Abstract
Optical coherence tomography angiography (OCTA) is a relatively new, non-invasive, dye-free imaging modality that provides a qualitative and quantitative assessment of the vasculature in the retina and optic nerve head. OCTA also enables visualization of the choriocapillaris, but only in areas of parapapillary atrophy. With OCTA, movement of red blood cells is used as a contrast to delineate blood vessels from static tissues. The features seen with OCTA in eyes with glaucoma are reduction in the superficial vessel density in the peripapillary and macular areas, and complete loss of choriocapillaris in localized regions of parapapillary atrophy (called deep-layer microvascular dropout). These OCTA changes correlate well topographically with the functional changes seen on visual field examination and structural changes seen on OCT (i.e., parapapillary retinal nerve fiber layer changes and inner retinal layer thickness changes at macula). The OCTA measurements also have acceptable test-retest variability and well differentiate glaucomatous from normal eyes. OCTA measurements can be affected by various subject-related, eye-related and disease-related factors. Vessel density reduction on OCTA reaches a base level (floor) at a more advanced disease stage than the structural changes on OCT and therefore has the potential to monitor progression in eyes with advanced glaucomatous damage. OCTA also adds information about glaucoma patients at risk of faster progression. OCTA, therefore, complements visual field and OCT examinations to diagnose glaucoma, detect progression and assess risk of progression.
Glaucoma is a chronic optic neuropathy characterized by progressive loss of retinal ganglion cells (RGCs).1 Although the exact pathogenesis of glaucoma is not fully understood, two main theories have been proposed to explain the death of RGCs in glaucoma.2 The “mechanical theory” postulates RGC death to be a consequence of raised intraocular pressure (IOP). It proposes that increased IOP causes a blockade of axoplasmic flow within the RGCs at the lamina cribrosa. As a result, there is a failure to deliver target-derived neurotrophic growth factors and this leads to RGC death.3 IOP is a leading risk factor for glaucoma and multiple studies have reported IOP also to be a major causal factor.1, 4–10 However, it is well accepted that the mechanical theory alone, fails to explain the entire pathogenic mechanism of glaucoma.11, 12 The “vascular theory”, an alternative theory to explain glaucoma pathogenesis, postulates RGC death to be a consequence of reduced blood supply.13
Measuring ocular blood flow in humans
Although numerous technologies, including fluorescein angiography (FA),14–18 indocyanine green angiography (ICGA),19 scanning laser ophthalmoscopy,20 laser Doppler flowmetry,21 and laser speckle flowgraphy,22 have been used to document the impairment of ocular blood flow and alterations of the retinal microvasculature in glaucoma, they have had diverse limitations and only minimal success in elucidating the role of vascular dysregulation in glaucoma.23 Moreover, many of these technologies have been unable to obtain accurate, reproducible and quantitative measurements.24
The search for a simple, non-invasive, reproducible method of evaluating the ocular blood flow led to the development of optical coherence tomography (OCT) angiography.
OCT angiography
OCT angiography (OCTA) is a non-invasive, dye-free technology that can image large vessels as well as microvasculature of the retina, optic nerve head (ONH) and some part of the choriocapillaris by performing multiple OCT scans of the same region. Moving particles, such as red blood cells in blood vessels, resulting in high variance of the OCT signal between scans and this variation in OCT signal is used to identify blood vessels. Several algorithms have been developed to interpret the variance in the OCT signals and to delineate the blood vessels, and these have been incorporated in various commercially available OCTA devices. The split spectrum amplitude decorrelation angiography (SSADA, Angiovue, RTVue-XR SD-OCT, Optovue Inc., Fremont, CA) uses the variation in the intensity of the OCT signal to identify blood vessels.25, 26 The full spectrum amplitude decorrelation angiography (FS-ADA, Spectralis OCT2 Module, Heidelberg Engineering, Heidelberg, Germany) uses the variation in the entire intensity spectrum of the OCT signal to identify blood vessels.27 Similarly, OCTA ratio analysis (OCTARA, DRI OCT Triton, Topcon, Japan) is another algorithm that uses the full spectrum of the OCT signal for blood vessel delineation, thereby preserving the axial resolution.28 The optical microangiography (OMAG), another OCTA algorithm (Angioplex, Cirrus HD-OCT, Carl Zeiss Meditec Inc., Dublin, CA), uses the variation in intensity as well as the phase difference of the OCT signals for vessel delineation.29–31 In addition to tracing the blood vessels, these algorithms are also designed to reduce motion artifacts and pulsatile bulk motion noise.26, 32
The current generation of OCTA can scan the optic disc region and the macula. The optic disc OCTA scan is performed using volumetric scans generally covering an area of 4.5 × 4.5 mm centered around the optic disc. The optic disc scan is divided into several slabs for further analysis. Segmentation of these slabs differs among commercially available OCTA devices mentioned above. Two slabs of the optic disc scan that are found to be useful in glaucoma are the radial peripapillary capillary (RPC) slab that delineates the vessels within the retinal nerve fiber layer (RNFL) layer and the choroidal slab that delineates the choroidal vessels in the parapapillary region. The RPC slab extends from the internal limiting membrane (ILM) to the posterior boundary of the RNFL (Figure 1a). Reduction in vasculature is reported to be more pronounced on the RPC slab compared to the deep retinal slabs in glaucomatous eyes.33 The “choroidal” slab is used to assess the deep retinal and choroidal vasculature. The choroidal slab on RTVue-XR SD-OCT extends from 75 μm below the retinal pigment epithelium (RPE, Figure 1b).34 The choroidal slab on Cirrus HDOCT extends from 64 μm below the RPE-fit line to 115 μm posterior (having a thickness of 51 μm).31
Figure 1.
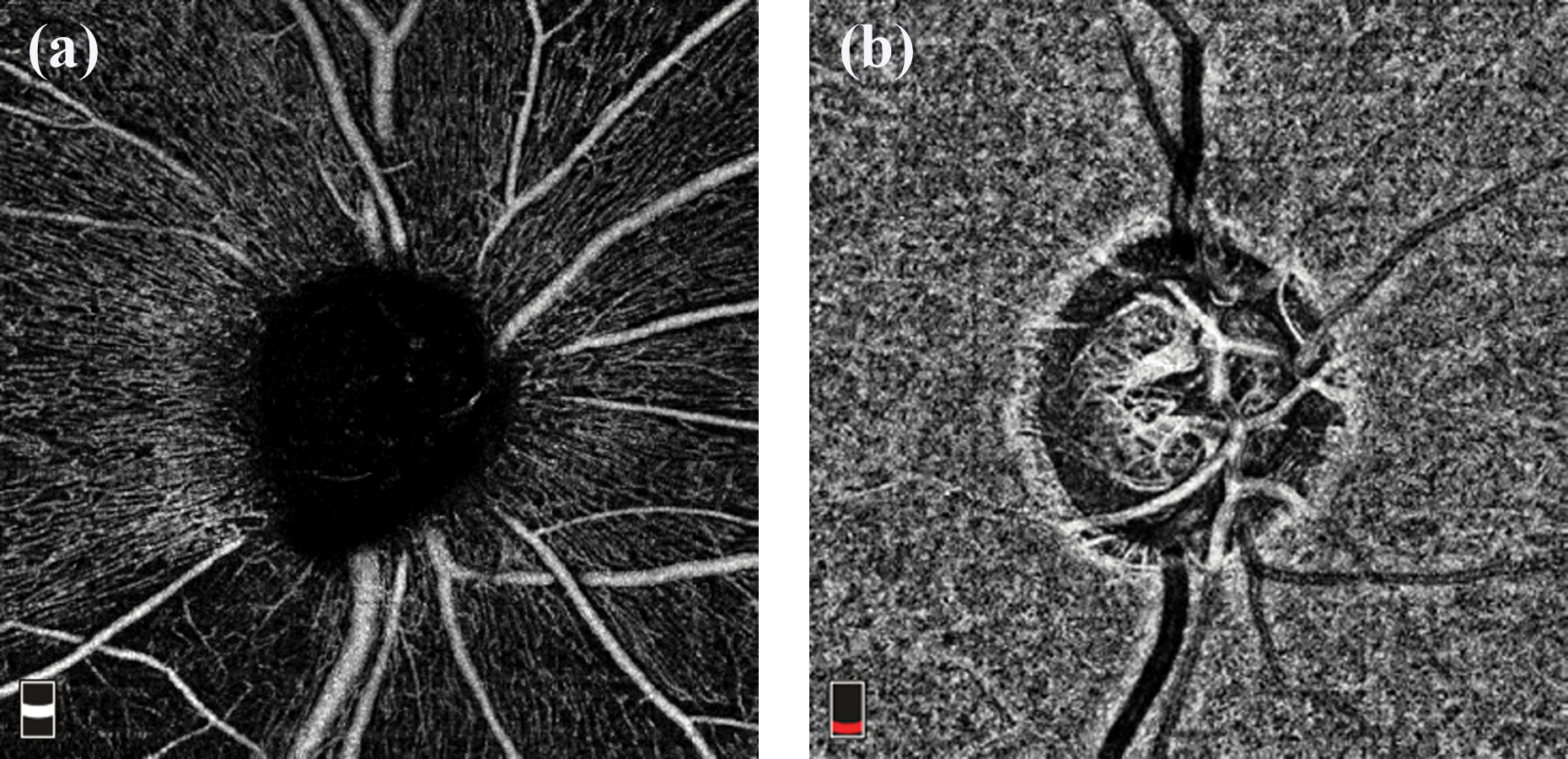
Angiography slabs of the optic nerve head scan obtained using spectral domain optical coherence tomography showing the radial peripapillary capillary, RPC (a) and choroid (b) slabs.
The macular OCTA scan is performed using a volumetric scan covering either a 3 × 3 mm (Figure 2a) or a 6 × 6 mm (Figure 2b) area of the macula. A comparative study has shown that the 6 × 6 mm scans better detect glaucomatous changes compared to the 3 × 3 mm scans (as in the example shown in Figure 3).35 The macular region is also divided into slabs for further analysis. Of these, superficial retinal slab is the one found to be useful in glaucoma. The superficial retinal slab on RTVue-XR SD-OCT extends from 3 μm below the ILM to 15 μm below the inner plexiform layer (IPL, Figure 2).34 The same slab on Cirrus HDOCT extends from ILM to IPL.31 Reduction in vasculature is reported to be more pronounced on the superficial retinal slab compared to the deep retinal slabs in glaucomatous eyes.36 Another important point to note is that segmentation errors are possible in the setting of normal anatomic variation or pathologic changes in the retinal layers due to the fact that fixed boundaries are assigned for the slabs; hence each OCTA B-scan should be reviewed prior to interpretation of the quantitative analysis.
Figure 2.
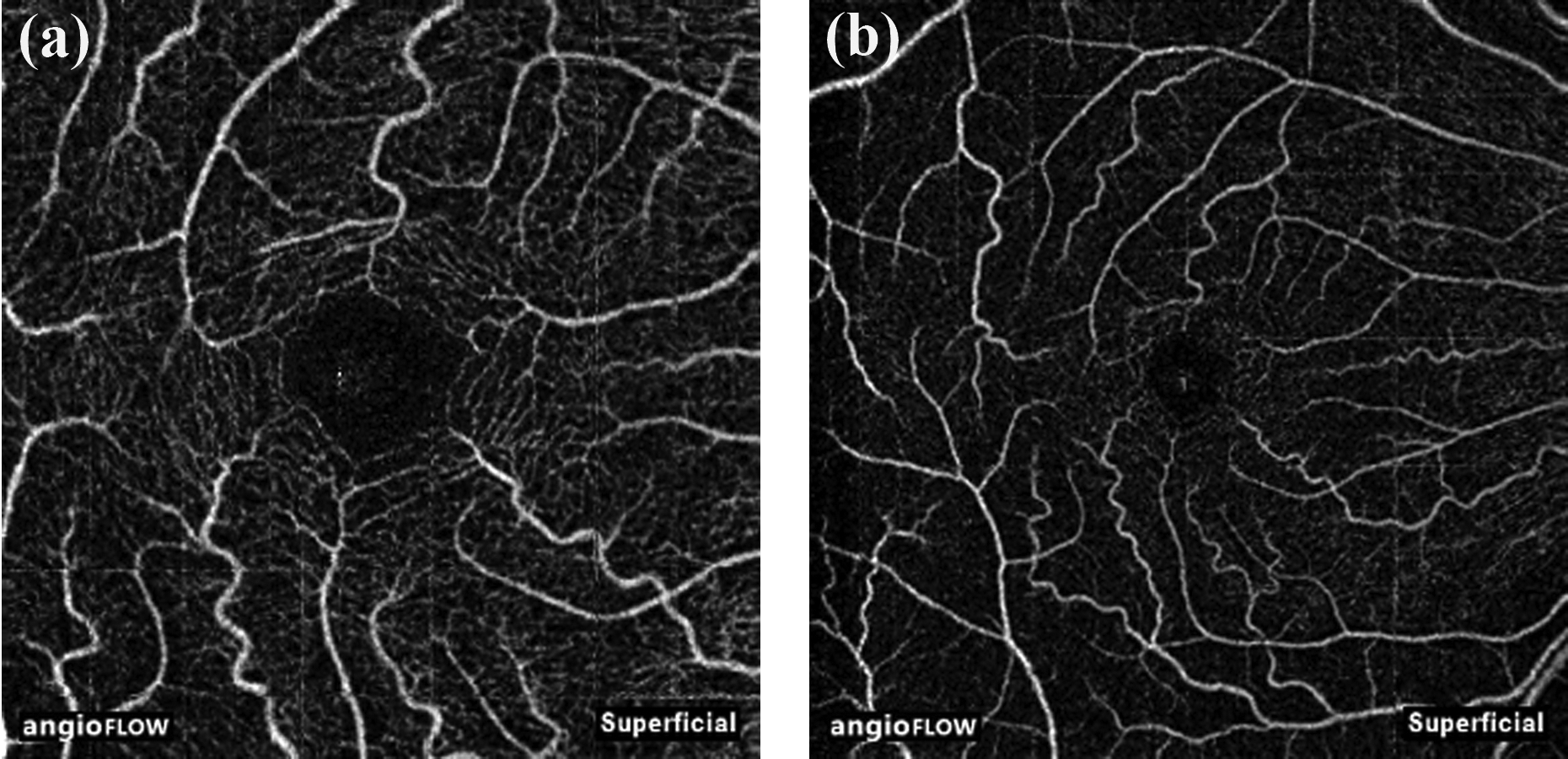
Superficial angiography slabs of the 3×3 (a) and 6×6 (b) mm macular scan obtained using spectral domain optical coherence tomography.
Figure 3.

OCTA features of a glaucomatous eye with mild disease. Optic disc photograph (a) shows inferior neuroretinal rim notch with a correlating superior nasal defect on the visual fields (b) and inferior retinal nerve fiber layer thinning on OCT (c). Composite peripapillary (4.5×4.5 mm scan) and macular (6×6 mm scan) OCTA scan shows reduced vessel density in the inferior region as indicated on the angiography (d) and heat map (e). Vessel density reduction on the macular OCTA scan is less obvious in the inner 3 mm region compared to the outer 3–6 mm region.
OCTA quantifies the ocular circulation using two parameters: flow index and vessel density. Flow index is defined as the average decorrelation values in the measured area. Vessel density, the most widely used OCTA parameter, is defined as the percentage area occupied by vessels in the measured area.25 The method of quantification of OCTA vessel density varied among different studies, especially until automated software were available; this might explain some discrepancies among investigations.37 Also, measurements from different OCTA algorithms (described above) in healthy eyes varied significantly, suggesting that the OCTA measurements from different algorithms cannot be used interchangeably.38
OCTA and POAG
(i). OCTA features in POAG
Initial studies with OCTA were performed in eyes with POAG and they showed reduced flow index and vessel density within the ONH (nerve head slab) and in the peripapillary region (RPC slab) of eyes with POAG compared to controls (Figure 3).25, 39–41 Subsequently, it was shown that OCTA vessel densities measured in the superficial macular regions were also reduced in eyes with glaucoma compared to control eyes (Figure 3).42, 43 Vessel densities showed a more pronounced decrease as the severity of glaucoma increased.36, 40, 44–52 More recently, deep-layer microvasculature dropout (MvD, Figure 4), defined as the complete loss of choriocapillaris in localized regions of parapapillary atrophy (PPA), has been observed on the choroidal slab in POAG eyes.53, 54 MvD has been shown to be a true perfusion defect using indocyanine green angiography.55
Figure 4.
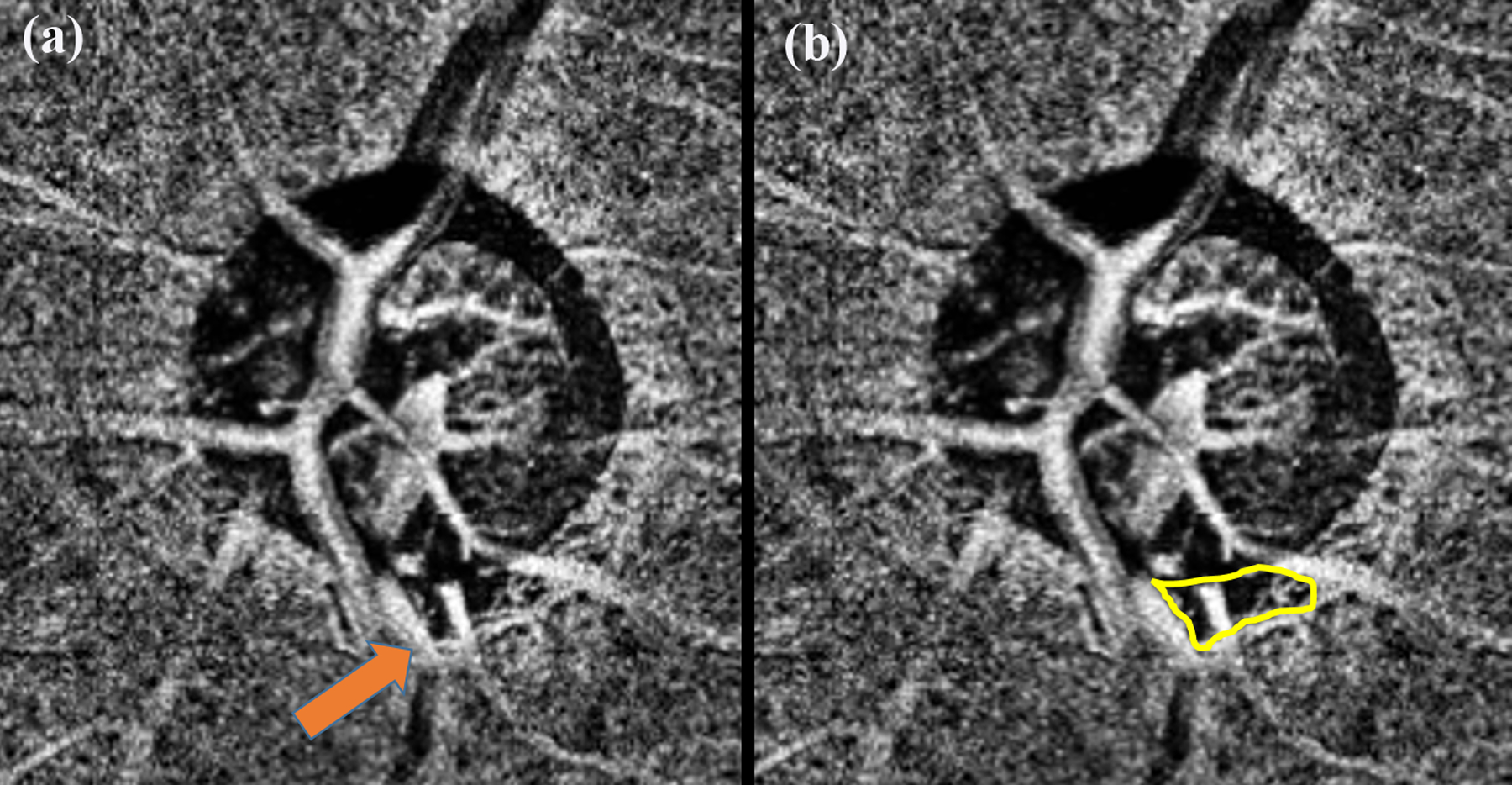
Choroidal OCTA slab of a glaucomatous eye showing the presence of deep-layer microvasculature dropout (MvD) in the inferior region (a). Arrow points to the MvD. Yellow line marks out the boundary of the MvD (b).
(ii). Repeatability and Reproducibility
Intra-visit repeatability and inter-visit reproducibility of OCTA measurements in the peripapillary and macular regions have been investigated by several groups. One study found that intra-visit coefficient of variation (CV) of OCTA peripapillary vessel density measurements (global and sectoral) ranged from 2.5% to 6.6% and that of superficial macular vessel density from 3.4% to 5.6%.56 Two other studies also showed similar CV for both intra-visit and inter-visit OCTA measurements.57, 58 Glaucoma eyes showed worse inter-visit repeatability than healthy eyes.57 In comparison, CV of the OCT measurements (RNFL and ganglion cell complex [GCC] thickness) were found to be less than that of OCTA measurements implying that the OCTA measurements were less reproducible than the OCT measurements.57 The intra-visit and inter-visit CVs of average RNFL and GCC thickness, for example, were around 1.5%, whereas that of average peripapillary and parafoveal vessel density were close to 4.0% (P< 0.001).57 This is an important consideration when using OCTA measurements to detect glaucoma progression.
(iii). Relationship of OCTA measurements with VF and OCT measurements
The relationship of the OCTA measurements to visual field (VF) and OCT measurements also has been evaluated using linear, quadratic and polynomial fits.59–69 This relationship was found to be good and non-linear fits described this relation better than linear fits.60, 62 Additionally, in glaucoma eyes with high myopia70 and in eyes with advanced glaucoma,71, 72 the association of VF parameters seemed to be stronger with OCTA compared to OCT thickness measurements. Furthermore, a recent study also found that the measurement floor, the value beyond which further change in the measurement becomes undetectable, was at a lower level for OCTA compared to OCT measurements.73 In fact, no detectable measurement floor was found for macular vessel density measurements and this showed that OCTA is a promising tool for monitoring progression in advanced disease.73
Studies have also reported a topographic association between the location of MvD and structural defects (RNFL thinning and lamina cribrosa defects) as well as functional defects (VF loss) in POAG eyes.53, 74–76
It is important to determine the temporal relationship of vessel density reduction on OCTA with respect to RNFL thinning and visual field defects. This would help develop strategies to detect the disease in the earliest stages. However, OCTA is a relatively new technology with only a few, small, longitudinal studies. As an alternative approach, studies have been performed in eyes with established perimetric glaucoma, whose VF defects are limited to one hemifield and the OCTA changes in regions corresponding to the intact hemifield have been examined. These studies have found reduced peripapillary vessel density and RNFL thickness in the hemiretina corresponding to the perimetrically intact hemifield compared to that of healthy eyes.77–79 One of these studies also found that the temporal sector of the perimetrically intact hemifield (corresponding to the region of papillomacular bundles) showed reduced vessel density in the presence of normal RNFL thickness.78 This suggested that there may be regional variations in the alterations of RNFL thickness and vessel density measurements, and OCTA changes may precede RNFL changes in some sectors. Another recent study reported that the OAG eyes with VF defects limited to one hemifield and also having a MvD showed significantly lower RNFL and GCIPL thickness in the hemiretina corresponding to the perimetrically intact hemifield than those without a MvD.80
(iv). Comparing OCTA with OCT measurements in diagnosing POAG
The diagnostic abilities of OCTA measurements (peripapillary and superficial macular vessel densities) have been compared with corresponding OCT measurements (RNFL and GCC thickness) in glaucoma.
A few studies comparing the diagnostic abilities (area under the receiver operating characteristic curves [AUC] and sensitivities at high specificities) of peripapillary vessel densities and RNFL thickness in POAG have found them too similar.40, 41, 61, 81, 82 Depending on the severity of glaucoma patients included in these studies, the AUCs of both peripapillary vessel density and RNFL thickness have ranged between 0.85 to 0.95. A few other studies have reported a better diagnostic ability of RNFL thickness compared to peripapillary vessel density in POAG.43, 83 In spite of the AUCs being similar, a few studies showed that the sensitivity to detect glaucoma in early stages of severity was better with RNFL thickness compared to peripapillary vessel density measurements.43, 84 Similar to the peripapillary measurements, diagnostic ability of superficial macular vessel density was found to be similar to that of macular GCC thickness by a few studies,61, 85 while the same was found to be inferior to GCC thickness in other studies.35, 43, 86, 87 One of these studies found that despite similar AUCs, more than one-third of early glaucoma eyes showed greater loss of vessel density than GCC thickness.85
(v). Factors associated with OCTA parameters
Factors associated with OCTA measurements can be classified into disease-related, subject-related, and eye-related factors. Clinicians evaluating the OCTA scans quantitatively, therefore, should consider all these factors during interpretation.
Disease-related Factors:
Variability in OCTA measurements has been reported in different subgroups of patients with POAG. It should be noted that that POAG eyes are not homogenous in terms of vascular density even at similar disease severities and the characteristics of glaucomatous eye might influence the OCTA measurements.
1. Glaucoma severity:
Vessel densities showed a more pronounced decrease as the severity of glaucoma increased.36, 40, 44–52 Prevalence and the size of MvD increased as the severity of disease increased.53, 74, 75, 88 MvD was also more commonly present when the VF defects were in the parafoveal region.88–91
2. Lamina cribrosa (LC) defect:
In eyes with similar severity of VF loss, the reduction in OCTA circumpapillary vessel density was greater in those with than those without focal LC defects. Further, reduction of vessel density was spatially correlated with the location of the LC defect.92 However, macular vessel density was not significantly different in severity-matched glaucoma eyes with and without focal LC defects.93 The presence of MvD is also reported to be strongly associated with LC defects.53, 94
3. Disc hemorrhage:
One cross-sectional study found that most of the vessel densities and structural measurements were similar (P>0.05) in POAG eyes with and without DH.95 In contrast, another study found that inferotemporal peripapillary vessel density was significantly lower in POAG eyes with DH compared to POAG eyes without DH. MvD, on the other hand, was found to be significantly associated with DH. Moreover, the prevalence of MvD was higher in POAG eyes with DH compared to POAG eyes without DH.88, 96
Subject-related Factors:
1. Demographics:
Most studies have reported older age to be associated with lower macular and peripapillary vessel density measurements.23, 36, 68, 97–100 In studies with a mixture of patients of African and European descent, the vessel density measurements were found to be lower in glaucomatous eyes of patients with European compared to African descent.99, 101
2. Diurnal change:
Studies have reported that the diurnal changes in OCTA vessel densities were small and clinically insignificant.102, 103
3. Exercise:
In a small cohort of 13 healthy people before and after exercise, it was found that increased physical activity induced significant reduction in OCTA vessel densities.104
4. Systemic conditions:
In hypertensive individuals (with no retinopathy), the peripapillary vessel densities were lower, while the macular vessel densities were higher. The vessel densities also were lower in subjects with diabetes (with no retinopathy)105 and the reduction in vessel density was associated with the duration of diabetes.100Another study evaluating the diurnal changes in OCTA measurements found a negative correlation of superficial macular and peripapillary vessel densities with heart rate, and a positive correlation of superficial vessel density with mean arterial pressure.103
4. Medication:
Effect of medications on OCTA measurements has not been studied well. Topical β-blocker administration has been reported to lead to 3.3% lower superficial macular vessel density compared to prostaglandin analogues, alpha agonists and carbonic anhydrase inhibitors, after adjusting for macular GCC thickness.36
Eye-related Factors:
1. Myopia:
OCTA measurements in the peripapillary region have been found to be significantly lesser in high myopic eyes compared to emmetropic eyes.106 Further, in myopia without glaucoma, peripapillary vessel density was lower than in normal eyes, and in myopic glaucoma, it was even more reduced.107 However, this may be partly related to image magnification in high myopic eyes.108
2. Disc area:
In a cross-sectional study, optic disc size was not found to affect the OCTA peripapillary measurements.105
3. Signal strength:
Several studies have reported that vessel densities were lesser in OCTA scans with lower signal strength.23, 56, 102, 105, 109 It is possible that the software does not differentiate between the static structures and blood vessels efficiently at low signal strengths.
4. Effect of IOP:
The potential relationship between IOP (or IOP reduction/increase) and ocular perfusion has been an important question in glaucoma management. Effect of IOP on OCTA vessel densities are inconclusive. While a few studies showed a significant increase in vessel densities after IOP reduction (either medically or surgically),110, 111 others found no change in vessel densities after IOP reduction.112, 113
(vi). Glaucoma Progression and its Risk Assessment using OCTA
As OCTA is a recently developed technique, there are no long-term studies evaluating its ability to detect progression. However, a few cases reports98, 99 and case series23, 114 have shown that OCTA is capable of detecting progressive decrease in superficial vessel densities in glaucomatous eyes even when monitored over short periods of time. It is important to note that vessel density is more variable than RNFL thickness and may reflect IOP changes, status of systemic perfusion, glaucomatous vascular dysregulation, retinal oxygenation, and hypercapnia at the time of measurements.97, 102, 104, 105, 110 A recent study by Kim et al also showed that increase in MvD area could be detected using serial OCTA scans.115 Future studies are needed to compare the progression detection ability of OCTA parameters with VF and OCT measurements.
In a longitudinal study assessing the risk of glaucoma progression, it was shown that lower baseline macular and peripapillary vessel densities were associated with a faster rate of RNFL progression in mild to moderate glaucoma over a mean follow-up of 27 months.116 Importantly, this association was independent of the baseline RNFL thickness, suggesting that OCTA may offer additional information to the evaluation of the risk of glaucoma progression and prediction of rates of disease worsening. Multiple studies have also found that the presence of MvD is associated with a faster rate of RNFL thinning117, 118 and VF progression.119 Of interest, a significant association between MvD and faster rate of central VF progression has been reported.120 Another recent study has reported an association between MvD enlargement and progressive RNFL thinning.115 All these studies suggest that assessment of OCTA parameters may add significant information to the evaluation of the risk of future glaucoma progression.
OCTA changes in other subtypes of glaucoma
Normal tension glaucoma: The vascular theory of glaucoma is considered to be more applicable in eyes developing glaucomatous damage at low IOP. A few studies compared the OCTA measurements in low pressure glaucoma (NTG) and high pressure glaucoma (POAG). However, no difference in OCTA measurements were seen between NTG and severity-matched POAG eyes.42, 121–123 In contrast, MvD was found to be more common in open-angle glaucoma eyes with lower pre-treatment/baseline IOP in one study.91
Primary angle-closure glaucoma (PACG): OCTA measurements are found to be reduced in eyes with PACG81, 124, 125 and after an acute primary angle-closure episode.126–128 Similar diagnostic abilities of OCTA vessel density measurements in PACG and POAG were found when accounting for the severity of disease, implying that the reduction of vessel density measurements in PACG is probably similar to that in POAG.81 However, another study reported that POAG and PACG eyes have a different vascular–function relationship when determined by OCTA.64 Another study found that the prevalence of MvD was lower in PACG compared to severity matched POAG eyes.91
Pseudoexfoliation glaucoma (PXG): A few studies have reported that the reduction of superficial vessel densities on OCTA was greater in PXG compared to POAG eyes of similar disease severity.129–132 One of these studies also reported that the prevalence of MvD, unlike superficial vessel densities, was significantly lower in PXG compared to POAG eyes of similar disease severity.131
Limitations and recent advances in OCTA
Motion artifacts are common with OCTA imaging due to the prolonged time required to acquire the scans despite methods available to account for the artifacts (Figure 5). Multiple studies have also reported a high number of poor quality images with OCTA.56, 58, 92, 133 Two significant improvements incorporated recently to overcome the issue of poor quality scans are (i) real time eye tracking technology, for controlling the motion artifacts more effectively134 and, (ii) high-density (HD) scanning mode, for improving the resolution of the scans. A recent study has reported that the number of poor quality scans significantly decreased with the incorporation of these improvements.135 Future advances in the technology should aim at reducing the acquisition time to obtain more precise measurements.
Figure 5.

Macular OCTA scan of a left eye showing two types of artifacts on the angiography map; motion artifacts, recognized as vertical bands temporally and duplication of vessels, recognized inferiorly and nasally.
Media opacities, especially vitreous opacities, can significantly affect the quality of OCTA scans and the quantification of vessel densities (Figure 6). Pupillary size also affects the quality of OCTA scans and dilation of pupil is necessary for good quality scans.
Figure 6.
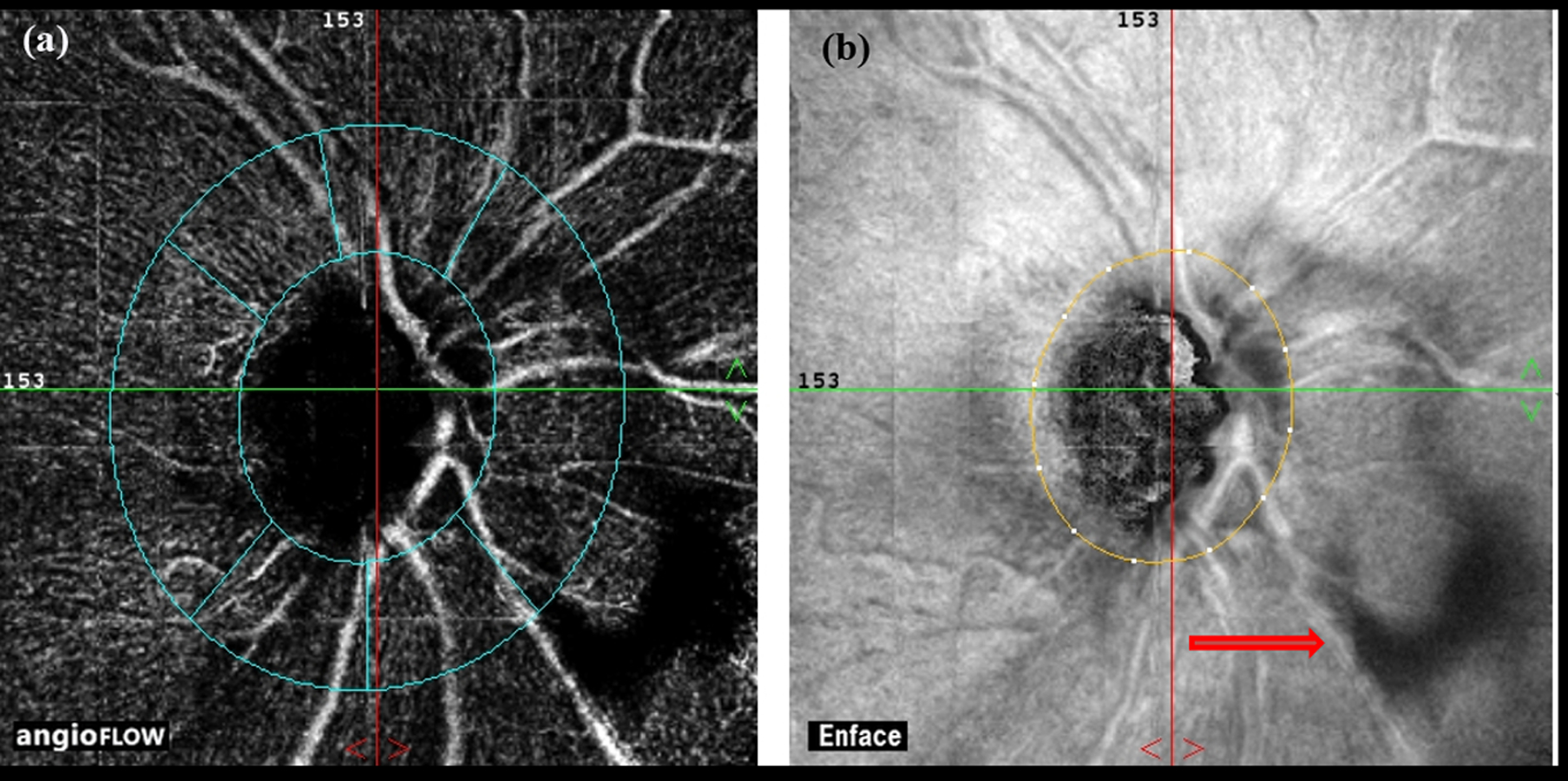
Vitreous opacity (red arrow on en face map, b) casting a shadow, as seen on the angiography map (a).
OCTA technology evaluates well the superficial retinal vessels, but not the deeper retinal and choroidal vasculature. This is because the signals from the superficial retinal vessels project on to the deeper layers causing projection artifacts.26 Detection of MvD, for example, is affected by the presence of projection artifacts. Newer methods of projection artifact correction have been tried and the newer generations of OCTA (projection resolved OCTA) are likely to evaluate the deeper retinal and choroidal vasculature better.136
Segmentation of the angiography slabs differs among commercially available OCTA devices. Campbell et al have recently used projection resolved OCTA to describe a rational segmentation algorithm that respects the normal distribution of vascular networks in the human retina.136 They demonstrated four unique vascular plexuses in the human retina with distinct vascular patterns, which vary based on depth and location from the optic nerve.136 Standardization of segmentation methods such as this could help comparability among different commercially available OCTA devices and improve the clinical applications of OCTA.
Conclusions
OCTA is a novel, noninvasive imaging technology that provides information regarding the severity of impaired perfusion in different depths of the retina and choroid that was not available in the past. Currently, researchers are still understanding the full potential of OCTA in clinical practice and hence, ophthalmologists need to be conservative in the application of this technology in treatment decisions. However, since OCTA is a safe, non-invasive test, it can be performed at the same time as OCT and can provide information that complements VF and OCT examinations for early diagnosis of glaucoma, detection of progression and its risk assessment. Future longitudinal studies should evaluate if OCTA can detect vascular changes earlier than RNFL thinning in glaucoma. If this were shown to be the case, then OCTA can lead to a paradigm shift in the way glaucoma is managed and can also lead to new ways of testing treatment outcomes in glaucoma.
Acknowledgments
Financial disclosures: Rao HL: Santen (C), Carl-Zeiss Meditec (C), Allergan (C); Pradhan ZS: none; Suh MH: none; Moghimi S: none; Mansouri K: Santen (C), Sensimed (C), Alcon (S), Allergan (S), ImplanData (C); Weinreb RN: Optovue (S), Meditec-Zeiss (S), Heidelberg Engineering (S), Allergan (C), Eyenovia (C), Bausch & Lomb (C), Unity (C), Centervue (S). Supported in part by NIH EY029058 (RNW) and an unrestricted grant by Research to Prevent Blindness (New York, NY).
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311:1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol 1994;39:23–42. [DOI] [PubMed] [Google Scholar]
- 3.Yan DB, Coloma FM, Metheetrairut A, et al. Deformation of the lamina cribrosa by elevated intraocular pressure. Br J Ophthalmol 1994;78:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis BA, Varma R, Chopra V, et al. Intraocular pressure, central corneal thickness, and prevalence of open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol 2008;146:741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol 1991;109:1090–5. [DOI] [PubMed] [Google Scholar]
- 6.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 2000;130:429–40. [DOI] [PubMed] [Google Scholar]
- 7.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108:1943–53. [DOI] [PubMed] [Google Scholar]
- 8.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–13; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48–56. [DOI] [PubMed] [Google Scholar]
- 10.Ernest PJ, Schouten JS, Beckers HJ, et al. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology 2013;120:512–9. [DOI] [PubMed] [Google Scholar]
- 11.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 2000;107:1287–93. [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114:1965–72. [DOI] [PubMed] [Google Scholar]
- 13.Flammer J The vascular concept of glaucoma. Surv Ophthalmol 1994;38 Suppl:S3–6. [DOI] [PubMed] [Google Scholar]
- 14.Arend O, Plange N, Sponsel WE, Remky A. Pathogenetic aspects of the glaucomatous optic neuropathy: fluorescein angiographic findings in patients with primary open angle glaucoma. Brain Res Bull 2004;62:517–24. [DOI] [PubMed] [Google Scholar]
- 15.Huber K, Plange N, Remky A, Arend O. Comparison of colour Doppler imaging and retinal scanning laser fluorescein angiography in healthy volunteers and normal pressure glaucoma patients. Acta Ophthalmol Scand 2004;82:426–31. [DOI] [PubMed] [Google Scholar]
- 16.Talusan E, Schwartz B. Specificity of fluorescein angiographic defects of the optic disc in glaucoma. Arch Ophthalmol 1977;95:2166–75. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz B, Rieser JC, Fishbein SL. Fluorescein angiographic defects of the optic disc in glaucoma. Arch Ophthalmol 1977;95:1961–74. [DOI] [PubMed] [Google Scholar]
- 18.Hitchings RA, Spaeth GL. Fluorescein angiography in chronic simple and low-tension glaucoma. Br J Ophthalmol 1977;61:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brart DP, de Souza Lima M, Bartsch DU, et al. Indocyanine green angiography of the peripapillary region in glaucomatous eyes by confocal scanning laser ophthalmoscopy. Am J Ophthalmol 1997;123:657–66. [DOI] [PubMed] [Google Scholar]
- 20.Rechtman E, Harris A, Kumar R, et al. An update on retinal circulation assessment technologies. Curr Eye Res 2003;27:329–43. [DOI] [PubMed] [Google Scholar]
- 21.Nicolela MT, Hnik P, Drance SM. Scanning laser Doppler flowmeter study of retinal and optic disk blood flow in glaucomatous patients. Am J Ophthalmol 1996;122:775–83. [DOI] [PubMed] [Google Scholar]
- 22.Yaoeda K, Shirakashi M, Funaki S, et al. Measurement of microcirculation in the optic nerve head by laser speckle flowgraphy and scanning laser Doppler flowmetry. Am J Ophthalmol 2000;129:734–9. [DOI] [PubMed] [Google Scholar]
- 23.Shoji T, Zangwill LM, Akagi T, et al. Progressive Macula Vessel Density Loss in Primary Open-Angle Glaucoma: A Longitudinal Study. Am J Ophthalmol 2017;182:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002;21:359–93. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 2012;3:3127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20:4710–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coscas G, Lupidi M, Coscas F. Heidelberg Spectralis Optical Coherence Tomography Angiography: Technical Aspects. Dev Ophthalmol 2016;56:1–5. [DOI] [PubMed] [Google Scholar]
- 28.Stanga PE, Tsamis E, Papayannis A, et al. Swept-Source Optical Coherence Tomography Angio (Topcon Corp, Japan): Technology Review. Dev Ophthalmol 2016;56:13–7. [DOI] [PubMed] [Google Scholar]
- 29.Wang RK, An L, Francis P, Wilson DJ. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Opt Lett 2010;35:1467–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An L, Johnstone M, Wang RK. Optical microangiography provides correlation between microstructure and microvasculature of optic nerve head in human subjects. J Biomed Opt 2012;17:116018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenfeld PJ, Durbin MK, Roisman L, et al. ZEISS Angioplex Spectral Domain Optical Coherence Tomography Angiography: Technical Aspects. Dev Ophthalmol 2016;56:18–29. [DOI] [PubMed] [Google Scholar]
- 32.Kraus MF, Potsaid B, Mayer MA, et al. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express 2012;3:1182–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Edmunds B, Takusagawa HL, et al. Projection-Resolved Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. Am J Ophthalmol 2019;207:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang D, Jia Y, Gao SS, et al. Optical Coherence Tomography Angiography Using the Optovue Device. Dev Ophthalmol 2016;56:6–12. [DOI] [PubMed] [Google Scholar]
- 35.Rao HL, Riyazuddin M, Dasari S, et al. Diagnostic Abilities of the Optical Microangiography Parameters of the 3×3 mm and 6×6 mm Macular Scans in Glaucoma. J Glaucoma 2018;27:496–503. [DOI] [PubMed] [Google Scholar]
- 36.Takusagawa HL, Liu L, Ma KN, et al. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology 2017;124:1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabiolo A, Gelormini F, Sacconi R, et al. Comparison of methods to quantify macular and peripapillary vessel density in optical coherence tomography angiography. PLoS One 2018;13:e0205773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei J, Pei C, Wen C, Abdelfattah NS. Repeatability and Reproducibility of Quantification of Superficial Peri-papillary Capillaries by four Different Optical Coherence Tomography Angiography Devices. Sci Rep 2018;8:17866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121:1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol 2015;133:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci 2016;57:OCT451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao HL, Pradhan ZS, Weinreb RN, et al. Regional Comparisons of Optical Coherence Tomography Angiography Vessel Density in Primary Open-Angle Glaucoma. Am J Ophthalmol 2016;171:75–83. [DOI] [PubMed] [Google Scholar]
- 43.Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS One 2017;12:e0173930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin JW, Lee J, Kwon J, et al. Regional vascular density-visual field sensitivity relationship in glaucoma according to disease severity. Br J Ophthalmol 2017;101:1666–72. [DOI] [PubMed] [Google Scholar]
- 45.Mansoori T, Sivaswamy J, Gamalapati JS, Balakrishna N. Radial Peripapillary Capillary Density Measurement Using Optical Coherence Tomography Angiography in Early Glaucoma. J Glaucoma 2017;26:438–43. [DOI] [PubMed] [Google Scholar]
- 46.Geyman LS, Garg RA, Suwan Y, et al. Peripapillary perfused capillary density in primary open-angle glaucoma across disease stage: an optical coherence tomography angiography study. Br J Ophthalmol 2017;101:1261–8. [DOI] [PubMed] [Google Scholar]
- 47.Akil H, Chopra V, Al-Sheikh M, et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br J Ophthalmol 2017. (In Press). [DOI] [PubMed] [Google Scholar]
- 48.Hollo G Vessel density calculated from OCT angiography in 3 peripapillary sectors in normal, ocular hypertensive, and glaucoma eyes. Eur J Ophthalmol 2016;26:e42–5. [DOI] [PubMed] [Google Scholar]
- 49.Kumar RS, Anegondi N, Chandapura RS, et al. Discriminant Function of Optical Coherence Tomography Angiography to Determine Disease Severity in Glaucoma. Invest Ophthalmol Vis Sci 2016;57:6079–88. [DOI] [PubMed] [Google Scholar]
- 50.Akagi T, Iida Y, Nakanishi H, et al. Microvascular Density in Glaucomatous Eyes With Hemifield Visual Field Defects: An Optical Coherence Tomography Angiography Study. Am J Ophthalmol 2016;168:237–49. [DOI] [PubMed] [Google Scholar]
- 51.Ichiyama Y, Minamikawa T, Niwa Y, Ohji M. Capillary Dropout at the Retinal Nerve Fiber Layer Defect in Glaucoma: An Optical Coherence Tomography Angiography Study. J Glaucoma 2017;26:e142–e5. [DOI] [PubMed] [Google Scholar]
- 52.Yarmohammadi A, Zangwill LM, Manalastas PIC, et al. Peripapillary and Macular Vessel Density in Patients with Primary Open-Angle Glaucoma and Unilateral Visual Field Loss. Ophthalmology 2017;125:578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suh MH, Zangwill LM, Manalastas PI, et al. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 2016;123:2509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee EJ, Kim TW, Lee SH, Kim JA. Underlying Microstructure of Parapapillary Deep-Layer Capillary Dropout Identified by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2017;58:1621–7. [DOI] [PubMed] [Google Scholar]
- 55.Lee EJ, Lee KM, Lee SH, Kim TW. Parapapillary Choroidal Microvasculature Dropout in Glaucoma: A Comparison between Optical Coherence Tomography Angiography and Indocyanine Green Angiography. Ophthalmology 2017;124:1209–17. [DOI] [PubMed] [Google Scholar]
- 56.Venugopal JP, Rao HL, Weinreb RN, et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. Br J Ophthalmol 2018;102:352–7. [DOI] [PubMed] [Google Scholar]
- 57.Manalastas PIC, Zangwill LM, Saunders LJ, et al. Reproducibility of Optical Coherence Tomography Angiography Macular and Optic Nerve Head Vascular Density in Glaucoma and Healthy Eyes. J Glaucoma 2017;26:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollo G Intrasession and Between-Visit Variability of Sector Peripapillary Angioflow Vessel Density Values Measured with the Angiovue Optical Coherence Tomograph in Different Retinal Layers in Ocular Hypertension and Glaucoma. PLoS One 2016;11:e0161631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Jiang C, Ko T, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 2015;253:1557–64. [DOI] [PubMed] [Google Scholar]
- 60.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016;123:2498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen HS, Liu CH, Wu WC, et al. Optical Coherence Tomography Angiography of the Superficial Microvasculature in the Macular and Peripapillary Areas in Glaucomatous and Healthy Eyes. Invest Ophthalmol Vis Sci 2017;58:3637–45. [DOI] [PubMed] [Google Scholar]
- 62.Rao HL, Pradhan ZS, Weinreb RN, et al. Relationship of Optic Nerve Structure and Function to Peripapillary Vessel Density Measurements of Optical Coherence Tomography Angiography in Glaucoma. J Glaucoma 2017;26:548–54. [DOI] [PubMed] [Google Scholar]
- 63.Igarashi R, Ochiai S, Sakaue Y, et al. Optical coherence tomography angiography of the peripapillary capillaries in primary open-angle and normal-tension glaucoma. PLoS One 2017;12:e0184301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jo YH, Sung KR, Yun SC. The Relationship Between Peripapillary Vascular Density and Visual Field Sensitivity in Primary Open-Angle and Angle-Closure Glaucoma. Invest Ophthalmol Vis Sci 2018;59:5862–7. [DOI] [PubMed] [Google Scholar]
- 65.Rao HL, Dasari S, Riyazuddin M, et al. Diagnostic Ability and Structure-function Relationship of Peripapillary Optical Microangiography Measurements in Glaucoma. J Glaucoma 2018;27:219–26. [DOI] [PubMed] [Google Scholar]
- 66.Sakaguchi K, Higashide T, Udagawa S, et al. Comparison of Sectoral Structure-Function Relationships in Glaucoma: Vessel Density Versus Thickness in the Peripapillary Retinal Nerve Fiber Layer. Invest Ophthalmol Vis Sci 2017;58:5251–62. [DOI] [PubMed] [Google Scholar]
- 67.Rao HL, Riyazuddin M, Dasari S, et al. Relationship of Macular Thickness and Function to Optical Microangiography Measurements in Glaucoma. J Glaucoma 2018;27:210–8. [DOI] [PubMed] [Google Scholar]
- 68.Penteado RC, Zangwill LM, Daga FB, et al. Optical Coherence Tomography Angiography Macular Vascular Density Measurements and the Central 10–2 Visual Field in Glaucoma. J Glaucoma 2018;27:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen A, Liu L, Wang J, et al. Measuring Glaucomatous Focal Perfusion Loss in the Peripapillary Retina Using OCT Angiography. Ophthalmology 2019. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin JW, Kwon J, Lee J, Kook MS. Relationship between vessel density and visual field sensitivity in glaucomatous eyes with high myopia. Br J Ophthalmol 2018. (In Press). [DOI] [PubMed] [Google Scholar]
- 71.Ghahari E, Bowd C, Zangwill LM, et al. Association of Macular and Circumpapillary Microvasculature with Visual Field Sensitivity in Advanced Glaucoma. Am J Ophthalmol 2019;204:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin JW, Lee J, Kwon J, et al. Relationship between macular vessel density and central visual field sensitivity at different glaucoma stages. Br J Ophthalmol 2019. (In Press). [DOI] [PubMed] [Google Scholar]
- 73.Moghimi S, Bowd C, Zangwill LM, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology 2019;126:980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee EJ, Lee SH, Kim JA, Kim TW. Parapapillary Deep-Layer Microvasculature Dropout in Glaucoma: Topographic Association With Glaucomatous Damage. Invest Ophthalmol Vis Sci 2017;58:3004–10. [DOI] [PubMed] [Google Scholar]
- 75.Shin JW, Kwon J, Lee J, Kook MS. Choroidal Microvasculature Dropout is Not Associated With Myopia, But is Associated With Glaucoma. J Glaucoma 2018;27:189–96. [DOI] [PubMed] [Google Scholar]
- 76.Suh MH, Park JW, Kim HR. Association Between the Deep-layer Microvasculature Dropout and the Visual Field Damage in Glaucoma. J Glaucoma 2018;27:543–51. [DOI] [PubMed] [Google Scholar]
- 77.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology 2017;124:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pradhan ZS, Dixit S, Sreenivasaiah S, et al. A Sectoral Analysis of Vessel Density Measurements in Perimetrically Intact Regions of Glaucomatous Eyes: An Optical Coherence Tomography Angiography Study. J Glaucoma 2018;27:525–31. [DOI] [PubMed] [Google Scholar]
- 79.Chen CL, Bojikian KD, Wen JC, et al. Peripapillary Retinal Nerve Fiber Layer Vascular Microcirculation in Eyes With Glaucoma and Single-Hemifield Visual Field Loss. JAMA Ophthalmol 2017;135:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jo YH, Kwon J, Shon K, et al. Greater Severity of Glaucomatous Damage in Eyes With Than Without Choroidal Microvasculature Dropout in Open-Angle Glaucoma. Invest Ophthalmol Vis Sci 2019;60:901–12. [DOI] [PubMed] [Google Scholar]
- 81.Rao HL, Kadambi SV, Weinreb RN, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol 2017;101:1066–70. [DOI] [PubMed] [Google Scholar]
- 82.Kurysheva NI, Maslova EV, Zolnikova IV, et al. A comparative study of structural, functional and circulatory parameters in glaucoma diagnostics. PLoS One 2018;13:e0201599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chihara E, Dimitrova G, Amano H, Chihara T. Discriminatory Power of Superficial Vessel Density and Prelaminar Vascular Flow Index in Eyes With Glaucoma and Ocular Hypertension and Normal Eyes. Invest Ophthalmol Vis Sci 2017;58:690–7. [DOI] [PubMed] [Google Scholar]
- 84.Chung JK, Hwang YH, Wi JM, et al. Glaucoma Diagnostic Ability of the Optical Coherence Tomography Angiography Vessel Density Parameters. Curr Eye Res 2017;42:1458–67. [DOI] [PubMed] [Google Scholar]
- 85.Hou H, Moghimi S, Zangwill LM, et al. Macula Vessel Density and Thickness in Early Primary Open-Angle Glaucoma. Am J Ophthalmol 2019;199:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wan KH, Lam AKN, Leung CK. Optical Coherence Tomography Angiography Compared With Optical Coherence Tomography Macular Measurements for Detection of Glaucoma. JAMA Ophthalmol 2018;136:866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Triolo G, Rabiolo A, Shemonski ND, et al. Optical Coherence Tomography Angiography Macular and Peripapillary Vessel Perfusion Density in Healthy Subjects, Glaucoma Suspects, and Glaucoma Patients. Invest Ophthalmol Vis Sci 2017;58:5713–22. [DOI] [PubMed] [Google Scholar]
- 88.Rao HL, Sreenivasaiah S, Dixit S, et al. Choroidal Microvascular Dropout in Primary Open-angle Glaucoma Eyes With Disc Hemorrhage. J Glaucoma 2019;28:181–7. [DOI] [PubMed] [Google Scholar]
- 89.Lee EJ, Kim TW, Kim JA, Kim JA. Central Visual Field Damage and Parapapillary Choroidal Microvasculature Dropout in Primary Open-Angle Glaucoma. Ophthalmology 2018;125:588–96. [DOI] [PubMed] [Google Scholar]
- 90.Kwon J, Shin JW, Lee J, Kook MS. Choroidal Microvasculature Dropout Is Associated With Parafoveal Visual Field Defects in Glaucoma. Am J Ophthalmol 2018;188:141–54. [DOI] [PubMed] [Google Scholar]
- 91.Rao HL, Sreenivasaiah S, Riyazuddin M, et al. Choroidal Microvascular Dropout in Primary Angle Closure Glaucoma. Am J Ophthalmol 2019;199:184–92. [DOI] [PubMed] [Google Scholar]
- 92.Suh MH, Zangwill LM, Manalastas PI, et al. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology 2016;123:2309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghahari E, Bowd C, Zangwill LM, et al. Macular Vessel Density in Glaucomatous Eyes With Focal Lamina Cribrosa Defects. J Glaucoma 2018;27:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han JC, Choi JH, Park DY, et al. Border Tissue Morphology Is Spatially Associated with Focal Lamina Cribrosa Defect and Deep-Layer Microvasculature Dropout in Open-Angle Glaucoma. Am J Ophthalmol 2019;203:89–102. [DOI] [PubMed] [Google Scholar]
- 95.Rao HL, Pradhan ZS, Weinreb RN, et al. Optical Coherence Tomography Angiography Vessel Density Measurements in Eyes With Primary Open-Angle Glaucoma and Disc Hemorrhage. J Glaucoma 2017;26:888–95. [DOI] [PubMed] [Google Scholar]
- 96.Park HL, Kim JW, Park CK. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology 2018;125:1003–13. [DOI] [PubMed] [Google Scholar]
- 97.Yu J, Jiang C, Wang X, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci 2015;56:3212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moghimi S, Zangwill LM, Penteado RC, et al. Macular and Optic Nerve Head Vessel Density and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Ophthalmology 2018;125:1720–8. [DOI] [PubMed] [Google Scholar]
- 99.Hou H, Moghimi S, Zangwill LM, et al. Macula Vessel Density and Thickness in Early Primary Open Angle Glaucoma. Am J Ophthalmol 2019;199:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang R, Nelson AJ, LeTran V, et al. Systemic Determinants of Peripapillary Vessel Density in Healthy African Americans: The African American Eye Disease Study. Am J Ophthalmol 2019;207:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology 2017;124:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mansouri K, Rao HL, Hoskens K, et al. Diurnal Variations of Peripapillary and Macular Vessel Density in Glaucomatous Eyes Using Optical Coherence Tomography Angiography. J Glaucoma 2018;27:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muller VC, Storp JJ, Kerschke L, et al. Diurnal variations in flow density measured using optical coherence tomography angiography and the impact of heart rate, mean arterial pressure and intraocular pressure on flow density in primary open-angle glaucoma patients. Acta Ophthalmol 2019;97:e844–e9. [DOI] [PubMed] [Google Scholar]
- 104.Alnawaiseh M, Lahme L, Treder M, et al. Short-term effects of exercise on optic nerve and macular perfusion measured by optical coherence tomography angiography. Retina 2017;37:1642–6. [DOI] [PubMed] [Google Scholar]
- 105.Rao HL, Pradhan ZS, Weinreb RN, et al. Determinants of Peripapillary and Macular Vessel Densities Measured by Optical Coherence Tomography Angiography in Normal Eyes. J Glaucoma 2017;26:491–7. [DOI] [PubMed] [Google Scholar]
- 106.Wang X, Kong X, Jiang C, et al. Is the peripapillary retinal perfusion related to myopia in healthy eyes? A prospective comparative study. BMJ open 2016;6:e010791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suwan Y, Fard MA, Geyman LS, et al. Association of Myopia With Peripapillary Perfused Capillary Density in Patients With Glaucoma: An Optical Coherence Tomography Angiography Study. JAMA Ophthalmol 2018;136:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sampson DM, Gong P, An D, et al. Axial Length Variation Impacts on Superficial Retinal Vessel Density and Foveal Avascular Zone Area Measurements Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci 2017;58:3065–72. [DOI] [PubMed] [Google Scholar]
- 109.Shahlaee A, Samara WA, Hsu J, et al. In Vivo Assessment of Macular Vascular Density in Healthy Human Eyes Using Optical Coherence Tomography Angiography. Am J Ophthalmol 2016;165:39–46. [DOI] [PubMed] [Google Scholar]
- 110.Hollo G Influence of Large Intraocular Pressure Reduction on Peripapillary OCT Vessel Density in Ocular Hypertensive and Glaucoma Eyes. J Glaucoma 2017;26:e7–e10. [DOI] [PubMed] [Google Scholar]
- 111.Shin JW, Sung KR, Uhm KB, et al. Peripapillary Microvascular Improvement and Lamina Cribrosa Depth Reduction After Trabeculectomy in Primary Open-Angle Glaucoma. Invest Ophthalmol Vis Sci 2017;58:5993–9. [DOI] [PubMed] [Google Scholar]
- 112.Zeboulon P, Leveque PM, Brasnu E, et al. Effect of Surgical Intraocular Pressure Lowering on Peripapillary and Macular Vessel Density in Glaucoma Patients: An Optical Coherence Tomography Angiography Study. J Glaucoma 2017;26:466–72. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Q, Jonas JB, Wang Q, et al. Optical Coherence Tomography Angiography Vessel Density Changes after Acute Intraocular Pressure Elevation. Sci Rep 2018;8:6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hollo G Influence of Removing the Large Retinal Vessels-related Effect on Peripapillary Vessel Density Progression Analysis in Glaucoma. J Glaucoma 2018;27:e137–e9. [DOI] [PubMed] [Google Scholar]
- 115.Kim JA, Lee EJ, Kim TW. Evaluation of Parapapillary Choroidal Microvasculature Dropout and Progressive Retinal Nerve Fiber Layer Thinning in Patients With Glaucoma. JAMA Ophthalmol 2019;137:810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moghimi S, Zangwill LM, Penteado RC, et al. Macular and Optic Nerve Head Vessel Density and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Ophthalmology 2018;125:1720–8. [DOI] [PubMed] [Google Scholar]
- 117.Park HL, Kim JW, Park CK. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology 2018;125:1003–13. [DOI] [PubMed] [Google Scholar]
- 118.Lin S, Cheng H, Zhang S, et al. Parapapillary Choroidal Microvasculature Dropout Is Associated With the Decrease in Retinal Nerve Fiber Layer Thickness: A Prospective Study. Invest Ophthalmol Vis Sci 2019;60:838–42. [DOI] [PubMed] [Google Scholar]
- 119.Kwon JM, Weinreb RN, Zangwill LM, Suh MH. Parapapillary Deep-Layer Microvasculature Dropout and Visual Field Progression in Glaucoma. Am J Ophthalmol 2019;200:65–75. [DOI] [PubMed] [Google Scholar]
- 120.Jo YH, Kwon J, Jeong D, et al. Rapid Central Visual Field Progression Rate in Eyes with Open-Angle Glaucoma and Choroidal Microvasculature Dropout. Sci Rep 2019;9:8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scripsema NK, Garcia PM, Bavier RD, et al. Optical Coherence Tomography Angiography Analysis of Perfused Peripapillary Capillaries in Primary Open-Angle Glaucoma and Normal-Tension Glaucoma. Invest Ophthalmol Vis Sci 2016;57:OCT611–OCT20. [DOI] [PubMed] [Google Scholar]
- 122.Bojikian KD, Chen CL, Wen JC, et al. Optic Disc Perfusion in Primary Open Angle and Normal Tension Glaucoma Eyes Using Optical Coherence Tomography-Based Microangiography. PLoS One 2016;11:e0154691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toshev AP, Schuster AK, Ul Hassan SN, et al. Optical Coherence Tomography Angiography of Optic Disc in Eyes With Primary Open-angle Glaucoma and Normal-tension Glaucoma. J Glaucoma 2019;28:243–51. [DOI] [PubMed] [Google Scholar]
- 124.Rao HL, Pradhan ZS, Weinreb RN, et al. Vessel Density and Structural Measurements of Optical Coherence Tomography in Primary Angle Closure and Primary Angle Closure Glaucoma. Am J Ophthalmol 2017;177:106–15. [DOI] [PubMed] [Google Scholar]
- 125.Zhu L, Zong Y, Yu J, et al. Reduced Retinal Vessel Density in Primary Angle Closure Glaucoma: A Quantitative Study Using Optical Coherence Tomography Angiography. J Glaucoma 2018;27:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang S, Wu C, Liu L, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Primary Angle-Closure Glaucoma. Am J Ophthalmol 2017;182:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moghimi S, SafiZadeh M, Fard MA, et al. Changes in Optic Nerve Head Vessel Density After Acute Primary Angle Closure Episode. Invest Ophthalmol Vis Sci 2019;60:552–8. [DOI] [PubMed] [Google Scholar]
- 128.Wang X, Jiang C, Kong X, et al. Peripapillary retinal vessel density in eyes with acute primary angle closure: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 2017;255:1013–8. [DOI] [PubMed] [Google Scholar]
- 129.Suwan Y, Geyman LS, Fard MA, et al. Peripapillary Perfused Capillary Density in Exfoliation Syndrome and Exfoliation Glaucoma versus POAG and Healthy Controls: An OCTA Study. Asia Pac J Ophthalmol (Phila) 2018;7:84–9. [DOI] [PubMed] [Google Scholar]
- 130.Park JH, Yoo C, Girard MJA, et al. Peripapillary Vessel Density in Glaucomatous Eyes: Comparison Between Pseudoexfoliation Glaucoma and Primary Open-angle Glaucoma. J Glaucoma 2018;27:1009–16. [DOI] [PubMed] [Google Scholar]
- 131.Pradhan ZS, Rao HL, Dixit S, et al. Choroidal Microvascular Dropout in Pseudoexfoliation Glaucoma. Invest Ophthalmol Vis Sci 2019;60:2146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Philip S, Najafi A, Tantraworasin A, et al. Macula Vessel Density and Foveal Avascular Zone Parameters in Exfoliation Glaucoma Compared to Primary Open-Angle Glaucoma. Invest Ophthalmol Vis Sci 2019;60:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spaide RF, Fujimoto JG, Waheed NK. Image Artifacts in Optical Coherence Tomography Angiography. Retina 2015;35:2163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Camino A, Zhang M, Gao SS, et al. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed Opt Express 2016;7:3905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Venugopal JP, Rao HL, Weinreb RN, et al. Repeatability and comparability of peripapillary vessel density measurements of high-density and non-high-density optical coherence tomography angiography scans in normal and glaucoma eyes. Br J Ophthalmol 2018. (in Press). [DOI] [PubMed] [Google Scholar]
- 136.Campbell JP, Zhang M, Hwang TS, et al. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep 2017;7:42201. [DOI] [PMC free article] [PubMed] [Google Scholar]


