et al. (2016) reported increases in baseline PEP in a mixed-age group sample across the early childhood period. Notably, given the limited work on continuity and change in PEP responsivity over time; it would be important to examine whether and how PEP responsivity towards to the same task changes over time.
The Current Study
In this study, we examined children’s sympathetic and parasympathetic cardiac ANS responses to two distinct categories of laboratory challenges – cognitive and negatively-valenced emotional challenges – in preschool, kindergarten and first grade. Within each of these two categories of challenges, children’s responses were further examined across 2 distinct types of tasks. The two types of negatively-valenced emotional tasks were: blocked-goal tasks, which were frustrating obstacles that are either too difficult or impossible to resolve, and unfairness tasks during which children experienced an unfair situation posed by the experimenter. The two types of cognitive tasks were a spatial problem-solving task (Tangrams) and a cognitive control task (Go/No-Go) that was long, repetitive, and required attentional and cognitive control. This study design, particularly the inclusion of two distinct types of cognitive and negatively-valenced emotional challenges was advantageous for understanding children’s sympathetic and parasympathetic ANS responses toward different types challenges.
Consistent with the notion that vagal withdrawal supports coping responses (Porges, 2011), we hypothesized that, on average, children would experience parasympathetic inhibition, as indexed by RSA withdrawal, across all laboratory challenges. Based on the idea that individuals experience sympathetic activation during challenges that require cognitive effort (Wright & Kirby, 2001) and empirical evidence showing that cognitively challenging tasks lead to sympathetic activation in adults (e.g., Berntson et al., 1996), we expected children to experience sympathetic activation (i.e., PEP shortening) during the cognitively challenging spatial problem-solving task and to some extent towards the cognitive control task given that it was a prolonged and repetitive task that may have been requiring less cognitive effort. However, given the previous evidence showing that children, unlike adults, do not experience sympathetic activation during cognitive challenges (Quigley & Stifter, 2006), we wanted to understand when during early childhood children start experiencing sympathetic activation towards cognitive challenges and whether there is a sample-level increase in children’s sympathetic activation over time. Consistent with the idea that individuals would experience sympathetic inhibition when they anticipate no means of escaping an aversive situation or its consequences (Obrist, 1981), as well as findings that have linked experiences of sadness with sympathetic inhibition (Kreibig, 2010), we expected children to experience sympathetic inhibition (i.e., lengthened PEP) during the two negatively-valenced emotional tasks, Toy Removal and Not Sharing, designed to evoke experiencing unfairness. Based on the proposition that individuals tend not to mobilize their resources if success on a task is unattainable (Richter et al., 2008), no mean-level change in sympathetic activity was expected during emotion regulation tasks that required children to solve frustrating and unattainable problems (e.g., Locked Box and Puzzle Box).
Consistent with previous findings, we expected moderate stability in baseline ANS activity (e.g., Gatzke-Kopp & Ram, 2018), modest stability in parasympathetic responsivity towards challenges (e.g., Calkins & Keane, 2004) but no stability in sympathetic responsivity across time (e.g., Alkon et al., 2011). We also expected an overall mean-level increase in baseline ANS activity (e.g., Esposito, Koss, Donzella, & Gunnar, 2016) and explored whether there would be sample-level developmental change in ANS responses towards the cognitive challenges over time.
Method
Participants
The sample for this study was 278 children (55% girls) who participated in a longitudinal study examining the physiological, emotional and cognitive predictors of early academic readiness. Children’s mean age at the preschool, kindergarten, and first grade assessments were 56.37 (SD=4.68), 70.80 (SD = 3.86), and 82.76 (SD=4.02) months, respectively. At the preschool assessment, approximately 61% of mothers had a 4-year college degree or higher, and the average income-to-needs ratio, calculated by dividing the total family income by the poverty threshold for that family size, was 2.11 (SD=1.41). Fifty-nine percent of the children were European American, 30% African American, and 11% other races; 6.5% of the sample was Hispanic. Of the original 278 participants, 249 returned for the kindergarten assessment and 240 returned for the first-grade assessment. Participants who did not return for the kindergarten visit did not differ from the remaining participants with respect to gender (p = .49), minority status (p = .81), income-to-needs (p = .17). and maternal education (p = .82), and those who did not return for the grade 1 visit did not differ from the remaining participants with respect to gender (p = .36) minority status (p = .23), income-to-needs (p = .10). and maternal education (p = .50). Likewise, participants missing RSA data at any wave did not differ from those with RSA data on these characteristics (p = .11–.99). In preschool, more males (n=28) than females (n=17) were missing PEP data (chi-square = 5.92, p =.015); however, participants missing PEP data did not differ from those with PEP data on any other characteristics at any wave (p =.41–.99).
Procedure
Overview.
Participants were recruited from daycare centers, local establishments (e.g., children’s museum) or via participant referral in a midsized Southeastern city in the United States. Children participated in laboratory assessments when they were at preschool, kindergarten and first grade; each that typically lasted for 2 hours. Before each visit, mothers provided written consent and were briefed about the study’s procedure. Mothers received monetary compensation for participation, and children selected a small toy at the completion of the visit. All procedures were approved by the university institutional review board.
Across all time points, a similar laboratory procedure took place. After consent, mothers left the room. Approximately 20–25 minutes into the session, the experimenter placed the physiological equipment onto the child. After the placement, children participated in a series of tasks during which their cardiac electrophysiological data were collected. In each assessment, children first participated in a baseline procedure, followed by 2 cognitive and 2 negatively-valenced emotional challenges, after which the equipment was removed and children received snacks (see Table 1 for a list of challenges used). To sustain children’s willingness to participate, children were asked to pick a sticker to put onto their sticker chart after each task.
Table 1.
Characteristics of the Laboratory Challenges used in the Study
| Cognitive Challenges | Negatively-Valenced Emotional Challenges | |||
|---|---|---|---|---|
| Problem-solving | Cognitive Control | Blocked Goal | Unfairness | |
|
Tangrams (Pre-K, Kindergarten, Grade 1) Children placed three-dimensional wooden shapes into pictures of shapes. This task required problem-solving as children needed to combine 2 shapes to fit into a larger shape and flip the shapes to make them fit in the lines. The shapes became progressively more difficult. |
Go/No-Go (Pre-K, Kindergarten, Grade 1) Children played a computer game during which they were asked press the button for all animals except for the dog. This task required attentional and cognitive control. |
Locked Box (Pre-K) Children were given a set of wrong keys and instructed to use the keys to unlock the lock to get to an attractive toy. Impossible Gift (Kindergarten) Children were presented with a gift for their hard work and encouraged the child to open the gift right away; however, the gift was sealed so it could not be opened. Puzzle Box (Grade 1) Children were asked to assemble a wooden puzzle in a large box without looking at it Broken Toy (Grade 1) Children were given a cool hand computer toy to play with, but the toy did not work. |
Toy Removal (Pre-K) The experimenter grabbed an attractive toy the child was playing with and did not return it for 2 minutes while commenting how fun it was to play with the toy. Not Sharing (Kindergarten) The experimenter did not share candy equally with the child and took away the candy initially given to the child and ate one piece without sharing. |
|
Note. Two main categories of challenges were used: cognitive and emotional. There were 2 types of cognitive challenges, problem-solving and cognitive control, and 2 types of emotional challenges, blocked goal and unfairness.
Laboratory Challenges
Baseline.
For the baseline tasks, children were seated on a chair in front of a computer monitor placed on a table. During the fish task (2 minutes), children watched a video of colorful fish swimming. Prior to the video, children were told that they were going to watch a fish video on the computer, and that they should stay in their seat and watch quietly so they don’t scare the fish. During the statue task (1 minute), children watched numbers decreasing gradually from 60 to 0 at the center of the screen. For this task, children were told to sit still and quietly until the number on the screen gets to zero.
Cognitive challenges.
Children participated in a spatial problem-solving task called Tangrams and a cognitive control task, Go/No-Go, in preschool, kindergarten, and first grade.
Problem-solving challenge (Tangrams).
During the cognitively demanding spatial problem-solving Tangrams task (10 minutes), children were asked to fit wooden shapes into the pictures of shapes presented on paper. For some pictures, children needed to combine two shapes to make a larger shape and flip a shape to make it fit in the lines. Following a brief demonstration, children were presented with puzzles of increasing difficulty and instructed to ask for help if needed. Trained coders coded children’s behaviors related to their engagement (for more information on the coded behaviors, see Halliday, Calkins, & Leerkes, 2018).
Cognitive control challenge (Go/No-Go).
During the cognitive control Go/No-Go task (approximately 8 minutes), children were asked to press the button for all animals except for the dog. This task is typically used to assess inhibitory control and can be characterized as a long and repetitive task that requires attentional and cognitive control. Children’s cognitive control was calculated via d prime calculation (for more information, see Swingler, Isbell, Zeytinoglu, Calkins, & Leerkes, 2018).
Negatively-valenced emotional challenges.
At each assessment, children participated in 2 emotion regulation tasks designed to elicit negative affect. Given that novelty of the task is important for eliciting negative affect, different emotion regulation tasks were used at each time point. Tasks were terminated early if the child became very upset or in rare instances if the child left the situation.
Preschool assessment (Locked Box & Toy Removal).
During the preschool assessment, there were two negative-emotion-eliciting tasks: a blocked-goal task known as Locked Box and an unfairness task named Toy Removal. The Locked Box task originates from Lab-TAB’s “transparent box” episode of distress (Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011). During this task, children were first demonstrated how to open a lock with a key. After ensuring that the child can use a key to unlock the lock, the child was asked to select one toy from three attractive toys. The selected toy was placed in a transparent box and locked with a padlock. The experimenter provided the child with a ring of wrong keys and instructed to use the keys to unlock the lock to get the toy. The experimenter prompted the child to open the box every 15 seconds throughout the 4-minute task. To terminate the task, the experimenter told the child that she has found the correct key and allowed the child to open the box. After allowing the child to play with the toy momentarily, during Toy Removal, the experimenter grabbed the toy from the child and played with it for 2 minutes. The experimenter periodically commented on how fun it was to play with the toy. To terminate, the experimenter returned the toy to the child.
Kindergarten assessment (Not Sharing & the Impossible to Open Gift).
During the kindergarten assessment, children participated in two negative-emotion-eliciting tasks: an unfairness task called Not Sharing and a blocked-goal task called Impossible to Open Gift. Not Sharing originates from Lab-TAB’s “I’m not sharing” episode of distress (Goldsmith, Reilly, Lemery, Longley, & Prescott, 1999). This task targeted the child’s feelings of being treated unjustly and was intended to be upsetting/frustrating for the child. The task started with the experimenter telling the child that the assistant has a surprise for them. The assistant came into the room with candy and instructed the experimenter to divide the candy evenly between them both. After the assistant left, the experimenter shared the first 6 candies equally. After that, the experimenter gave herself more candy than the child multiple times, and at one point ate a piece of the child’s candy. At the end of the unfair episode (2-minutes), the experimenter took all of the child’s candy. To terminate, the experimenter allowed the child to pick and eat 2 pieces of their favorite candy. The Impossible to Open Gift task (1-minute), adapted from Carlson and Wang (2007) and Goldsmith et al. (1999) was administered. In this task, the experimenter presented the child with a gift for their hard work and encouraged the child to open the gift right away; however, the gift was sealed so it could not be opened.
First-grade assessment (Puzzle Box & Broken Toy).
During the first-grade assessment, there were two frustrating blocked-goal tasks: Puzzle Box and Broken Toy. In the Puzzle Box task (4-minutes), children were asked to assemble a wooden puzzle in a large box without looking at it (Eisenberg et al., 2000). One side of the box had plexiglass through which the experimenter could observe the child’s hand movements and the other side had two sleeves through which the children were asked to slip their arms to access the puzzle. The sleeves were covered by a cloth that could be lifted if a child wanted to peek at the puzzle. Children were told to work on the puzzle without peeking and that it was an easy puzzle so they had only 4 minutes to finish it. Children were told that once they finished the puzzle, there was a surprise for them. The experimenter watched the child and made comments such as “finish the puzzle” in 15 second intervals. In Broken Toy (2-minutes), the experimenter told the child that she had a really cool toy for them to play with and left the room to bring 2 hand computer toys. Next, the experimenter brought toys, demonstrated how to turn on and pick a game, and gave the child the toy that does not work. The experimenter played with her toy periodically making comments like “I really like this game!” The experimenter terminated the task and gave her own toy to the child.
Physiological Measures
The cardiovascular data were collected using Mindware BioNex 8SLT Chassis (Gahanna, OH), which measured ECG and impedance cardiogram (ICG) signals simultaneously. Seven spot electrodes were placed on participants to record cardiogram signals. ECG signals were obtained using the modified Lead II configuration with two ECG electrodes placed on the distal end of the right clavicle and lower left rib, with a ground electrode placed on the lower right rib. To quantify the heart rate (HR) data, the ECG signal was passed through an A/D converter with ECG sampled at 1,000 Hz and Zo sampled at 500 Hz. ICG signals were recorded using four electrodes. Two impedance electrodes were placed on the front of the participants’ body, specifically on the left collarbone horizontal to the jugular notch and at the bottom of the sternum. Two current electrodes were placed on the back, specifically on the participants’ neck and approximately one inch below the lower receiving electrode.
Respiratory sinus arrhythmia (RSA).
RSA is heart rate variability measured in the interbeat interval (IBI) series associated with the phases of breathing. RSA was derived from the IBI series over the course of each 30 second epoch, using Mindware Technologies HRV 3.0 analysis software. This program calculates RSA by subjecting the IBI series for each epoch to Fast Fourier Transform (FFT) and applying a Hamming window for the .24–1.04 Hz frequency range, which has been used in children this age (e.g., Kolacz, Holochwost, Gariépy, & Mills-Koonce, 2016; Miller, Kahle, & Hastings, 2016). The integral of the power of this band was extracted and the natural logarithm of this measure was the RSA statistic. The RSA data files were cleaned and edited using the HRV software to derive mean RSA for each epoch. Trained researchers have visually inspected each epoch to correct misidentified beats manually, identify and exclude spurious data due to equipment or sticker problems or child movement. Scores derived from these epochs were averaged to create mean RSA scores for each task. Participants’ task scores were retained if 50% or more of the epochs were artifact-free (e.g., a minimum of 2 artifact-free epochs for a 4-epoch task).
Preejection period (PEP).
PEP, derived from the ICG signals, refers to the time interval in milliseconds between the onset of ventricular depolarization (Q wave of the ECG) and onset of left ventricular ejection (B point of the dz/dt wave). The Q and B points were identified automatically using algorithms provided by the MindWare IMP 3.1 analysis software. The Q point was identified at the lowest point of the signal appearing within 25 milliseconds prior to the R-point (Bush, Caron, Blackburn, & Alkon, 2016). The B point was estimated using Lozano’s method, which approximates the B- point based on the dz/dt peak (percent dz/dt was identified as 55%, plus 4; Lozano et al., 2007). The PEP data files were cleaned and edited using the IMP 3.1 software to derive mean PEP for each epoch. Trained researchers have visually inspected each epoch to identify and exclude spurious data due to equipment or sticker problems or child movement. Epoch scores were averaged to create mean PEP scores for each task. For short tasks, two epochs and for the long tasks 50% of the epochs needed to be artifact-free to be retained.
Data Analytic Strategy
The initial analyses included checking normality of the distributions and identifying outliers. In case of outliers, the validity of the data was checked and outliers were removed if there was artifact. Following preliminary analyses, we conducted a series of random-intercept hierarchical linear models (HLM) to examine which laboratory challenges elicited a change in ANS activity from baseline to task and compared the magnitude of the fixed effects using the hypotheses testing function of the HLM software. At each time point (preschool, kindergarten, and grade 1), two random intercept models were tested: one for RSA and one for PEP. In each model, ANS activity (e.g., RSA or PEP) was the outcome variable, the intercept reflected ANS activity during baseline, and each laboratory task was entered as a predictor of ANS activity. At each time point, 2 cognitive challenges (Tangrams & Go/No-Go) and 2 emotional challenges (e.g., Locked Box & Toy Removal) were entered as predictors. For example, in preschool, the following two models were tested:
The intercept (γ00) reflected average baseline ANS activity and a significant p-value associated with this coefficient suggested that baseline activity was significantly different from zero. The fixed effect of each laboratory challenge indicated the extent to which ANS activity during the laboratory challenge was different than the intercept or baseline ANS activity. For example, in the RSA models, the coefficient for Tangrams (γ10) reflected the magnitude of RSA responsivity during Tangrams and a significant p-value linked with this coefficient suggested that there was a significant change in RSA from baseline to Tangrams. Negative values indicated decreases in RSA from baseline to task (parasympathetic withdrawal), whereas positive values indicated increases in RSA from baseline to task (parasympathetic augmentation). In the PEP models, negative values indicated decreases in PEP from baseline to task (sympathetic activation), whereas positive values indicated increases in PEP from baseline to task (sympathetic inhibition). Although a repeated-measures ANOVA followed by post-hoc paired t-tests could also be conducted, the random intercept HLM provided two main advantages. The first was that missing data was handled by full-information maximum likelihood (FIML). Thus, all individuals who had data for at least one laboratory task were included the in the analyses. The second advantage was that the magnitude of the responses across the 4 challenges could be compared within the same model, accounting for the dependency across the measures.
Finally, we examined longitudinal stability/instability and sample-level continuity and change over time. We examined longitudinal stability in baseline RSA/PEP and RSA/PEP responsivity via bivariate correlations. Next, we examined sample level continuity and change in RSA and PEP scores across time for measures that were repeated over time (baseline RSA/PEP scores and RSA/PEP responsivity during the two cognitive challenges). We also examined sample-level change in children’s behavioral responses towards the two cognitive tasks with the goal to understand if the mean-level changes in physiology and behavior were similar. In order to characterize sample level change over time, we conducted repeated-measures analyses of variance (ANOVAs) using a multi-level framework in SPSS, which allowed us to include all participants who had available data in analyses.
Results
Table 2 includes descriptive information for mean RSA and PEP obtained during baseline and laboratory challenges conducted in preschool, kindergarten, and grade 1.
Table 2.
Descriptive Information for Task RSA and PEP across Challenges
| RSA |
PEP |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | ||
| Preschool | |||||||||||
| Baseline | 259 | 3.65 | 9.56 | 7.21 | 1.11 | 233 | 71.63 | 107.50 | 91.14 | 6.43 | |
| Cognitive 1: Problem-Solving (Tangrams) | 256 | 3.48 | 9.27 | 6.27 | 1.17 | 222 | 75.00 | 105.79 | 90.67 | 6.09 | |
| Cognitive 2: Cognitive Control (Go/No-Go) | 249 | 3.66 | 9.54 | 6.83 | 1.06 | 224 | 74.31 | 107.25 | 90.87 | 6.25 | |
| Emotional 1: Blocked Goal (Locked Box) | 250 | 3.26 | 9.63 | 6.17 | 1.14 | 221 | 75.25 | 107.63 | 90.87 | 6.59 | |
| Emotional 2: Unfairness (Toy Removal) | 244 | 3.69 | 9.55 | 6.84 | 1.13 | 216 | 75.25 | 111.75 | 91.71 | 6.57 | |
| Kindergarten | |||||||||||
| Baseline | 233 | 4.07 | 9.87 | 7.35 | 1.05 | 219 | 73.50 | 112.63 | 92.67 | 6.72 | |
| Cognitive 1: Problem-Solving (Tangrams) | 231 | 2.44 | 9.39 | 6.44 | 1.17 | 210 | 66.35 | 108.37 | 92.09 | 6.68 | |
| Cognitive 2: Cognitive Control (Go/No-Go) | 225 | 3.66 | 9.26 | 6.95 | 1.02 | 207 | 75.50 | 108.25 | 92.56 | 6.15 | |
| Emotional 1: Blocked Goal (Impossible Gift) | 224 | 3.39 | 9.29 | 5.96 | 1.18 | 195 | 67.50 | 118.00 | 92.50 | 7.47 | |
| Emotional 2: Unfairness (Not Sharing) | 227 | 4.37 | 9.91 | 6.89 | 1.11 | 206 | 71.00 | 111.00 | 93.00 | 7.09 | |
| Grade 1 | |||||||||||
| Baseline | 229 | 4.83 | 10.15 | 7.36 | .98 | 217 | 71.25 | 108.25 | 93.70 | 6.74 | |
| Cognitive 1: Problem-Solving (Tangrams) | 228 | 4.07 | 9.64 | 6.47 | 1.10 | 211 | 73.00 | 106.33 | 92.75 | 6.52 | |
| Cognitive 2: Cognitive Control (Go/No-Go) | 223 | 4.40 | 9.97 | 7.10 | .95 | 206 | 77.38 | 107.00 | 92.47 | 6.20 | |
| Emotional 1: Blocked Goal (Puzzle Box) | 226 | 4.00 | 9.82 | 6.52 | 1.05 | 206 | 78.71 | 110.00 | 93.41 | 6.37 | |
| Emotional 2: Blocked Goal (Broken Toy) | 224 | 4.35 | 9.88 | 6.87 | 1.04 | 202 | 77.00 | 109.25 | 93.29 | 6.72 | |
The Effect of Challenge on ANS Functioning
Parasympathetic Responsivity.
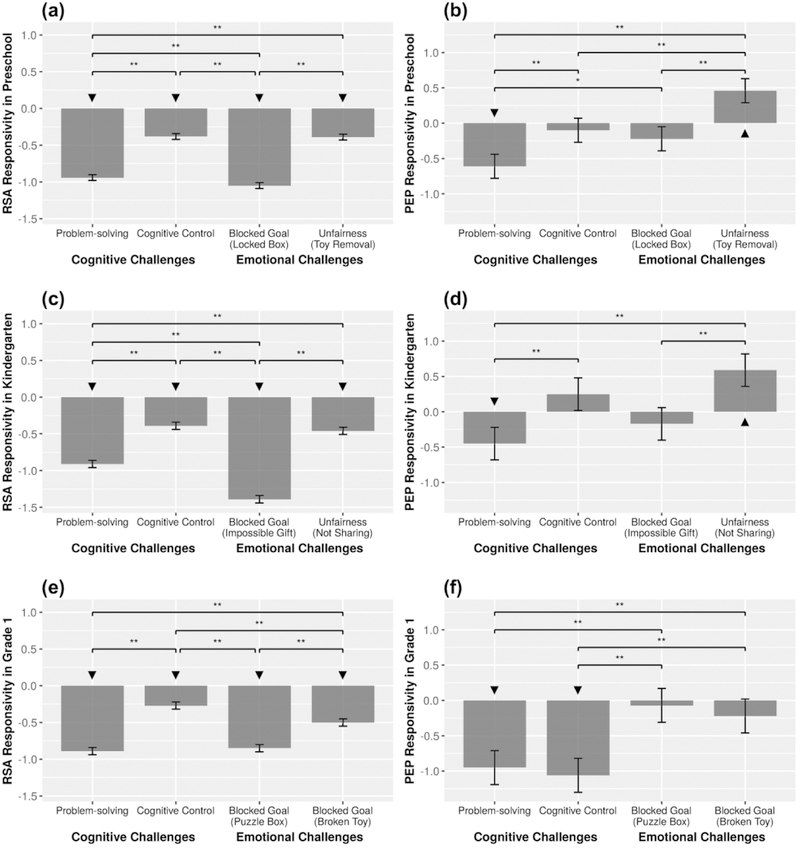
As presented in Table 3, three HLM models were conducted to test the effect of laboratory challenge on RSA in preschool, kindergarten, and first grade (see Models 1, 2, & 3). Across all time points, all fixed effect coefficients were negative and significant, p < .001, suggesting that all laboratory challenges elicited RSA withdrawal or led to a reduction in children’s RSA relative to baseline. Follow up tests comparing the cognitive challenges suggested that, across all assessments the problem-solving task (Tangrams) elicited greater RSA withdrawal than the cognitive control task (Go/No-Go; see Figure 1a, 1c, & 1e). Comparisons among the negative-emotion-eliciting challenges showed that, across assessments, the blocked goal challenges elicited greater RSA withdrawal than the unfairness challenges. Specifically, in preschool, Locked Box elicited greater RSA withdrawal than Toy Removal (see Figure 1a); and in kindergarten, Impossible Gift elicited greater RSA withdrawal than Not Sharing (see Figure 1c). In grade 1, RSA responsivity across the two blocked goal tasks also differed, such that Puzzle Box elicited greater RSA withdrawal than Broken Toy (see Figure 1e).
Table 3.
Fixed effect coefficients from the random intercept HLM models examining task ANS activity in relation to baseline ANS activity
| RSA Models |
PEP Models |
||||||
|---|---|---|---|---|---|---|---|
| coefficient | SE | p | coefficient | SE | p | ||
| Preschool | Model 1 | Model 4 | |||||
| Intercept (baseline) | 7.21 | 0.07 | <0.001 | 91.10 | 0.41 | <0.001 | |
| Cognitive 1: Problem-Solving (Tangrams) | −0.94 | 0.04 | <0.001 | −0.61 | 0.17 | <0.001 | |
| Cognitive 2: Cognitive Control (Go/No-Go) | −0.38 | 0.04 | <0.001 | −0.10 | 0.17 | 0.562 | |
| Emotional 1: Blocked Goal (Locked Box) | −1.05 | 0.04 | <0.001 | −0.22 | 0.17 | 0.213 | |
| Emotional 2: Unfairness (Toy Removal) | −0.39 | 0.04 | <0.001 | 0.46 | 0.17 | 0.009 | |
| Kindergarten | Model 2 | Model 5 | |||||
| Intercept (baseline) | 7.35 | 0.07 | <0.001 | 92.57 | 0.46 | <0.001 | |
| Cognitive 1: Problem-Solving (Tangrams) | −0.91 | 0.05 | <0.001 | −0.45 | 0.23 | 0.052 | |
| Cognitive 2: Cognitive Control (Go/No-Go) | −0.39 | 0.05 | <0.001 | 0.25 | 0.23 | 0.279 | |
| Emotional 1: Blocked Goal (Impossible Gift) | −1.39 | 0.05 | <0.001 | −0.17 | 0.23 | 0.481 | |
| Emotional 2: Unfairness (Not Sharing) | −0.46 | 0.05 | <0.001 | 0.59 | 0.23 | 0.010 | |
| Grade 1 | Model 3 | Model 6 | |||||
| Intercept (baseline) | 7.36 | 0.07 | <0.001 | 93.65 | 0.45 | <0.001 | |
| Cognitive 1: Problem-Solving (Tangrams) | −0.89 | 0.04 | <0.001 | −0.95 | 0.24 | <0.001 | |
| Cognitive 2: Cognitive Control (Go/No-Go) | −0.27 | 0.04 | <0.001 | −1.06 | 0.24 | <0.001 | |
| Emotional 1: Blocked Goal (Puzzle Box) | −0.85 | 0.04 | <0.001 | −0.07 | 0.24 | 0.784 | |
| Emotional 2: Blocked Goal (Broken Toy) | −0.50 | 0.04 | <0.001 | −0.22 | 0.24 | 0.359 | |
Figure 1.
Results from the HLM hypothesis tests comparing the magnitude of the fixed effects reflecting ANS responsivity toward challenges in preschool. Asterisks (*) indicate the significance level of the chi-square tests comparing fixed effects. *p < .05, **p <.01. Negative RSA responsivity scores reflect RSA withdrawal. Negative PEP responsivity scores reflect PEP shortening (sympathetic activation) and positive PEP responsivity scores reflect PEP lengthening (sympathetic inhibition). “Downward arrow” indicates a significant decrease in ANS responsivity (e.g., RSA withdrawal) and the “upward arrow” indicates significant increase in ANS responsivity (e.g., PEP lengthening). Error bars represent standard errors of the fixed effects.
Comparisons among emotional and cognitive challenges indicated that, across all assessments, the cognitive control challenge (Go/No-Go) elicited lower RSA withdrawal than the blocked goal challenges (Locked Box in preschool, Impossible Gift in kindergarten, and Puzzle Box and Broken Toy in grade 1). The magnitude of RSA responsivity did not differ among the cognitive control (Go/No-Go) and unfairness tasks (Toy Removal in preschool & Not Sharing in kindergarten). In preschool and kindergarten assessments, the problem-solving challenge elicited lower RSA withdrawal than the blocked goal challenges (Locked Box in preschool & Impossible Gift in kindergarten; see Figures 1a & 1c respectively). However, in first grade, the problem-solving challenge elicited greater RSA withdrawal than the Broken Toy task, but did not elicit a significantly different response than Puzzle Box (see Figure 1e).
Sympathetic Responsivity.
As presented in Table 3, the effect of laboratory challenge on PEP were tested in preschool, kindergarten, and first grade (see Models 4, 5 & 6). The problem-solving challenge led to a reduction in PEP values from baseline (PEP shortening) in preschool (p <.001), in kindergarten (p =.052), and in grade 1 (p <.001), suggesting that this challenge elicited sympathetic activation across all time points. The cognitive control challenge led to an increase in PEP values from baseline (PEP shortening) reflecting sympathetic activation only in grade 1. Among the negative-emotion-eliciting tasks, the two unfairness challenges – Toy Removal in preschool and Not Sharing in kindergarten – led to an increase in PEP from baseline to task (PEP lengthening), reflecting sympathetic inhibition. The other 4 negative-emotion-eliciting tasks (Locked Box, Impossible to Open Gift, Puzzle Box & Broken Toy) did not lead to a change in PEP relative to baseline.
Follow up tests comparing the cognitive challenges indicated that the problem-solving challenge elicited greater PEP shortening than the cognitive control task in preschool (see Figure 1b) and in kindergarten (see Figure 1d); however, in the first grade, there was no difference in PEP responsivity across these two challenges (see Figure 1f). Comparisons among the negative-emotion-eliciting tasks indicated that, the unfairness challenges led to increases in PEP (PEP lengthening) than the blocked goal challenges: Toy Removal elicited greater PEP lengthening than Locked Box in preschool (see Figure 1b), and Not Sharing elicited greater PEP lengthening than Impossible Gift in kindergarten (see Figure 1d). The two negative-emotion-eliciting blocked goal challenges in first grade, Puzzle Box and Broken Toy, did not differ in relation to PEP responsivity (see Figure 1f).
Comparisons between and among emotional and cognitive challenges showed that the problem-solving task led to greater reductions in PEP (PEP shortening) than the unfairness tasks in preschool and kindergarten (see Figure 1b & 1d). In preschool, the problem-solving challenge led to a greater reduction in PEP than the blocked goal, Locked Box challenge (see Figure 1b). Likewise, in grade 1, the problem-solving and cognitive control challenges elicited greater reductions in PEP than the two frustrating, blocked-goal challenges (see Figure 1f). Finally, although not the primary focus of this paper, correlations among physiological responsivity scores and task-related behaviors have been reported (see footnote 1).
Stability in ANS Functioning over Time
As presented in Table 4, there was moderate stability in both baseline RSA and baseline PEP over time. There was modest stability in RSA responsivity toward the problem-solving challenge (Tangrams) from preschool to kindergarten, and kindergarten to first grade. RSA responsivity towards the cognitive control task (Go/No-Go) showed modest stability only from kindergarten to grade 1 but not from preschool to kindergarten or first grade. From preschool to kindergarten, children’s RSA responsivity towards distinct emotional challenges showed modest stability (except for the non-significant relation between RSA responsivity during the blocked-goal Locked Box and unfairness Not Sharing tasks). From the kindergarten to the first-grade assessment, children’s RSA responsivity was modestly stable across all emotional challenges. Except for the modest stability in PEP responsivity towards the problem-solving challenge from kindergarten to first grade, there was no stability in PEP responsivity across time.
Table 4.
Longitudinal stability in ANS baseline and responsivity scores from preschool to grade 1
| Stability in RSA |
Stability in PEP |
||||||
|---|---|---|---|---|---|---|---|
| PreK to K | Pre-K to G1 | K to G1 | PreK to K | Pre-K to G1 | K to G1 | ||
| Baseline & Cognitive Tasks | |||||||
| Baseline | .66** | .56** | .66** | .56** | .57** | .49** | |
| Problem-solving (Tangrams) | .32** | .31** | .46** | .12 | −.04 | .26** | |
| Cognitive Control (Go/No-Go) | .11 | .13 | .26** | .10 | −.04 | .07 | |
| Negatively-Valenced Emotional Tasks | |||||||
| Stability in Emotional Composite | .25** | .21** | .30** | .10 | .07 | .12 | |
| Blocked Goal to Unfairness (Locked Box, Not Sharing) | .08 | .11 | |||||
| Stability in Blocked Goal (Locked Box, Impossible Gift) | .24** | .14 | |||||
| Stability in Blocked Goal (Locked Box, Puzzle Box) | .21** | .14 | |||||
| Stability in Blocked Goal (Locked Box, Broken Toy) | .08 | .14 | |||||
| Stability in Unfairness (Toy Removal, Not Sharing) | .20** | .00 | |||||
| Unfairness to Blocked Goal (Toy Removal, Imp Gift) | .16* | .06 | |||||
| Unfairness to Blocked Goal (Toy Removal, Puzzle Box) | .17* | .01 | |||||
| Unfairness to Blocked Goal (Toy Removal, Broken Toy) | .11 | .04 | |||||
| Unfairness to Blocked Goal (Not Sharing, Puzzle Box) | .21** | .12 | |||||
| Unfairness to Blocked Goal (Not Sharing, Broken Toy) | .20** | .08 | |||||
| Stability in Blocked Goal (Impossible Gift, Puzzle Box) | .30** | .09 | |||||
| Stability in Blocked Goal (Impossible Gift, Broken Toy) | .22** | .04 | |||||
Note. N = 221–259.
p < .05
p <.01.
Among the emotional tasks, Locked Box in preschool, Impossible Gift in kindergarten, Puzzle Box and Broken Toy in first grade were considered as “blocked goal” challenges, whereas Toy Removal in preschool and Not Sharing in kindergarten were considered as “unfairness” challenges.
Sample-Level Continuity and Change in ANS Functioning Over Time
Baseline RSA scores demonstrated a small increase over time, F(2, 243) = 4.85, ηp2= .04, p = .009. Baseline RSA in kindergarten (M = 7.32) was higher than baseline RSA in preschool (M = 7.15), p = .004. Baseline RSA in first grade (M = 7.37) was greater than baseline RSA in preschool, p = .01; but not different from baseline RSA in kindergarten, p = .53. Baseline PEP scores also showed an increase over time, F(2, 220) = 19.20, ηp2 = .15, p < .001. Baseline PEP in kindergarten (M = 92.52) was significantly higher than baseline RSA in preschool (M = 91.31), p < .001, and baseline PEP in first grade (M = 94.01) was higher than baseline PEP in kindergarten, p = .028. These results suggest that there was a sample-level increase in baseline RSA and PEP over time.
Given that the two cognitive tasks have been used across all assessments, we examined sample-level change in ANS responsivity toward these challenges over time. There was no mean-level change in RSA or PEP responsivity toward the problem-solving challenge (Tangrams) across time; F(2, 240) = .55, p = .58 and F(2, 222) = 2.49, p =.085, respectively There was a sample-level change in RSA responsivity towards the cognitive control challenge (Go/No-Go) over time, F(2, 235) = 4.72, ηp2= .04, p = .01. Although there was no mean-level change from preschool to kindergarten, p = .54, children showed lower RSA withdrawal in first grade (M = −.26) compared to kindergarten (M = −.40), p = .004, and preschool (M = −.36), p = .024. There was also a sample-level decrease in PEP responsivity towards the cognitive control challenge (Go/No-Go) across time, F(2, 211) = 11.51, ηp2 = .10, p < .001, such that PEP responsivity did not change from preschool (M = −.27) to kindergarten (M = −.41), p = .15; but decreased from kindergarten to first grade (M = −1.23), p < .001. These results suggest that children experienced lower RSA withdrawal and greater PEP shortening during the cognitive control challenge at grade 1, compared to kindergarten. Notably, children’s cognitive control (d’prime scores) showed a sample-level increase over time F(2, 250) = 173.15, ηp2 = .58, p < .001, such that cognitive control increased from preschool (M = 2.23) to kindergarten (M = 2.96), p <.001; and from kindergarten to first grade (M = 3.41), p < .001.
Discussion
Children respond to challenges at the biological, psychological, and behavioral levels (Calkins & Marcovitch, 2010). Although the majority of research on children’s self-regulation has focused on children’s behavioral responses towards distinct challenges, understanding children’s physiological responses to such challenges can reveal important information regarding children’s inner experiences during challenging situations. As one of the stress response systems that most readily and pervasively responds to challenges, ANS plays an important role in regulating the homeostatic function of the body by suppressing the body’s internal demands to respond to effectively external challenges, and promoting growth and restoration during calm states (Porges, 2011). Given that autonomic nervous system (ANS) responses have been proposed to be “context-dependent” (e.g., Porges et al., 2011) understanding children’s sympathetic and parasympathetic autonomic responses towards distinct challenges can help advance our understanding of children’s physiological responses toward everyday challenges and help identify tasks that effectively elicit a stress response. Because autonomic responsivity to emotional and cognitive challenges may reflect trait-like characteristics that mediate pathways towards adaptive functioning (Obradović et al., 2011), it is important to understand whether and the extent to which autonomic responsivity towards specific challenges show stability across time. Moreover, understanding the continuity and change in children’s group-level responses to distinct challenges would be important to understand whether children’s physiological responses increase, decrease, or show continuity over time.
The cognitively demanding problem-solving challenge (Tangrams) elicited sympathetic activation and high levels of parasympathetic inhibition at all assessments, suggesting that this challenging task likely demands the recruitment of both branches of the ANS. Interestingly, there was no sample-level change in the sympathetic or parasympathetic responsivity to the problem-solving challenge across time, suggesting that the overall magnitude of children’s autonomic responses toward this task showed continuity from preschool to first grade. Notably, certain behaviors children engaged in during this challenge showed increases from preschool to first grade. For example, children’s attention to task instructions, on-task behavior, and persistence showed improvements over time, but their energy/enthusiasm showed continuity during this period (see Halliday, 2019). These findings suggest that children’s autonomic responses and observed behaviors may not follow a similar developmental trajectory from preschool to first grade. One possibility is that although children’s autonomic responses may support children’s ability to engage in these behaviors (e.g., persistence), children may show better task performance with a similar level of autonomic response as they get older. A second possibility is that children’s autonomic responses may not be directly related to the specific behaviors observed during this task.
The second cognitive challenge, the cognitive control task (Go/No-Go) did not lead to a significant level of responsivity (change from baseline to task) in preschool or kindergarten but led to sympathetic activation or PEP shortening in grade 1. Although there were no changes in the magnitude of children’s sample-level sympathetic and parasympathetic responsivity toward the cognitive control task from preschool to kindergarten, children showed greater sympathetic activation (PEP shortening) and greater parasympathetic inhibition (RSA withdrawal) in first grade relative to kindergarten. With respect to children’s behavioral performance, children’s task performance reflecting cognitive control showed sample-level increases from preschool kindergarten, and from kindergarten to first grade (also see Isbell, Calkins, Cole, Swingler, & Leerkes, 2019). These findings suggest that children’s autonomic responses and observed behaviors during the cognitive control task follow a relatively similar developmental trajectory, especially from kindergarten to first grade.
In comparing the magnitude of children’s autonomic responses toward the two cognitive challenges, the problem-solving challenge led to greater sympathetic activation than the cognitive control challenge in preschool and kindergarten, and led to greater parasympathetic withdrawal than the cognitive control challenge at all assessments. The systematic difference in the magnitude of RSA withdrawal across the two cognitive tasks may be due to the differences in the cognitive demands posed by the two challenges; such that the problem-solving task may have demanded greater cognitive effort than the cognitive control task. However, given that the cognitive demands of these challenges in this study were not comparable, in future work, it would be important to examine autonomic responsivity towards cognitive tasks that can be compared in relation to how much cognitive effort they demand. Given that physical activity has been related to parasympathetic withdrawal or decreases in RSA (Bush et al., 2011; Porges et al., 2007), the differences in parasympathetic responses towards the challenges may also be due to differences in gross motor behavior. In the problem-solving task, children were asked to place wooden tangrams into pictures of shapes on a paper; whereas in the cognitive control task, children were asked to press a button for each animal. Given that the problem-solving task likely demanded greater motor behavior than the cognitive control task, the problem-solving challenge may have elicited greater RSA withdrawal than the cognitive control due to these differences in physical demands.
The two types of negatively-valenced emotional challenges – the interpersonal challenges involving unfairness and the frustrating blocked-goal challenges – elicited ANS responses differing in magnitude. The two unfairness tasks (Toy Removal and Not Sharing) elicited sympathetic inhibition as reflected by PEP lengthening, whereas the other four blocked-goal challenges (i.e., Locked Box, Impossible to Open Gift, Puzzle Box & Broken Toy) did not lead to a change in sympathetic ANS activity. Moreover, unfairness tasks elicited greater sympathetic inhibition and lower parasympathetic withdrawal than the blocked goal tasks. Specifically, in preschool, Toy Removal elicited greater sympathetic inhibition and lower parasympathetic withdrawal than Locked Box, and in kindergarten Not Sharing elicited greater sympathetic inhibition and lower parasympathetic withdrawal than Impossible to Open Gift.
These findings provide support for the idea that children’s experiences during the two types of negatively-valenced emotional challenges (unfairness & blocked goal) may be qualitatively different. The unfairness challenges have been designed to evoke negative emotions by making children experience injustice (i.e., in Toy Removal, the experimenter takes away a toy that the child chose to play with; in Not Sharing, the experimenter does not share candy equally and takes away the child’s candy). However, the four blocked goal challenges have been designed for children to actively solve a problem to reach a goal (e.g., in Locked Box, children used keys to open a box; in Impossible Gift, children strived to open a wrapped gift). Given that sympathetic inhibition has been proposed to be experienced when individuals anticipate no means of escaping an aversive situation (Obrist, 1981) and has been associated with sadness (Kreibig, 2010), it is possible that the unfairness tasks, Toy Removal and Not Sharing, led children to experience sadness and/or perceive the circumstances as more aversive and difficult to escape. Consistent with this idea, in previous research, adults experienced sympathetic inhibition (an increase in PEP) in response to a sadness eliciting film (Kreibig, Wilhelm, Roth, & Gross, 2007) and children experienced sympathetic inhibition during an emotionally evocative video (Quigley & Stifter, 2006). Based on these findings, it is possible that children may experience sympathetic inhibition towards tasks designed to elicit sadness or passive coping responses. The differences in parasympathetic responses towards the two types of emotional challenges may also be due to the differences in gross motor behavior demanded by the tasks. The blocked goal tasks may have led to greater motor movement as children were engaged in solving a problem to reach a blocked goal (e.g., using keys to open a Locked Box), whereas the unfairness tasks may have lower demands for gross motor behavior (e.g., in Not Sharing, looking at the experimenter as the experimenter ate the candy taken from the child). In future research, it would be important to tease apart the roles of gross motor movement and emotional responses in physiological responses. For example, it would be important to use challenges that are equivalent with respect to the gross motor demands but different with respect to emotional demands to understand if physiological responses differ with respect to the emotional demands of the challenges (see Bush et al., 2011).
In regards to the longitudinal stability and sample-level change in baseline ANS functioning, baseline sympathetic and parasympathetic ANS activity showed moderate-to-high stability and a small mean-level increase across early childhood. These findings are consistent with previous research showing modest-to-moderate levels of stability in baseline levels of RSA (e.g., Alkon, Boyce, Davis, & Eskenazi, 2011; Esposito et al., 2016; Patriquin, Lorenzi, Scarpa, Calkins, & Bell, 2015; Perry et al., 2013), modest-to-moderate levels of stability in baseline PEP across early childhood (Alkon et al., 2011; Esposito et al., 2016), and mean-level increases in baseline ANS activity (e.g., Esposito et al., 2016). Evidence of moderate levels of longitudinal stability in baseline sympathetic and parasympathetic functioning suggest that these two aspects of ANS functioning show trait-like characteristics around 4 to 6 years of age.
There was modest-to-moderate stability in parasympathetic responsivity to the problem-solving challenge from preschool to kindergarten, and kindergarten to first grade. There was no stability in parasympathetic responsivity to the cognitive control challenge from preschool to kindergarten but there was modest stability from kindergarten to first grade. In regards to emotion tasks, there was modest stability in parasympathetic responsivity in most tasks across time. These findings are consistent with previous findings showing modest levels of stability in certain indices of parasympathetic responsivity during early childhood (e.g., Calkins & Keane, 2004; Perry et al., 2012). In contrast to these findings, there was no stability in sympathetic responsivity scores across time, which is consistent with the findings of Alkon et al. (2011) who did not find stability in sympathetic responsivity across infancy and early childhood. Thus, children’s sympathetic ANS responsivity toward challenges may not show trait-like patterns from around 4 to 6 years of age. There are a number of possible explanations to this finding. First, it may be that children’s sympathetic ANS responsivity towards challenges is not stable during early childhood and only become stable later in development. Second, it is possible that PEP responsivity may not be a stable measure; however, recent evidence suggests that there is even less stability in electrodermal activity, as measured by skin conductance, compared to cardiac measures of ANS functioning (Gatzke-Kopp & Ram, 2018). Based on Polyvagal Theory’s proposition that individuals rely mostly on parasympathetic system in responding to mildly stressful challenges (Porges, 2011), a third explanation is that there should be lower levels of stability in sympathetic responsivity towards mildly stressful challenges. As such, it would be important to examine and rule out these alternative explanations to better understand the development of sympathetic responsivity toward everyday challenges.
The current study supported theoretical propositions suggesting that autonomic responsivity is context-dependent. Consistent the notion that individuals rely mostly on their parasympathetic system when responding to everyday challenges, all laboratory challenges elicited parasympathetic inhibition or RSA withdrawal, a response pattern that has been proposed to reflect coping responses (Porges, 2011); however, the magnitude of the parasympathetic response depended on the task. The magnitude of sympathetic responses toward challenges also depended on the task, but in addition, the direction of the sympathetic response (i.e., activation, inhibition, no change relative to baseline) was also task-depended. The problem-solving task led to sympathetic activation, the unfairness tasks led to sympathetic inhibition, and the blocked goal tasks did not lead to a change in sympathetic activity relative to baseline.
Second, this study had important implications for selection of tasks in ANS research. Our results showing differences within both emotional (i.e., blocked goal vs. interpersonally upsetting) and cognitively challenges (e.g., cognitively more vs. less challenging) highlight the importance of critically evaluating the type of emotional or cognitive tasks that would be conducive for examining certain questions. For example, a heightened parasympathetic withdrawal response toward a more challenging task seem to be a normative response; however, the same type of response toward a less challenging task may reflect a more “reactive” way of responding to less demanding tasks. Moreover, given that only two types of challenges (i.e., challenging problem-solving task, interpersonal/unfairness task) elicited mean-level change in sympathetic activity, researchers interested in examining sympathetic ANS functioning may consider selecting such tasks that elicit change in children’s sympathetic activity.
An important strength of this study was that although most research on children’s ANS functioning focused more on parasympathetic functioning, this study examined both parasympathetic and sympathetic ANS functioning in early childhood. As such, the design of the study was advantageous for investigating basic yet under-investigated questions such as normative sympathetic responses toward laboratory challenges across development. Moreover, examining parasympathetic and sympathetic ANS responses within the same study allowed us to evaluate normative response patterns of these branches to the same challenges. This study design was advantageous for examining questions such as whether certain challenges (e.g., problem-solving) lead to heightened responses in both ANS branches relative to other challenges (e.g, cognitive control) or whether they lead to only heightened responses in one branch only.
This study had notable limitations. An important limitation was that although the same cognitively demanding challenges were administered across all three assessments, different emotional challenges were administered at each assessment. The rationale behind this decision was that children could potentially remember important components of the emotional challenges and therefore not become frustrated the second or third time they encountered the challenge. For example, if the Locked Box task was used at each time point, children may have remembered that “none of the keys work” and that the experimenter did not give the right key to solve the task. Alternatively, only a subset of children, perhaps with better memory, could remember these details and not get as upset or frustrated as the other participants, which could have introduced an important confound (i.e., child cognitive ability/memory) to the design. Given that different emotionally frustrating tasks were used across time, we did not examine mean-level change in these tasks over time as increases or decreases across time may be due to the selection of different tasks rather than due to developmental change. It is also important to note that none of the laboratory challenges were specifically designed to elicit fear, joy, or disgust responses and therefore normative ANS responses toward tasks eliciting these emotions were not examined. Another possible limitation was that baseline ANS activity was assessed during video tasks, which may lower heart children’s physiological arousal compared to other baseline conditions (e.g., book reading or listening to a story) and therefore physiological responsivity scores may have been overestimated.
Moreover, recent work has raised concerns regarding applying historically used frequency-band parameters when scoring RSA in children. Using HR data previously collected from 5 samples of children/adolescents of different ages, Shader and colleagues (2018) recalculated RSA scores, which have originally been calculated using adult respiratory frequency bands, using age-appropriate frequency bands. Their results indicated that, when using FFT as in this study, scoring children’s RSA values using adult frequency band parameters leads to over-estimation of RSA values. The current study used frequency band parameters appropriate for older children (rather than adults). Therefore, although Shader et al.’s findings cannot directly elude to whether or the extent to which using parameters better suited for older children would have a significant impact on the findings of this study, it certainly highlights the importance of using age-appropriate frequency bands when calculating RSA scores.
This study has implications for understanding the functioning of children’s sympathetic and parasympathetic ANS in everyday life. Consistent with the Polyvagal Theory’s propositions (Porges, 2011), results support the idea that children typically rely on their phylogenetically newer, myelinated vagal system in responding to challenges that pose psychosocial and cognitive demands, as this system likely supports the ability to cope and respond to challenges effectively. Consistent with Obrist’s perspective (Obrist, 1981), children also likely experience sympathetic activation during challenges require high levels of cognitive effort such as during problem-solving tasks and experience sympathetic inhibition during interpersonally upsetting challenges that involve unfairness. Evidence showing that distinct kinds of challenges pose different levels of physiological demands highlight the importance of understanding how experiencing such challenges pervasively may influence children’s development in the long-term.
Acknowledgements:
This research was supported by Grant 5R01HD071957 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. The authors wish to express their thanks to the students and staff who assisted with data collection, the families who participated in the study, and Dr. Marion O’Brien who was instrumental in the planning and implementation of this study prior to her death.
Footnote
Although not the primary focus of this paper, correlations among physiological responsivity scores and task-related behaviors were examined for each assessment to understand whether individual differences in physiological responses were related to behaviors. RSA and PEP responsivity scores obtained during the problem-solving task (Tangrams) were not related to global experimenter ratings on attention to instructions (p =.12–.58), persistence (p = .41–.99), and on-task behavior (p =.16–.64) at any of the assessments. Responsivity scores from the Go/No-Go task were not related to task performance (d’) in preschool and kindergarten (p =.52–.99). Greater RSA withdrawal during Go/No-Go was modestly related to better task performance in grade 1 (r = −.15, p = .02); however, PEP responsivity was not related to task performance (p = .93). RSA responsivity was negatively related to emotion regulation (composite of global regulation, verbal negativity and latency to distress) during Toy Removal in preschool (r =−.15, p = .02), Not Sharing in kindergarten (r = −.14, p =.04), Impossible Gift in kindergarten (r = −.15, p = .02) and Puzzle Box in grade 1 (r = −.13, p = .05), such that greater RSA withdrawal was linked with worse regulation. RSA responsivity was not related to observed emotion regulation during Locked Box in preschool (p = .90) and Broken Toy in grade 1 (p = .29), and PEP responsivity was not related to observed emotion regulation (p = 22–.81).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alkon A., Boyce WT., Davis NV., & Eskenazi B. (2011). Developmental Changes in Autonomic Nervous System Resting and Reactivity Measures in Latino Children from 6 to 60 Months of Age. Journal of Developmental & Behavioral Pediatrics, 32, 668–677. doi: 10.1097/DBP.0b013e3182331fa6 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT., & Quigley KS. (1991). Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), 459–487. doi: 10.1037/0033-295X.98.4.459 [DOI] [PubMed] [Google Scholar]
- Berntson Gary G., Cacioppo JT., & Fieldstone A. (1996). Illusions, arithmetic, and the bidirectional modulation of vagal control of the heart. Biological Psychology, 44(1), 1–17. doi: 10.1016/S0301-0511(96)05197-6 [DOI] [PubMed] [Google Scholar]
- Bush NR., Alkon A., Obradović J., Stamperdahl J., & Thomas Boyce W. (2011). Differentiating challenge reactivity from psychomotor activity in studies of children’s psychophysiology: Considerations for theory and measurement. Journal of Experimental Child Psychology, 110, 62–79. doi: 10.1016/j.jecp.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR., Caron ZK., Blackburn KS., & Alkon A. (2016). Measuring Cardiac Autonomic Nervous System (ANS) Activity in Toddlers - Resting and Developmental Challenges. Journal of Visualized Experiments, 108, e53652. doi: 10.3791/53652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA., Goldsmith HH., & Davidson RJ. (2005). Cardiac reactivity is associated with changes in negative emotion in 24-month-olds. Developmental Psychobiology, 46(2), 118–132. doi: 10.1002/dev.20048 [DOI] [PubMed] [Google Scholar]
- Carlson SM., & Wang TS. (2007). Inhibitory control and emotion regulation in preschool children. Cognitive Development, 22, 489–510. doi: 10.1016/j.cogdev.2007.08.002 [DOI] [Google Scholar]
- Eisenberg N., Guthrie IK., Fabes RA., Shepard S., Losoya S., Murphy BC., … Reiser M. (2000). Prediction of elementary school children’s externalizing problem behaviors from attentional and behavioral regulation and negative emotionality. Child Development, 71(5), 1367–1382. doi: 10.1111/1467-8624.00233 [DOI] [PubMed] [Google Scholar]
- Esposito EA., Koss KJ., Donzella B., & Gunnar MR. (2016). Early deprivation and autonomic nervous system functioning in post-institutionalized children. Developmental Psychobiology, 58(3), 328–340. doi: 10.1002/dev.21373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR., Van Hulle CA., Aksan N., Essex MJ., & Goldsmith HH. (2011). Deriving Childhood Temperament Measures From Emotion-Eliciting Behavioral Episodes: Scale Construction and Initial Validation. Psychological Assessment, 23, 337–353. doi: 10.1037/a0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L., & Ram N. (2018). Developmental dynamics of autonomic function in childhood. Psychophysiology, 55(11), e13218. doi: 10.1111/psyp.13218 [DOI] [PubMed] [Google Scholar]
- Halliday SE., Calkins SD., & Leerkes EM. (2018). Measuring preschool learning engagement in the laboratory. Journal of Experimental Child Psychology, 167, 93–116. doi: 10.1016/j.jecp.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell E., Calkins SD., Cole VT., Swingler MM., & Leerkes EM. (2019). Longitudinal associations between conflict monitoring and emergent academic skills: An event-related potentials study. Developmental Psychobiology, 61(4), 495–512. doi: 10.1002/dev.21809 [DOI] [PubMed] [Google Scholar]
- Kolacz J., Holochwost SJ., Gariépy JL., & Mills-Koonce WR. (2016). Patterns of joint parasympathetic, sympathetic, and adrenocortical activity and their associations with temperament in early childhood. Developmental Psychobiology, 58(8), 990–1001. doi: 10.1002/dev.21429 [DOI] [PubMed] [Google Scholar]
- Lozano DL., Norman G., Knox D., Wood BL., Miller BD., Emery CF., & Berntson GG. (2007). Where to B in dZ/dt. Psychophysiology, 44(1), 113–119. doi: 10.1111/j.1469-8986.2006.00468 [DOI] [PubMed] [Google Scholar]
- Miller JG., Kahle S., & Hastings PD. (2016). Moderate baseline vagal tone predicts greater prosociality in children. Developmental Psychology, 53(2), 274–289. doi: 10.1037/dev0000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM., & Rohleder N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology, 34(4), 486–496. 10.1016/j.psyneuen.2009.01.014 [DOI] [PubMed] [Google Scholar]
- Nunan D., Sandercock GRH., & Brodie DA. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. PACE - Pacing and Clinical Electrophysiology, 33(11), 1407–1417. doi: 10.1111/j.1540-8159.2010.02841 [DOI] [PubMed] [Google Scholar]
- Obradović J., Bush NR., & Boyce WT. (2011). The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors, 23(1), 101–114. Development and Psychopathology. doi: 10.1017/S0954579410000672 [DOI] [PubMed] [Google Scholar]
- Obrist PA. (1981). Cardiovascular psychophysiology: A perspective. New York: Plenum. [Google Scholar]
- Patriquin MA., Lorenzi J., Scarpa A., Calkins SD., & Bell MA. (2015). Broad implications for respiratory sinus arrhythmia development: Associations with childhood symptoms of psychopathology in a community sample. Developmental Psychobiology, 57(1), 120–130. doi: 10.1002/dev.21269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB., Nelson JA., Swingler MM., Leerkes EM., Calkins SD., Marcovitch S., & O’Brien M. (2013). The relation between maternal emotional support and child physiological regulation across the preschool years. Developmental Psychobiology, 55(4), 382–394. doi: 10.1002/dev.21042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. (1995). Cardiac vagal tone: A physiological index of stress. Neuroscience & Biobehavioral Reviews, 19(2), 225–233. 10.1016/0149-7634(94)00066-A [DOI] [PubMed] [Google Scholar]
- Porges SW. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. doi: 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW., Heilman KJ., Bazhenova OV., Bal E., Doussard-Roosevelt JA., & Koledin M. (2007). Does motor activity during psychophysiological paradigms confound the quantification and interpretation of heart rate and heart rate variability measures in young children? Developmental Psychobiology, 49(5), 485–494. doi: 10.1002/dev.20228 [DOI] [PubMed] [Google Scholar]
- Porges SW. (2011). The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-regulation (Norton Series on Interpersonal Neurobiology). New York, NY: W. W. Norton & Company. [Google Scholar]
- Quigley KS., & Stifter C. a. (2006). A comparative validation of sympathetic reactivity in children and adults. Psychophysiology, 43, 357–365. doi: 10.1111/j.1469-8986.2006.00405 [DOI] [PubMed] [Google Scholar]
- Richter M., Friedrich A., & Gendolla GH. (2008). Task difficulty effects on cardiac activity. Psychophysiology, 45(5), 869–875. doi: 10.1111/j.1469-8986.2008.00688 [DOI] [PubMed] [Google Scholar]
- Saul J. (1990). Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. Physiology, 5(1), 32–37. doi: 10.1152/physiologyonline.1990.5.1.32 [DOI] [Google Scholar]
- Shader TM., Gatzke-Kopp LM., Crowell SE., Jamila Reid M., Thayer JF., Vasey MW., … Beauchaine TP. (2018). Quantifying respiratory sinus arrhythmia: Effects of misspecifying breathing frequencies across development. Development and Psychopathology, 30(1), 351–366. doi: 10.1017/S0954579417000669 [DOI] [PubMed] [Google Scholar]
- Smith R., Thayer JF., Khalsa SS., & Lane RD. (2017). The hierarchical basis of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 75, 274–296. doi: 10.1016/j.neubiorev.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Swingler MM., Isbell E., Zeytinoglu S., Calkins SD., & Leerkes EM. (2018). Maternal behavior predicts neural underpinnings of inhibitory control in preschoolers. Developmental Psychobiology, 60(6), 692–706. doi: 10.1002/dev.21742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RA., & Kirby LD. (2001). Effort determination of cardiovascular response: An integrative analysis with applications in social psychology. Advances in Experimental Social Psychology, 33, 255–307. doi: 10.1016/S0065-2601(01)80007-1 [DOI] [Google Scholar]