Abstract
Consumption of a high fat diet (HFD) increases circulating free fatty acids, which can enter the brain and promote a state of microgliosis, as defined by a change in microglia number and/or morphology. Most studies investigating diet-induced microgliosis have been conducted in male rodents despite well-documented sex differences in the neural control of food intake and neuroimmune signaling. This highlights the need to investigate how sex hormones may modulate the behavioral and cellular response to HFD consumption. Estradiol is of particular interest since it exerts a potent anorexigenic effect and has both anti-inflammatory and neuroprotective effects in the brain. As such, the aim of the current study was to investigate whether estradiol attenuates the development of HFD-induced microgliosis in female rats. Estradiol- and vehicle-treated ovariectomized rats were fed either a low-fat chow diet or a 60% HFD for 4 days, after which they were perfused and brain sections were processed via immunohistochemistry for microglia-specific Iba1 protein. Four days of HFD consumption promoted microgliosis, as measured via an increase in the number of microglia in the arcuate nucleus (ARC) of the hypothalamus and nucleus of the solitary tract (NTS), and a decrease in microglial branching in the ARC, NTS, lateral hypothalamus (LH), and ventromedial hypothalamus. Estradiol replacement attenuated the HFD-induced changes in microglia accumulation and morphology in the ARC, LH, and NTS. We conclude that estradiol has protective effects against HFD-induced microgliosis in a region-specific manner in hypothalamic and hindbrain areas implicated in the neural control of food intake.
Keywords: Hypothalamus, Nucleus of the solitary tract, Inflammation, Microglia, Obesity, Female rats
1. Introduction
The escalating prevalence of obesity and its associated medical complications, including cardiovascular disease, diabetes, certain cancers, and chronic respiratory and gastrointestinal diseases (Malnick and Knobler, 2006), represents a growing public health concern. The rise in obesity over the past few decades appears to be mediated primarily by environmental factors, including the overabundance of highly palatable, energy-dense foods and the shift toward an increasingly sedentary lifestyle (Lake and Townshend, 2006). Rodent models have been critical to understanding how our current obesogenic environment affects the biological and behavioral factors that control food intake and regulate body weight (Lutz and Woods, 2012). These models of diet-induced obesity (DIO) involve switching rodents from a chow diet that is low in fat and refined sugar but high in complex carbohydrates and fiber to a more palatable, energy-dense diet that is high in fat and/or sugar (Butler and Eckel, 2018).
Consumption of a HFD increases circulating free fatty acids, which enter the brain via passive diffusion or a variety of transport protein-mediated pathways (Tracey et al., 2018). Central fatty acids act on toll like receptors (TLRs) on glial cells (Huang et al., 2012) to promote a state of inflammation in the brain (De Souza et al., 2005; Guillemot-Legris and Muccioli, 2017; Posey et al., 2008; Thaler et al., 2012; Valdearcos et al., 2015). This pro-inflammatory response is mediated predominantly by microglia, which serve as the resident macrophages of the central nervous system (Valdearcos et al., 2014). Following a central insult, microglia transition rapidly from a ramified state, characterized by long secondary and tertiary branches extending from the soma, to an activated, pro-inflammatory state with retracted and thickened processes (Mor et al., 1999; Streit et al., 1999). Activated microglia promote central inflammation through the release of pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α(TNFα) (Mor et al., 1999; Streit et al., 1999).
Studies have shown that limited (1–3 days) exposure to a HFD is sufficient to promote a state of microgliosis and an increase in the release of pro-inflammatory cytokines in the mediobasal hypothalamus (e.g., Thaler et al., 2012), similar to that observed following weeks to months of HFD exposure (Valdearcos et al., 2014). This rapid (within days) HFD-induced pro-inflammatory response is particularly evident in the arcuate nucleus of the hypothalamus (ARC) (Thaler et al., 2012), which contains both anorexigenic and orexigenic neurons that play a critical role in the neural control of food intake (Sohn, 2015). Because this rapid HFD-induced neuroimmune response in the ARC precedes significant weight gain, it is thought to play a causal role in the pathophysiology of obesity. In support of this notion, it has been shown that inhibiting inflammation through depletion of microglial cells in the mediobasal hypothalamus prevents DIO in mice consuming a HFD (Valdearcos et al., 2014). While the ARC has been a major focus of diet-induced neuroinflammation research, it is likely that other brain regions are also adversely impacted by high levels of free fatty acids. In particular, other hypothalamic and hindbrain regions implicated in the neural control of food intake, such as the ventromedial hypothalamus (VMH), lateral hypothalamus (LH), and nucleus of the solitary tract (NTS) may also be responsive to HFD but are understudied in this context (Blouet and Schwartz, 2012; Sohn, 2015; Stuber and Wise, 2016).
Most studies investigating DIO have been conducted in male rodents despite well-documented sex differences in the neural control of food intake and prevalence of eating disorders (Chowen et al., 2017; Klump et al., 2012). While few studies have been conducted in females, the available data suggest that females are less susceptible than males to diet-induced obesity (Dorfman et al., 2017; Hong et al., 2009; Lainez et al., 2018; Underwood and Thompson, 2016). This has led researchers to investigate how sex hormones may modulate the development of DIO. In this regard, estradiol has emerged as a leading candidate based on its well documented anorexigenic effect in many species including humans (Eckel, 2011), and the weight gain associated with declining levels of estradiol in ovariectomized (OVX) animals and peri-menopausal women (Asarian and Geary, 2002; Wing et al., 1991).
Estradiol exerts anti-inflammatory and neuroprotective effects in the brain that may be mediated, at least in part, by intrinsic sex differences in the microglial response to inflammatory stimuli (Yanguas-Casás et al., 2018). Estradiol’s ability to modulate neuroinflammation has been well-studied in the context of aging and neurodegenerative disease (Vegeto et al., 2008), where it has been shown to decrease inflammation by acting directly on microglia and neurons via estrogen receptor-dependent mechanisms (Morselli et al., 2014). However, the impact of estradiol on diet-induced inflammation is not well characterized. In vitro studies have shown that estradiol can protect against saturated fatty acid-induced inflammation in cultured hypothalamic neurons (Morselli et al., 2014), but the effect of circulating estradiol in animals maintained on a HFD is unknown.
The current study was designed to investigate the impact of estradiol on the microglial response to short-term HFD consumption. Groups of estradiol- and vehicle-treated OVX rats were maintained on either a standard (low fat) diet or a HFD for 4 days, after which the number and morphology of microglial cells were assessed in hypothalamic and hindbrain areas implicated in the neural control of food intake. This short-term exposure to HFD was chosen because we were specifically interested in investigating whether estradiol can attenuate the rapid development of diet-induced microgliosis at a time point that precedes classic signs of an obesity phenotype such as insulin and leptin resistance. We hypothesized that 4 days of HFD consumption would promote microgliosis, and that estradiol replacement would attenuate this reactive, neuroimmune response to the HFD.
2. Methods
2.1. Animals and housing
Female Sprague-Dawley rats (Charles River Breeding Laboratory, Raleigh, NC), weighing 225–250 g at study onset, were housed individually in polycarbonate cages (Ancare; 19 x 10.5 x 8 inches). Throughout the study, animals were given unrestricted access to pelleted chow (5001 Rodent Diet, LabDiet; St. Louis, MO; 3.36 kcal/g), presented in stainless-steel food hoppers placed on top of the cage lids, and tap water unless otherwise specified. Animal rooms were maintained at 20 ± 2°C with a 12:12 h light-dark cycle (dark onset = 1900 h). Animal usage and all procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
2.2. Surgery
Animals were anesthetized with 3% isoflurane (Butler Schein Animal Health, Dublin, Ohio), delivered at a rate of 1 L/min, and bilaterally OVX using an intra-abdominal approach. Following surgery, animals received intraperitoneal injections of butorphanol (0.5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) to minimize postoperative pain. Behavioral testing commenced after two weeks of postoperative recovery.
2.3. Hormone treatment and dietary manipulation
At study onset, OVX animals were randomly assigned to one of four groups matched for body weight: chow-vehicle (n=7), chow-estradiol (n=7), HFD-vehicle (n=8), or HFD-estradiol (n=8). Animals received subcutaneous injections of either 2 μg estradiol (estradiol benzoate; E8515; Sigma), dissolved in 0.1 mL of 100% dimethyl sulfoxide (DMSO; Sigma), or 0.1 mL DMSO vehicle alone according to group assignment. Injections were administered during the mid-light cycle on days 4 and 8 of the 10-day testing protocol. This cyclic regimen of estradiol replacement was chosen because it has been shown to model the changes in endogenous estradiol secretion observed across the estrous cycle in gonadally-intact female rats, and reinstate estrous-related decreases in food intake 48 h after each injection of estradiol (i.e., on test days 6 and 10 in the current study) (Asarian and Geary, 2002). The dietary manipulation began during the mid-light phase on test day 6, when animals assigned to the HFD group were switched from the chow (low fat) diet (13% fat; 3.36 kcal/g) to a HFD (D12492, Research Diets, New Brunswick, NJ; 60% fat; 5.21 kcal/g). The HFD pellets were presented in stainless-steel food hoppers placed on top of the cage lids for 4 days. During this same 4-day interval, animals in the control group remained on the chow diet. Food intake and body weight were measured (Ohaus Scout Pro; ± 0.1 g) at the same time each day during the mid-light phase for the duration of the experiment. Any spillage inside the cage was collected and accounted for in the daily food intake measurements. Immediately following the diet manipulation (on test day 10), animals were deeply anesthetized with Somnasol (100 mg/kg, Henry Schein) and, when unresponsive, perfused transcardially with a 0.15 M saline solution followed by 4% paraformaldehyde. Brains were dissected and post-fixed in 4% paraformaldehyde for an additional 24 h at 4°C and then cryoprotected in 30% sucrose until processed as described below. Immediately following the perfusion, uterine horns of 22 animals were removed (n = 11/hormone treatment group), trimmed bilaterally to 10 mm in length, and weighed. Because estradiol has been shown to increase uterine horn wet weight (Davis et al., 2008; Hewitt et al., 2003), this served as a physiological assay of hormone treatment.
2.4. Immunohistochemistry
Coronal sections were cut on a freezing, sliding microtome at 35 μm through the hypothalamus (−1.56 to −3.96 from bregma) and hindbrain (−13.44 to −15.00 from bregma) and then processed for the expression of ionized calcium binding adaptor molecule (Iba1), a cytoplasmic protein that is specific to microglia (Ito et al., 1998). Free-floating sections were washed with 0.1M PB followed by incubation in 0.5% sodium borohydride (20 min) and 0.3% triton (30 min; all in 0.1M PB). Tissue sections were incubated in Iba1 primary antibody (019–19741, Wako Chemicals), at a dilution of 1:40,000 in 1% goat serum + 0.3% triton in 0.1M PB overnight (20 h) at room temperature. On the following day, the tissue was washed in 0.1M PB and then incubated for 1 h in biotinylated goat anti-rabbit secondary antibody (BA-1000, Jackson Immuno-Research) at a dilution of 1:500 in 0.3% triton followed by incubation in an avidin-biotin mixture (PK-6100, Vector Labs) for 90 min. To visualize Iba1 immunoreactivity, tissue sections were stained with nickel-intensified DAB (SK-4100, Vector Labs) for 11 min. Stained tissue sections were mounted on microscope slides and dipped in xylene before being coverslipped.
2.5. Iba1 quantification
Image Pro Plus software was used to capture images of Iba1-processed hypothalamic and hindbrain tissue at 20x magnification using an Olympus AX70 light microscope equipped with a digital camera. The number and morphology of Iba1-positive cells were quantified within a 300 x 300 pixel sample from each brain region of interest (ROI), including the ARC, VMH, LH, and NTS as in previous studies (Spencer et al., 2019; Thaler et al., 2012) (Fig. 1A,B). Within each ROI, the number of Iba1-positive cells was counted manually. Only those cells with a distinct soma were included in the manual counts. To investigate cell morphology, microglial branch number and length were quantified via a skeletonization analysis using ImageJ software as described previously (Young and Morrison, 2018). In this analysis, ImageJ processes each image so that pixels are subtracted sequentially from thresholded material within a given ROI until each structure is only one pixel wide (Fig. 1C,D). The software then automatically calculates the number of branches and summed branch length within the ROI. For each animal, the total number of branches and summed branch length was divided by the number of microglia within each ROI, as determined by the manual counts. The ARC, VMH, and LH were quantified at the level of the median eminence (−2.4 to −3.12 from bregma; n = 2–3 sections/ROI), and the NTS was quantified at the level of the area postrema (−13.68 to −14.4 from bregma; n = 2–3 sections/brain). Some animals were excluded from analysis due to tissue damage. For the LH, one animal was excluded from each of the chow-vehicle, chow-estradiol, and HFD-estradiol groups. For the VMH, one animal was excluded from the chow-estradiol group. For the NTS, one animal was excluded from each of the chow-estradiol, HFD-vehicle, and HFD-estradiol groups.
Fig. 1.
The number and morphology of Iba1-positive cells was assessed in the ARC, VMH, LH, and NTS. Boxes represent the area of quantification within each brain region of interest (A,B). Representative images for assessment of Iba1 morphology (number of branches and summed branch length) before (C) and after (D) applying the skeletonization function in Image J. Scale bars are 100μm (A,B) and 10μm (C). Abbreviations: ARC; arcuate nucleus, VMH; ventromedial hypothalamus, LH; lateral hypothalamus, III; third ventricle, NTS; nucleus of the solitary tract, AP; area postrema, CC; central canal.
2.6. Statistical analysis
Data are presented as mean + SEM. Group differences in uterine horn weights were analyzed via an independent t-test (estradiol versus vehicle treatment). Initial body weight across the four treatment groups at study onset was analyzed via a one-way ANOVA. Food intake, body weight, number of Iba1-positive cells, average branch number, and average summed branch length were analyzed via two-factor ANOVAs (diet x hormone). Group differences following significant (p < 0.05) ANOVA effects were examined using Student Newman-Keuls post-hoc tests. Effect size estimates were calculated for significant ANOVAs and post-hoc pairwise comparisons via partial Eta squared (η2) and Cohen’s d, respectively. The relationship between cumulative food intake/weight gain and the number of Iba1-positive cells in HFD-fed animals was assessed via Pearson correlations. These correlational analyses were restricted to brain areas in which the HFD increased the number of Iba1-positive cells. Data were analyzed using SPSS statistical software.
3. Results
3.1. Effects of diet and hormone on uterine horn weight, caloric intake, and weight gain
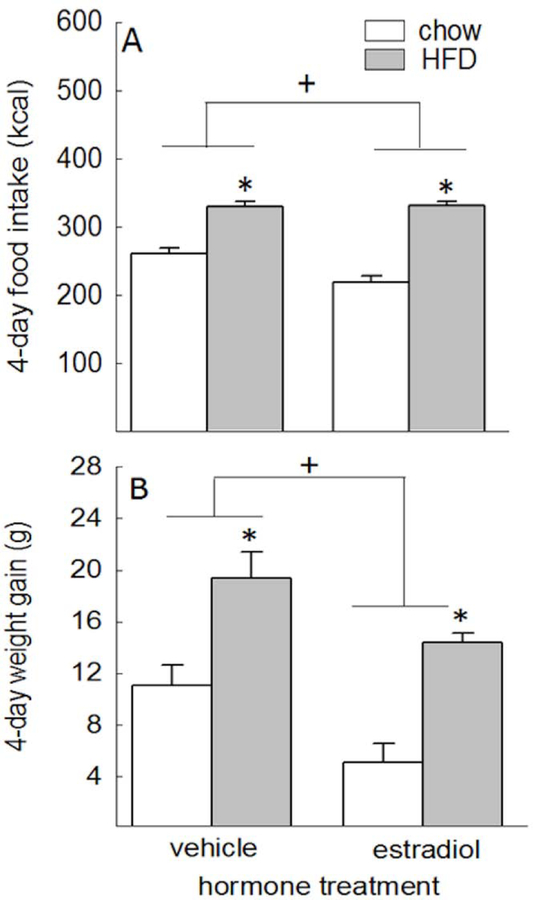
Estradiol treatment increased uterine horn weight, relative to vehicle treatment (66.8 ± 4.4 mg vs. 25.9 ± 3.5 mg, respectively; t(21) = 7.37, p < 0.001). Analysis of caloric intake across the 4-day diet manipulation revealed main effects of diet and hormone treatment, F(1,23) = 143.85 and 4.81, respectively, p < 0.05, η2 = 0.86 and 0.17, respectively. Animals receiving the HFD ate more than animals receiving chow, p < 0.05, d = 4.33, and estradiol-treated animals ate less than vehicle-treated animals, p < 0.05, d = −0.26 (Fig. 2A). The starting body weights for each group at the beginning of the experiment did not differ from each other, F(3,26) = 0.42, p = 0.742 (data not shown). Analysis of weight gain at the end of the 4-day diet manipulation revealed main effects of diet and hormone treatment, F(1,23) = 43.06 and 15.76, respectively, p < 0.05, η2 = 0.65 and 0.41, respectively. Animals receiving the HFD gained more weight than animals receiving chow, p < 0.05, d = 2.02, and estradiol-treated animals gained less weight than vehicle-treated animals, p < 0.05, d = −0.86 (Fig. 2B).
Fig. 2.
(A) Total caloric intake across the 4-day diet manipulation was influenced by diet, with increased caloric intake in HFD-fed versus chow-fed animals, and hormone treatment, with decreased caloric intake in estradiol- versus vehicle-treated animals. (B) Weight gain across this same period was similarly influenced by diet, with greater weight gain in HFD-fed versus chow-fed animals, and hormone treatment, with less weight gain in estradiol- versus vehicle-treated animals. *HFD-fed animals greater than chow-fed animals, p < 0.05. +Estradiol-treated animals < vehicle-treated animals, p < 0.05.
3.2. Estradiol blocked HFD-induced changes in microglia number and morphology in the hypothalamus
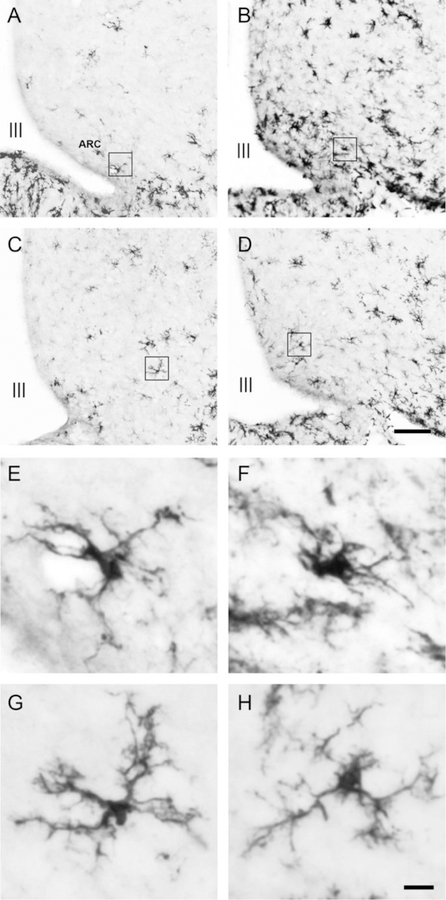
Immunohistochemistry was used to detect the microglia-specific cytoplasmic marker Iba1. Representative images of Iba1-positive cells within the ARC for each treatment condition are shown in Fig. 3. Analysis of the number of Iba1-positive cells in the ARC revealed a diet x hormone interaction, F(1,26) = 5.30, p < 0.05, η2 = 0.17. Consumption of the HFD increased the number of Iba1-positive cells in vehicle-treated animals, p < 0.05, d = 1.57, but had no effect in estradiol-treated animals (Fig. 4A). The average microglial branch number and summed branch length within the ARC were also influenced by diet x hormone interactions, F(1,24) = 6.29 and 6.86, respectively, p < 0.05, η2 = 0.21 and 0.22, respectively. Consumption of the HFD decreased the number and length of branches in Iba1-positive cells in vehicle-treated animals, p < 0.05, d = −1.47 and −1.54, respectively, but not in estradiol-treated animals (Fig. 4B,C).
Fig. 3.
Representative photomicrographs of Iba1-positive cells in the ARC of (A) chow-fed/vehicle-treated animals, (B) HFD-fed/vehicle-treated animals, (C) chow-fed/estradiol-treated animals, and (D) HFD-fed/estradiol-treated animals. Representative photomicrographs depicting the morphology of individual microglia from (E) chow-fed/vehicle-treated animals, (F) HFD-fed/vehicle-treated animals, (G) chow-fed/estradiol-treated animals, and (H) HFD-fed/estradiol-treated animals. The individual microglial cells depicted in E-H are higher magnification images of the cells outlined by the boxes in A-D. Scale bars are 100μm (D) and 10μm (H). Abbreviations: ARC; arcuate nucleus, III; third ventricle.
Fig. 4.
Estradiol treatment blocked HFD-induced changes in both the number and morphology of Iba1-positive cells in the ARC. HFD consumption increased the number of Iba1-positive cells in the ARC of vehicle-treated, but not estradiol-treated, animals (A). HFD consumption also decreased the average number of branches (B) and average summed branch length (C) of Iba1-positive cells in vehicle-treated, but not estradiol-treated, animals. **Different from all other means, p < 0.05.
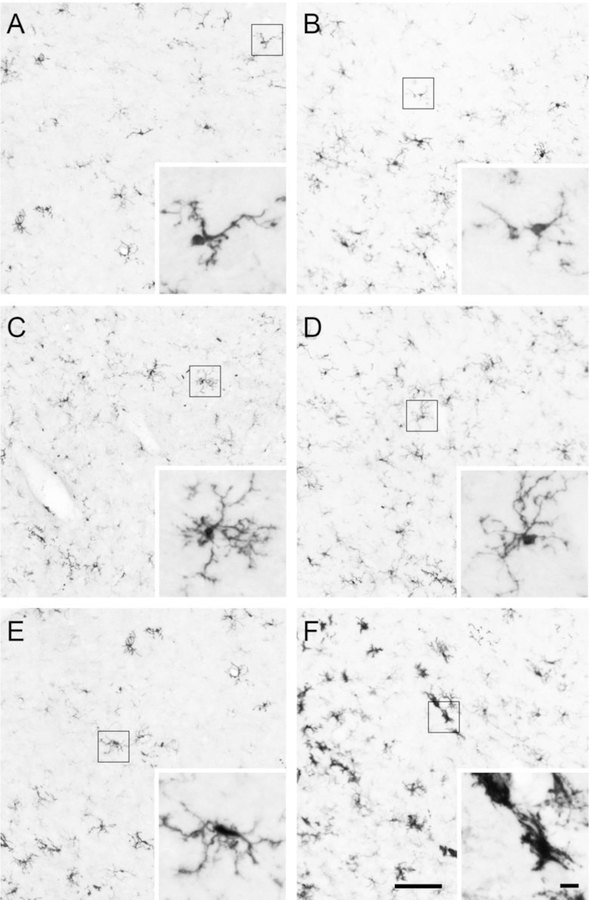
Representative images of Iba1-positive cells within the LH and VMH are shown in Fig. 5. The number of Iba1-positive cells in the LH was not affected by diet or hormone treatment, F(1,23) = 0.01 – 0.69, p = 0.41 – 0.91 (Fig. 6A). The average branch number of Iba1-positive cells was influenced by a diet x hormone interaction, F(1,21) = 4.42, p < 0.05, η2 = 0.17. Consumption of the HFD decreased average branch number in vehicle-treated animals, p < 0.05, d = −0.63, but not in estradiol-treated animals (Fig. 6B). While there was a trend for the average summed branch length of Iba1-positive cells to be influenced by a diet x hormone interaction, this effect failed to reach statistical significance, F(1,21) = 3.77, p = 0.06 (Fig. 6C).
Fig. 5.
Representative photomicrographs of iba1-positive cells in the LH of (A) chow-fed/vehicle-treated animals, (B) HFD-fed/vehicle-treated animals, (C) chow-fed/estradiol-treated animals, (D) and HFD-fed/estradiol-treated animals. Representative photomicrographs of iba1-positive cells in the VMH of (E) chow-fed/vehicle-treated animals and (F) HFD-fed/vehicle-treated animals. The inset images are higher magnification of the individual Iba1-positive cells within the boxes in A-F. Scale bar of LH and VMH images is 100μm. Scale bar of inset images is 10μm.
Fig. 6.
Estradiol blocked HFD-induced changes in microglia morphology in the LH but not the VMH. There were no differences in the number of iba1-positive cells in the LH (A). HFD decreased the number of branches per cell in the LH in vehicle-treated, but not estradiol-treated, animals (B). HFD did not affect summed branch length per cell in the LH (C). In the VMH, HFD did not affect the number of iba1-positive cells (D) or average branch number (E) but did decrease the summed branch length per cell (F) in both vehicle- and estradiol-treated animals (F). + HFD-vehicle < chow-vehicle, p < 0.05. *HFD-fed animals < chow-fed animals, p < 0.05.
Within the VMH, neither the number of Iba1-positive cells nor the average branch number were affected by diet or hormone treatment, F(1,25) = 0.04 – 3.11, p = 0.297 – 0.091 (Fig. 6D,E). There was a main effect of diet on average summed branch length, F(1,24) = 4.62, p < 0.05, η2 = 0.16. Consumption of the HFD decreased the average summed branch length in both vehicle- and estradiol-treated animals, p < 0.05, d = −0.59 (Fig. 6F).
3.3. Estradiol blocked the HFD-induced increase in microglia number in the NTS
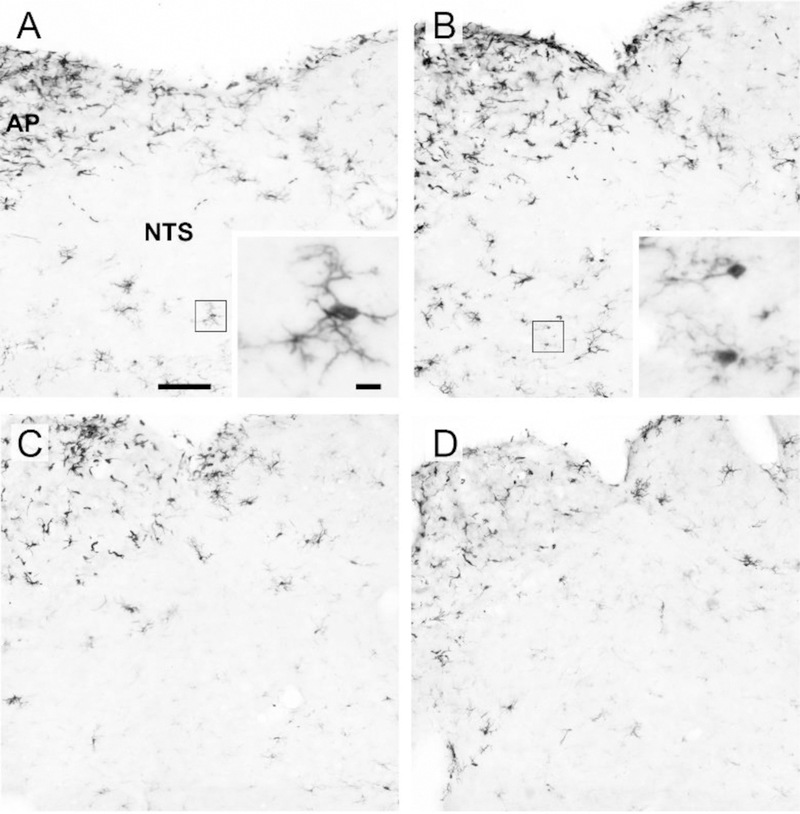
Representative images of Iba1-positive cells within the NTS for each treatment condition are shown in Fig. 7. The number of Iba1-positive cells was influenced by a diet x hormone interaction, F(1, 23) = 4.69, p < 0.05, η2 = 0.17. Consumption of the HFD increased the number of Iba1-positive cells in vehicle-treated animals, p < 0.05, d = 1.94, but had no effect in estradiol-treated animals (Fig. 8A). Both the average branch number and the average summed branch length were influenced by diet, F(1,23) = 14.32 and 9.51, respectively, p < 0.05, η2 = 0.38 and 0.29, respectively. Consumption of the HFD promoted a similar decrease in both morphological measures in vehicle- and estradiol-treated animals, p < 0.05, d = −1.44 and −1.17, for branch number and length, respectively (Fig. 8B,C).
Fig. 7.
Representative photomicrographs of iba1-positive cells in the NTS of (A) chow-fed/-vehicle-treated animals, (B) HFD-fed/vehicle-treated animals, (C) chow-fed/estradiol-treated animals, and (D) HFD-fed/estradiol-treated animals. Insets in A and B are of a representative cell depicting microglia morphology. Boxes in A and B indicate cell shown in the inset image. Scale bar of NTS images is 100μm. Scale bar of inset images is 10μm. Abbreviations: AP; area postrema, NTS; nucleus of the solitary tract.
Figure 8.

Estradiol blocked HFD-induced changes in microglia number, but not morphology, in the NTS. HFD increased iba1-positive cells in the NTS in vehicle-treated, but not estradiol-treated, animals (A). HFD decreased branch number and summed branch length per cell in the NTS in both vehicle- and estradiol-treated animals (B and C). +HFD-vehicle group > chow-vehicle group, p < 0.05. *HFD-fed animals < chow-fed animals, p < 0.05
3.4. Correlation between cumulative food intake/weight gain and Iba1 expression in HFD-fed animals.
The relationship between food intake/weight gain and Iba1 expression was examined in the two brain areas in which HFD consumption increased the number of Iba1-positive cells. These correlational analyses revealed no association between the amount of HFD consumed and the number of Iba1-positive cells in the ARC, r(15) = −0.001, p = 0.998, or NTS, r(13) = 0.301, p = 0.296 (data not shown). There was also no association between weight gain and the number of Iba1-postive cells in the ARC, r(13) = 0.102, p = 0.730, or NTS, r(11) = 0.538, p = 0.731 (data not shown).
4. Discussion
This study provides evidence that estradiol has a protective effect against the development of diet-induced microgliosis in multiple brain areas implicated in the neural control of food intake. Our findings extend previous studies in male rodents by showing that 4 days of HFD consumption promotes microgliosis in the ARC, VMH, LH and NTS of estradiol-deficient OVX animals. Our data also show that these diet-induced changes in microglial number and morphology were attenuated by estradiol treatment in a region-specific manner. Taken together, these findings suggest that sex-differences in the development of DIO may be mediated, at least in part, via estradiol’s ability to protect the brain against an initial HFD insult.
In the current study, we used a cyclic regimen of estradiol treatment that models the fluctuations in endogenous estradiol observed in ovarian-intact, cycling female rats (Asarian and Geary, 2002). This physiological regimen of estradiol treatment decreased food intake and weight gain, relative to vehicle-treated OVX animals. This finding is consistent with previous studies documenting estradiol’s anorexigenic effect in OVX animals fed a similar chow diet (Butera, 2010; Eckel, 2011) or a more palatable, energy-dense diet (Butera et al., 2010). In addition to affecting food intake and body weight, our regimen of estradiol treatment increased uterine weight, relative to vehicle-treated OVX animals, consistent with previous studies that have shown estrous-related increases in uterine weight in cycling animals (Davis et al., 2008; Hewitt et al., 2003). Taken together, these findings suggest that our assessment of the effect of estradiol on diet-induced changes in microglial expression is physiologically relevant and conducted at a time that would model estrus in cycling female rats.
Limited (4-day) access to a HFD promoted microglial accumulation in the ARC, as measured by an increase in the number of Iba1-positive cells in vehicle-treated OVX animals. Because activated microglia retract their processes and reduce their complexity (Streit et al., 1999), changes in microglia morphology can be used as one measure of microglial activation. However, it is worth noting that expression of cell surface proteins and cytokine release was not evaluated in the current study so conclusions regarding microglia activation or microglial-mediated neuroinflammation are somewhat limited. Here, we found that HFD consumption not only increased the number of microglia in the ARC but it also decreased both the number and length of microglial branches, implying an activated morphology, in vehicle-treated OVX animals. These findings are consistent with a previous report of microglial accumulation and reduced microglial branching in the ARC of male rats consuming a HFD (Thaler et al., 2012), and studies showing similar changes in microglia morphology in response to other stimuli that are known to activate microglia, such as lipopolysaccharide (LPS) treatment and traumatic brain injury (Streit et al., 1999). While HFD consumption promoted microgliosis in the ARC of vehicle-treated OVX animals, a similar effect was not observed in estradiol-treated OVX animals. This suggests that estradiol protects against diet-induced microglial accumulation and changes in microglia morphology that are consistent with an activated phenotype in this region. To the best of our knowledge, this is the first demonstration that estradiol can block HFD-induced microgliosis in the ARC. Our finding is consistent, however, with previous research showing inherent sex differences in neuroinflammation at the level of the microglia. For example, palmitic acid treatment has been shown to increase the expression of pro-inflammatory cytokines in male-, but not female-, derived microglia (Yanguas-Casás et al., 2018), and 12 weeks of HFD consumption increased microglial accumulation and the release of pro-inflammatory cytokines in the ARC of male, but not female, mice (Lainez et al., 2018). Our data extend these findings by showing that estradiol can modulate the microglial response to HFD.
We extend previous work on diet-induced hypothalamic inflammation by examining the number and morphology of microglia in hypothalamic areas beyond the ARC, including the LH and VMH, which are also involved in the neural control of food intake. While HFD consumption did not alter the number of microglia in the LH, it did decrease microglial branch number in vehicle-treated, but not estradiol-treated, animals. This suggests that estradiol prevents diet-induced changes in microglial morphology within the LH. While our study is the first to investigate HFD-induced microgliosis in the LH of male or female rats, a previous study reported an increase in TNFα in the LH of male rats consuming HFD for 16 weeks (De Souza et al., 2005). The LH plays an important role in controlling food intake as it receives input from the ARC (Betley et al., 2013), which is sensitive to homeostatic signals that affect appetite, and sends both excitatory and inhibitory projections to the ventral tegmental area (VTA), which regulates dopamine signaling and the motivation to consume food (Kempadoo et al., 2013; van Zessen et al., 2012). Thus, HFD-induced activation of microglia in the LH could modulate LH neuronal function and the hypothalamic circuitry controlling both the homeostatic and hedonic control of food intake in ways that could promote overeating and weight gain.
Similar to that observed in the LH, HFD consumption did not alter the number of microglia in the VMH. However, microglia branch length was decreased by HFD in vehicle-treated OVX animals but this effect was not attenuated by estradiol treatment. We believe this is the first demonstration of HFD-induced microgliosis within the VMH. Taken together, our findings show that HFD consumption promotes microglial activation in multiple hypothalamic areas whereas microglial accumulation was limited to the ARC. This likely reflects microglial migration from the LH and VMH since others have shown that HFD-induced microgliosis in the ARC is not due to newly proliferated cells (Gao et al., 2014). Given their migratory nature, an increase in the number of microglia in the ARC is most likely due to microglial accumulation driven by migration of activated microglia from the LH and VMH. The sensitivity of the ARC to the HFD could be due to higher levels of saturated fatty acids in this brain area, which lies adjacent to the median eminence, a circumventricular organ that may facilitate greater accumulation of free fatty acids in the ARC.
Given the complex neural circuits that control food intake, it is reasonable to hypothesize that extra-hypothalamic brain areas may also be affected by HFD. One region that may be particularly susceptible to diet-induced inflammation is the NTS. The NTS plays an important role in regulating energy balance and responds to peripheral metabolic signals and circulating hormones, including estradiol (Asarian and Geary, 2007; Blouet and Schwartz, 2012). Here, we found that HFD consumption promoted an increase in microglial number and a decrease in microglial branching and summed branched length in the NTS of vehicle-treated OVX animals. This extends a previous study in which chronic (21-day) HFD consumption increased the number of microglia within the NTS of male rats (Vaughn et al., 2017). Interestingly, estradiol treatment blocked the HFD-induced increase in microglial number in the NTS but the changes in microglia morphology, consistent with an activated phenotype, were similar in vehicle- and estradiol-treated animals. This suggests that estradiol’s anti-inflammatory effect in the NTS is specific to microglial accumulation under the experimental conditions used here. HFD has also been shown to impair vagal innervation of the NTS (Sen et al., 2017) and, given the role the NTS plays in regulating energy homeostasis and its connections to the hypothalamus (Schneeberger et al., 2014), NTS microgliosis has the potential to disrupt the neural circuits controlling food intake.
This differential effect on microglial morphology between the hypothalamus and NTS could be due to differential estrogen receptor expression between hypothalamic and hindbrain microglia, although studies investigating the regional specificity of microglial estrogen receptor expression are lacking. However, like neurons, there is precedent for microglia to have region-specific functions and reactions to similar stimuli (Spencer et al., 2019) and these differences could be estrogen receptor-dependent.
Correlational analyses were performed to examine the relationship between food intake/weight gain and changes in Iba1 expression in the two brain areas in which HFD consumption increased the number of Iba1-positive cells (ARC and NTS). These analyses revealed that Iba1 expression was not correlated with either cumulative HFD intake or weight gain across the 4 days of HFD exposure. To the best of our knowledge, this is the first investigation of the relationship between HFD consumption and changes in Iba1 expression. The lack of association between these two variables suggests that the rapid, HFD-induced increase in Iba1 expression observed here is mediated by the accumulation of free fatty acids in the ARC and NTS, rather than total caloric intake. While a positive correlation between weight gain and Iba1 expression in the ARC was reported in male rats following 8 weeks of HFD exposure (Thaler et al., 2012), we found no similar relationship between HFD-induced weight gain and ARC/NTS Iba1 expression in our study. These discrepant findings, which are likely related to differences in the duration of HFD exposure, suggest that more than 4 days of HFD intake are required before the amount of HFD-induced weight gain is predictive of increases in microglial accumulation.
The mechanism by which estradiol modulates diet-induced microgliosis is unclear. Microglia express multiple estrogen receptor subtypes, including ERα, ERβ, and G protein-coupled estrogen receptor-1 (GPER-1), all of which have been implicated in mediating estradiol’s anti-inflammatory effect in response to LPS stimulation (Baker et al., 2004; Vegeto et al., 2003; Zhao et al., 2016). As such, estradiol may be acting directly on microglia to reduce microglial migration and the morphological changes in response to the HFD in our study. Estradiol has a well-documented anti-inflammatory effect in the brain, and has been shown to rapidly attenuate microgliosis induced by other types of non-feeding related brain insult such as ischemic stroke (Vegeto et al., 2008; Zhao et al., 2016). Here, we demonstrate that estradiol rapidly alters the microglial response to HFD in a similar manner to other types of central insults that promote microglia accumulation and activation. Moreover, estrogen receptor activation inhibits signaling pathways that promote the synthesis and release of pro-inflammatory cytokines, including the c-Jun N-terminal kinase (JNK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway in microglia (Bruce-Keller et al., 2000; Ghisletti et al., 2005). These studies suggest that estradiol acts directly on microglia via canonical inflammatory pathways to attenuate their pro-inflammatory effects.
Given estradiol’s anorexigenic effect (Eckel, 2011), one must also consider whether estradiol acted indirectly to reduce diet-induced microgliosis in the current study. Because estradiol treatment reduced caloric intake and body weight independent of diet condition, it is possible that estradiol may have decreased HFD-induced microgliosis indirectly by decreasing food intake, and thus the central accumulation of fatty acids in HFD-fed animals. While this is plausible, we believe it is unlikely. A careful examination of the main effect of estradiol on food intake reveals that it was primarily driven by a decrease in caloric intake in the chow-fed animals with negligent differences in caloric intake in the HFD-fed animals (Fig. 2A). We also ran a correlational analysis between total HFD consumption and the number of Iba1-positive cells in the ARC and NTS and found no significant association between these two variables. The lack of a positive association between HFD intake and Iba1 expression suggests that estradiol’s ability to block HFD-induced microgliosis in the ARC and NTS is not secondary to reduced consumption of the HFD.
Previous work has investigated the relative contribution of caloric overconsumption versus macronutrient composition in the development of diet-induced hypothalamic inflammation. For example, mice receiving saturated fatty acids from milk fat via enteric gavage had an influx of palmitic acid in the brain within 1 h, and increased hypothalamic levels of inflammatory markers and microgliosis in the mediobasal hypothalamus after 3 days (Valdearcos et al., 2014). Because these effects were specific to saturated fatty acid intake, as caloric load was held constant across groups, this study suggests that it is the excess dietary saturated fatty acids, rather than increased caloric intake or weight gain, that promotes a state of hypothalamic inflammation. Thus, it appears that the brain’s rapid pro-inflammatory response to HFD is predominantly mediated by diet composition (i.e., accumulation of saturated fatty acids in the brain) and not by increases in caloric intake or adiposity. These findings provide further support for the notion that estradiol is acting directly to attenuate HFD-induced microgliosis in the ARC and NTS, rather than indirectly through reductions in caloric intake or weight gain.
Estradiol also acts directly on neurons to reduce inflammatory signaling (Morselli et al., 2014). Thus, estradiol’s attenuation of diet-induced microgliosis in the current study may not be mediated solely at the level of the microglia. Given that hypothalamic neurons also express TLRs and can directly sense free fatty acids via receptor binding and/or intracellular metabolism (Tran et al., 2016), neurons may initiate an inflammatory response by signaling to neighboring microglia to become activated, which, in turn, further perpetuates the inflammatory response (Masson et al., 2015; Tang et al., 2007; Tran et al., 2016). Since hypothalamic and NTS neurons express multiple estrogen receptor subtypes (Rainbow et al., 1982; Thammacharoen et al., 2008), one cannot rule out the possibility of estradiol acting on neurons and interfering with neuronal-glial crosstalk to reduce inflammation.
We have shown, for the first time, that limited (4-day) exposure to a HFD promotes microgliosis in the ARC, LH, VMH, and NTS of female rats, and that estradiol can exert a protective effect against HFD insult within both the hypothalamus and NTS. Estradiol’s attenuation of microglial activity in these regions could potentially protect females against HFD insults to anorexigenic neurons (Thaler et al., 2012) and thus promote less weight gain in DIO models. These findings have significant implications regarding sex differences in the development of obesity and the neuroimmune response in disease. Future studies are needed to identify the estrogen receptor subtype(s) that mediate this protective effect of estradiol against HFD-induced microgliosis, as well as the underlying cellular mechanism.
Highlights.
High fat diet promotes microgliosis in the hypothalamus and hindbrain of female rats
Diet-induced microgliosis is most evident in the ARC and NTS
Estradiol attenuates diet-induced microglial accumulation in the ARC and NTS
Estradiol attenuates diet-induced changes in microglial morphology in the ARC and LH
Acknowledgements
The authors would like to acknowledge Dr. Frank Johnson for his expertise and technical assistance in imaging brain tissue.
Funding
This study was supported by an FSU Planning grant awarded to LAE. MJB was supported by a T32 training grant funded by the National Institute of Mental Health (MH093311).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
None.
References
- Asarian L, Geary N, 2007. Estradiol Enhances Cholecystokinin-Dependent Lipid-Induced Satiation and Activates Estrogen Receptor-α-Expressing Cells in the Nucleus Tractus Solitarius of Ovariectomized Rats. Endocrinology 148, 5656–5666. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N, 2002. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav 42, 461–471. [DOI] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ, 2004. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor β. Endocrinology 145, 5021–5032. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZFH, Ritola KD, Sternson SM, 2013. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ, 2012. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 16, 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP, 2000. Antiinflammatory effects of estrogen on microglial activation. Endocrinology 141, 3646–3656. [DOI] [PubMed] [Google Scholar]
- Butera PC, 2010. Estradiol and the control of food intake. Physiol. Behav 99, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Wojcik DM, Clough SJ, 2010. Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physiol. Behav 99, 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MJ, Eckel LA, 2018. Eating as a motivated behavior: modulatory effect of high fat diets on energy homeostasis, reward processing and neuroinflammation. Integr. Zool [DOI] [PMC free article] [PubMed]
- Chowen JA, Argente-Arizón P, Freire-Regatillo A, Argente J, 2017. Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Front. Neuroendocrinol 48, 3–12. [DOI] [PubMed] [Google Scholar]
- Davis AM, Mao J, Naz B, Kohl JA, Rosenfeld CS, 2008. Comparative effects of estradiol, methyl-piperidino-pyrazole, raloxifene, and ICI 182 780 on gene expression in the murine uterus. J. Mol. Endocrinol 41, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJA, Velloso LA, 2005. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199. [DOI] [PubMed] [Google Scholar]
- Dorfman MD, Krull JE, Douglass JD, Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT, Matsen ME, Morton GJ, Thaler JP, 2017. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun 8, 14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA, 2011. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 104, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Ottaway N, Schriever SC, Legutko B, García-Cáceres C, de la Fuente E, Mergen C, Bour S, Thaler JP, Seeley RJ, Filosa J, Stern JE, Perez-Tilve D, Schwartz MW, Tschöp MH, Yi C-X, 2014. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, Vegeto E, 2005. 17 -Estradiol Inhibits Inflammatory Gene Expression by Controlling NF- B Intracellular Localization. Mol. Cell. Biol 25, 2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot-Legris O, Muccioli GG, 2017. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci 40, 237–253. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS, 2003. Estrogen Receptor-Dependent Genomic Responses in the Uterus Mirror the Biphasic Physiological Response to Estrogen. Mol. Endocrinol 17, 2070–2083. [DOI] [PubMed] [Google Scholar]
- Hong J, Stubbins RE, Smith RR, Harvey AE, Núñez NP, 2009. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH, 2012. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res 53, 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S, 1998. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol. Brain Res 57, 1–9. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger G-M, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A, 2013. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J. Neurosci 33, 7618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT, 2012. The effects of puberty on genetic risk for disordered eating: Evidence for a sex difference. Psychol. Med 42, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainez NM, Jonak CR, Nair MG, Ethell IM, Wilson EH, Carson MJ, Coss D, 2018. Diet-Induced Obesity Elicits Macrophage Infiltration and Reduction in Spine Density in the Hypothalami of Male but Not Female Mice. Front. Immunol 9, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake A, Townshend T, 2006. Obesogenic environments: exploring the built and food environments. J. R. Soc. Promot. Health 126, 262–7. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Woods SC, 2012. Overview of animal models of obesity. Curr. Protoc. Pharmacol 5.61.1–5.61.18. [DOI] [PMC free article] [PubMed]
- Malnick SDH, Knobler H, 2006. The medical complications of obesity. QJM 9, 565–579. [DOI] [PubMed] [Google Scholar]
- Masson GS, Nair AR, Dange RB, Silva-Soares PP, Michelini LC, Francis J, 2015. Toll-like receptor 4 promotes autonomic dysfunction, inflammation and microglia activation in the hypothalamic paraventricular nucleus: Role of endoplasmic reticulum stress. PLoS One 10, e0122850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, Naftolin F, 1999. Estrogen and microglia: A regulatory system that affects the brain. J. Neurobiol 40, 484–496. [DOI] [PubMed] [Google Scholar]
- Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi C-X, Hahner L, Palmer BF, Tschöp MH, Clegg DJ, 2014. Hypothalamic PGC-1α Protects Against High-Fat Diet Exposure by Regulating ERα. Cell Rep 9, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD, 2008. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Metab 296, E1003–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, MacLusky NJ, McEwen BS, 1982. Estradiol receptor levels in rat hypothalamic and limbic nuclei. J. Neurosci 2, 1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Gomis R, Claret M, 2014. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol 220, T25–T46. [DOI] [PubMed] [Google Scholar]
- Sen T, Cawthon CR, Ihde BT, Hajnal A, DiLorenzo PM, de La Serre CB, Czaja K, 2017. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav 173, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, 2015. Network of hypothalamic neurons that control appetite. BMB Rep 48, 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Basri B, Sominsky L, Soch A, Ayala MT, Reineck P, Gibson BC, Barrientos RM, 2019. High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner. Neurobiol. Aging 74, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA, 1999. Reactive microgliosis. Prog. Neurobiol 57, 563–581. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Wise RA, 2016. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci 19, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S-C, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP, 2007. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci 104, 13798–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW, 2012. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest 122, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammacharoen S, Lutz TA, Geary N, Asarian L, 2008. Hindbrain Administration of Estradiol Inhibits Feeding and Activates Estrogen Receptor-α-Expressing Cells in the Nucleus Tractus Solitarius of Ovariectomized Rats. Endocrinology 149, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST, 2018. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DQ, Tse EK, Kim MH, Belsham DD, 2016. Diet-induced cellular neuroinflammation in the hypothalamus: Mechanistic insights from investigation of neurons and microglia. Mol. Cell. Endocrinol 438, 18–26. [DOI] [PubMed] [Google Scholar]
- Underwood EL, Thompson LT, 2016. A High-Fat Diet Causes Impairment in Hippocampal Memory and Sex-Dependent Alterations in Peripheral Metabolism. Neural Plast 2016, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK, 2014. Microglia Dictate the Impact of Saturated Fat Consumption on Hypothalamic Inflammation and Neuronal Function. Cell Rep 9, 2124–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Xu AW, Koliwad SK, 2015. Hypothalamic Inflammation in the Control of Metabolic Function. Annu. Rev. Physiol 77, 131–160. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD, 2012. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AC, Cooper EM, DiLorenzo PM, O’Loughlin LJ, Konkel ME, Peters JH, Hajnal A, Sen T, Lee SH, de La Serre CB, Czaja K, 2017. Energy-dense diet triggers changes in gut microbiota, reorganization of gut‑brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. (Wars) 77, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A, 2003. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci 100, 9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Benedusi V, Maggi A, 2008. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrinol [DOI] [PMC free article] [PubMed]
- Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL, 1991. Weight Gain at the Time of Menopause. Arch. Intern. Med 151, 97. [PubMed] [Google Scholar]
- Yanguas-Casás N, Crespo-Castrillo A, de Ceballos ML, Chowen JA, Azcoitia I, Arevalo MA, Garcia-Segura LM, 2018. Sex differences in the phagocytic and migratory activity of microglia and their impairment by palmitic acid. Glia 66, 522–537. [DOI] [PubMed] [Google Scholar]
- Young K, Morrison H, 2018. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp e57648. [DOI] [PMC free article] [PubMed]
- Zhao TZ, Ding Q, Hu J, He SM, Shi F, Ma LT, 2016. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]