Abstract
Incidence of human papillomavirus (HPV) attributable oropharyngeal cancers (OPCs) has been increasing globally, especially among men in high-income countries. There is a lack of studies comparing oral HPV prevalence by age and country among healthy men. The purpose of our study was to assess oral HPV prevalence by country and age. Participants of the HPV Infection in Men Study (HIM), a cohort of 3,098 healthy men from São Paulo, Brazil, Cuernavaca, Mexico and Tampa, USA, were studied. Oral HPV prevalence and type distribution were assessed using the SPF10 PCR-DEIA-LiPA25 system. The prevalence of any HPV in Brazil, Mexico and the US was 8.7% (95% CI: 7.1%, 10.4%), 10.0% (95% CI: 8.3%, 12.1%) and 7.6% (95% CI: 5.9%, 9.5%), respectively, while the prevalence of high-risk HPV was 5.3% (95% CI: 4.1%, 6.7%), 7.3% (95% CI: 5.7%, 9.0%) and 5.4% (95% CI: 4.0%, 7.0%), respectively. No significant differences in prevalence of grouped HPV types were observed by country despite significant differences in sexual behaviors. However, the age-specific prevalence of oral HPV differed by country. Brazilian (6.0% [95% CI: 3.4%, 9.7%]) and Mexican (9.2% [95% CI: 5.6%, 14.0%]) participants had peak high-risk HPV prevalence among men aged 41–50 years whereas the US participants had peak prevalence at ages 31–40 years (11.0% [95% CI: 6.4%, 17.3%]). In conclusion, oral HPV prevalence was low with no difference in overall prevalence observed by country. Factors associated with the differences in oral HPV age-patterning by country and sexual orientation require further study.
Keywords: oral HPV, HIM study, Brazil, Mexico, USA
Introduction
Human papillomavirus (HPV) is a known causal agent of cervical,1 penile, vaginal and anal cancers.2–4 HPV is also the cause of a subset of oropharyngeal cancers (OPCs).2 With a decline in tobacco smoking, previously the most common cause of OPCs, the proportion of OPCs attributed to HPV has been increasing, most notably among men. In the USA, OPC incidence is 4–5 times higher among men compared to women.5 Approximately 20–70% of OPCs have been attributed to HPV infection,6,7 including 70% in the USA.8–10
There have been many cross-sectional and a few longitudinal studies reporting the prevalence and incidence of oral HPV infections in the USA.11,12 However, little is known about oral HPV prevalence among healthy men in other countries such as Brazil and Mexico, where oral HPV prevalence has mostly been estimated in oral cancer and OPC tumor tissue, and among women.13–18 Few studies have examined the association between age and oral HPV prevalence, with most conducted among USA populations only.11,19
In the current study, we assessed oral HPV prevalence and type distribution in three countries (Brazil, Mexico and USA) and assessed the variation in prevalence by age and sexual orientation among 3,098 men participating in the HPV Infection in Men (HIM) study oral subcohort.
Materials and Methods
The oral subcohort is nested within the HIM study, which has been previously described.20 Men were recruited from São Paulo (Brazil), Cuernavaca (Mexico) and Tampa (USA) and its surrounding areas from March 2005 to December 2009. Oral specimens included in our study were collected from each of the clinical sites as follows: Brazil, December, 2007–November, 2009; Mexico, April, 2008–March, 2012; USA, November, 2007–September, 2009. Approval of study procedures prior to the study was obtained from the Human Subject Committees of the Centro de Referência e Treinamento de Doenças Sexualmente Transmissíveis e AIDS in Brazil, National Institute of Public Health in Mexico and the University of South Florida in USA. All participants gave written consent.
Population
The following were the eligibility criteria for the men included in the HIM study: (i) Age 18–73 years; (ii) Resident of one of the three recruitment sites in Brazil, Mexico or USA; (iii) Did not report previous diagnoses of anal or penile cancer; (iv) Never diagnosed with genital or anal warts prior to the study; (v) No current reports of a sexually transmitted infection or treatment of a sexually transmitted infection; (vi) Not participating in a HPV vaccine study; (vii) No history of HIV or AIDS; (viii) No history of imprisonment, homelessness or drug treatment during the past 6 months; (ix) Willing to comply with 10 scheduled visits every 6 months for 4 years with no plans to relocate within the next 4 years.
Overall, men were recruited from different population sources to obtain access to a broad range of ages, sexual behaviors, and HPV risk. In Brazil, men were recruited from the general population at a facility for urogenital care (Centro de Referência e Treinamento de Doenças Sexualmente Transmissíveis e AIDS) and through general media advertising. Only men reporting for nonsexually transmitted infections were enrolled. In addition, the spouses and partners of women participating in a large cohort study of the natural history of HPV infection and risk of cervical neoplasia conducted in São Paulo since 1993 were also recruited. At the Mexico site, employees and beneficiaries of the Instituto Mexicano del Seguro Social, factory employees, and officials of the Mexican army that were permanently assigned to that geographical area were recruited. In the USA, participants were recruited from the University of South Florida and the Tampa metropolitan area by distributing flyers and posters throughout the campus and providing monthly educational presentations. In addition, utilizing brochures and flyers as well as advertisements in local and university papers, men from the broader Tampa Bay, FL community were recruited.
Study protocol
The HIM Study protocol included a pre-enrollment visit, a baseline (enrollment) visit, and 8–13 additional visits after enrollment scheduled 6 months apart. Oral gargle collection was initiated approximately 2 years after enrollment into the HIM cohort commenced. Therefore, the first oral specimen collected (not necessarily at the enrollment visit) was utilized in the current study. Study participants who had two or more archived oral gargle specimens collected ≥6 months apart were included in the HIM Study Oral Subcohort (n = 3,166).
DNA extraction and HPV Testing
Oral gargle samples were HPV genotyped using the SPF10 PCR-DEIA-LiPA25 system. DNA was extracted from oral gargle cell pellets using the automated BioRobot MDx (Qiagen, Inc.) following the manufacturer’s instructions. HPV genotyping was performed utilizing the RHA Kit HPV SPF10-LiPA25 (DDL Diagnostic Laboratory, Rijswijk, The Netherlands), an in vitro reverse hybridization assay (RHA). The LiPA25 targets a 65 base pair fragment of the L1 region of the HPV genome and requires a three-step process: (i) qPCR that determines sample adequacy based on detection of RNAse P; (ii) a DNA enzyme immunoassay (DEIA) or ELISA method that detects 65 HPV types; and (iii) a LiPA25 genotyping multiplex PCR that selectively identifies the following HPV types by reverse hybridization: 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70 and 74. Samples that were considered adequate via qPCR in step (i) were further analyzed using steps (ii) and (iii).
Classification of HPV types
The following 13 HPV types were categorized as high-risk, defined as oncogenic and potentially oncogenic for cancer: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68/73. The other HPV types detected with the SPF10 LiPA25 protocol were categorized as low-risk: 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70 and 74. A participant was considered positive for any HPV genotype if his sample amplified HPV on PCR and hybridized with a specific HPV type on genotyping. Those who were positive for only high-risk genotypes or both high-risk and low-risk genotypes were included in the “high-risk HPV” category. Single or multiple infections with only low-risk HPV types were categorized as “only low-risk HPV”. Those who were positive for single or multiple HPV 6, 11, 16 or 18 were categorized as “4 vaccine types” (4vHPV) while those who were positive for single or multiple HPV 6, 11, 16, 18, 31, 33, 45, 52 or 58 were categorized as “9 vaccine types” (9vHPV). Individuals who had more than one type of HPV infection were classified as “multiple HPV types”. Individuals who had HPV 16 as well as any other HPV type were categorized as “HPV 16 and other types”. Those with more than one type of high-risk HPV infection were included in the “multiple high-risk types” category. Individuals with both low-risk and high-risk HPV infections were classified as “both high-risk and low-risk types.”
Statistical analysis
Only specimens that were deemed adequate for HPV evaluation (RNase P positive samples) were included in the analyses (n = 3,098). The distribution of sociodemographic and sexual behavioral characteristics was evaluated in Table 1, grouped oral HPV genotype distribution by country in Table 2 and individual oral HPV genotype distribution in Table 3. Associations between covariates and country of residence were tested using Pearson’s χ2 test or Fisher’s exact test depending on the sample size. p-values were corrected for multiple comparisons in Tables 1–3. The age-specific prevalence of high-risk HPV, 9vHPV, 4vHPV and HPV 16 was examined by country (Figure 1), and interactions between age and country of residence were examined using logistic regression models. Age-specific prevalence of high-risk HPV, low-risk HPV and nine-valent vaccine types was assessed among MSW (men having sex with women only) participants and combined MSM (men having sex with men only) and MSWM (men having sex with men and women) participants (Figure 2) using semi-parametric, thin plate penalized regression spline models21 in R software (version 3.4.4) and potential interactions between smoothed age and sexual orientation were examined using the method utilized in Rose et al. 22 article.
Table 1.
Sociodemographic and sexual behavioral characteristics of HIM Study oral subcohort by country
| Brazil (N = 1,198) n (%) | Mexico (N = 1,005) n (%) | USA (N = 895) n (%) | |
|---|---|---|---|
| Age (years) | |||
| 18–30 | 439 (36.6) | 359 (35.7) | 550 (61.5) |
| 31–40 | 421 (35.1) | 355 (35.3) | 145 (16.2) |
| 41–50 | 251 (21.0) | 206 (20.5) | 99 (11.1) |
| 51–73 | 87 (7.3) | 84 (8.4) | 100 (11.2) |
| Missing | 0 (0.0) | 1 (0.1) | 1 (0.1) |
| Race | |||
| Asian/PI | 19 (1.6) | 0 (0.0) | 51 (5.7) |
| Black | 347 (29.0) | 1 (0.1) | 155 (17.3) |
| Mexican | 0 (0.0) | 916 (91.1) | 0 (0.0) |
| Other | 90 (7.5) | 0 (0.0) | 69 (7.7) |
| White | 728 (60.8) | 56 (5.6) | 613 (68.5) |
| Refused | 13 (1.1) | 30 (3.0) | 6 (0.7) |
| Missing | 1 (0.1) | 2 (0.2) | 1 (0.1) |
| Ethnicity | |||
| Hispanic | 257 (21.5) | 999 (99.4) | 126 (14.1) |
| Non-Hispanic | 923 (77.0) | 2 (0.2) | 669 (74.7) |
| Missing | 18 (1.5) | 4 (0.4) | 100 (11.2) |
| Sexual orientation | |||
| MSW | 906 (75.6) | 891 (88.7) | 787 (87.9) |
| MSWM | 126 (10.5) | 30 (3.0) | 25 (2.8) |
| MSM | 105 (8.8) | 18 (1.8) | 17 (1.9) |
| No sex | 59 (4.9) | 64 (6.4) | 64 (7.2) |
| Missing | 2 (0.2) | 2 (0.2) | 2 (0.2) |
| Lifetime no. of sexual partners | |||
| 0–2 | 246 (20.5) | 297 (29.6) | 266 (29.7) |
| 3–7 | 189 (15.8) | 363 (36.1) | 232 (25.9) |
| 8–19 | 321 (26.8) | 242 (24.1) | 190 (21.2) |
| >19 | 428 (35.7) | 97 (9.7) | 203 (22.7) |
| Missing | 14 (1.2) | 6 (0.60) | 4 (0. 5) |
| Number of different females with whom vaginal intercourse was performed in last 6 months | |||
| None | 340 (28.4) | 350 (34.8) | 224 (25.0) |
| 1 | 413 (34.5) | 392 (39.0) | 462 (51.6) |
| 2+ | 408 (34.1) | 198 (19.7) | 201 (22.5) |
| Missing | 37 (3.1) | 65 (6.5) | 8 (0.9) |
| Number of different males with whom anal intercourse was performed in last 6 months | |||
| None | 1,033 (86.2) | 961 (95.6) | 858 (95.9) |
| 1 | 53 (4.4) | 17 (1.7) | 13 (1.5) |
| 2+ | 91 (7.6) | 8 (0.8) | 12 (1.3) |
| Missing | 21 (1.8) | 19 (1.9) | 12 (1.3) |
| Ever performed or received oral sex during lifetime | |||
| Yes | 1,060 (88.5) | 807 (80.3) | 781 (87.3) |
| No | 123 (10.3) | 178 (17.7) | 106 (11.8) |
| Missing | 15 (1.3) | 20 (2.0) | 8 (0.9) |
p-value < 0.01 for all variables; p-values were corrected for multiple comparisons using Benjamini and Hochberg method.
Table 2.
Prevalence of oral HPV by country in the HIM study oral subcohort
| Brazil (N = 1,198) n (%) | Mexico (N = 1,005) n (%) | USA (N = 895) n (%) | p-value1 | |
|---|---|---|---|---|
| Any HPV genotype | 104 (8.7) | 101 (10.0) | 68 (7.6) | 0.17 |
| Any high-risk HPV | 63 (5.3) | 73 (7.3) | 48 (5.4) | 0.10 |
| Only low-risk HPV | 49 (4.1) | 34 (3.4) | 23 (2.6) | 0.17 |
| HPV 6 and/or 11 and/or 16 and/or 18 (4 vaccine types) | 42 (3.5) | 40 (4.0) | 20 (2.2) | 0.09 |
| HPV 6 and/or 11 and/or 16 and/or 18 and/or 31 and/or 33 and/or 45 and/or 52 and/or 58 (9 vaccine types) | 54 (4.5) | 55 (5.5) | 33 (3.7) | 0.18 |
| HPV 16 and/or 18 and/or 31 and/or 33 and/or 45 and/or 52 and/or 58 (high-risk types within the 9 vaccine types) | 41 (3.4) | 28 (2.8) | 20 (2.2) | 0.73 |
| Multiple HPV types | 10 (1.1) | 18 (1.5) | 10 (1.0) | 0.53 |
| HPV 16 and other types | 1 (0.1) | 7 (0.6) | 2 (0.2) | 0.17 |
| Multiple high-risk types | 3 (0.3) | 8 (0.7) | 7 (0.7) | 0.52 |
| Both high-risk and low risk-types | 3 (0.3) | 8 (0.7) | 6 (0.6) | 0.62 |
p-values were corrected for multiple comparisons using Benjamini and Hochberg method.
Table 3.
Oral HPV genotype distribution by country in the HIM study oral subcohort
| Brazil (N = 1,198) n (%) | Mexico (N = 1,005) n (%) | USA (N =895) n (%) | p-value1 | |
|---|---|---|---|---|
| High-risk HPV types | ||||
| HPV 16 | 20 (1.7) | 18 (1.8) | 14 (1.6) | 0.93 |
| HPV 18 | 8 (0.7) | 5 (0.5) | 2 (0.2) | 0.39 |
| HPV 31 | 2 (0.2) | 4 (0.4) | 2 (0.2) | 0.61 |
| HPV 33 | 1 (0.1) | 0 (0.0) | 4 (0.5) | 0.04 |
| HPV 35 | 1 (0.1) | 3 (0.3) | 4 (0.5) | 0.23 |
| HPV 39 | 3 (0.3) | 15 (1.5) | 4 (0.5) | <0.01 |
| HPV 45 | 2 (0.2) | 5 (0.5) | 1 (0.1) | 0.26 |
| HPV 51 | 9 (0.8) | 15 (1.5) | 7 (0.8) | 0.16 |
| HPV 52 | 10 (0.8) | 5 (0.5) | 7 (0.8) | 0.61 |
| HPV 56 | 10 (0.8) | 4 (0.4) | 3 (0.3) | 0.28 |
| HPV 58 | 3 (0.3) | 3 (0.3) | 1 (0.1) | 0.80 |
| HPV 59 | 2 (0.2) | 3 (0.3) | 2 (0.2) | 0.89 |
| HPV 68 | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0.61 |
| Low-risk HPV types | ||||
| HPV 6 | 13 (1.1) | 13 (1.3) | 4 (0.5) | 0.15 |
| HPV 11 | 2 (0.2) | 6 (0.6) | 0 (0.0) | 0.03 |
| HPV 34 | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a |
| HPV 40 | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a |
| HPV 42 | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a |
| HPV 43 | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0.29 |
| HPV 44 | 2 (0.2) | 2 (0.2) | 1 (0.1) | 1.00 |
| HPV 53 | 9 (0.8) | 4 (0.4) | 6 (0.7) | 0.55 |
| HPV 54 | 4 (0.3) | 0 (0.0) | 0 (0.0) | 0.04 |
| HPV 66 | 13 (1.1) | 4 (0.4) | 10 (1.1) | 0.14 |
| HPV 70 | 0 (0.0) | 1 (0.1) | 1 (0.1) | 0.53 |
| HPV 74 | 9 (0.8) | 5 (0.5) | 6 (0.7) | 0.76 |
p-values were corrected for multiple comparisons using Benjamini and Hochberg method.
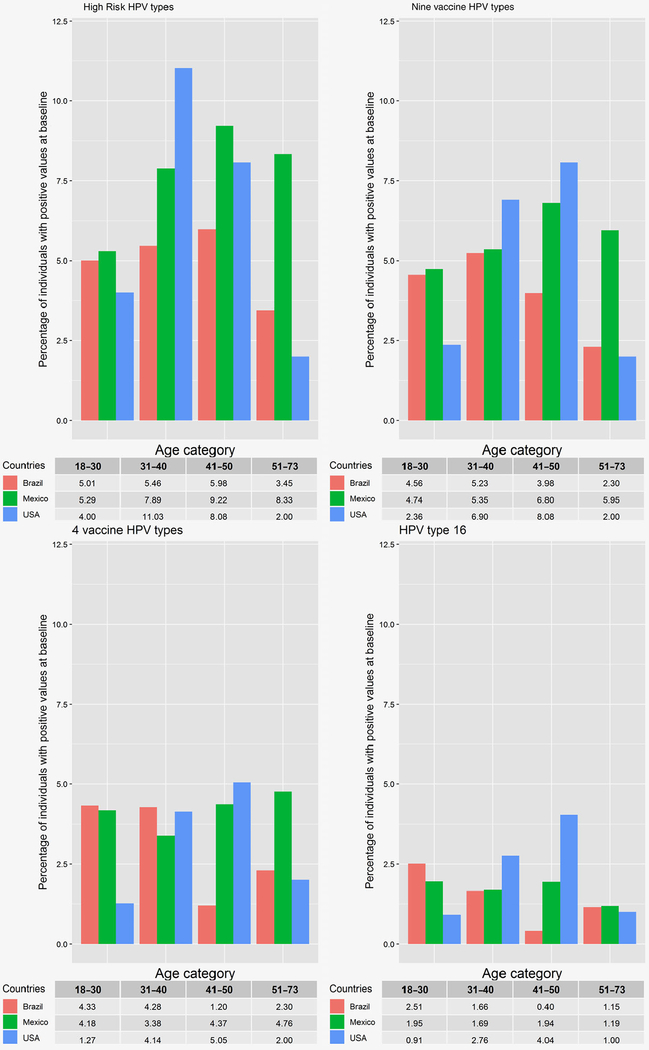
Figure 1.
Age-specific oral HPV prevalence by country in the HIM study oral cohort. Interaction between age group and country of residence was significant for high-risk HPV at 31–40 years, and 9 vaccine HPV types, 4 vaccine HPV types, and HPV 16 at 41–50 years age group.
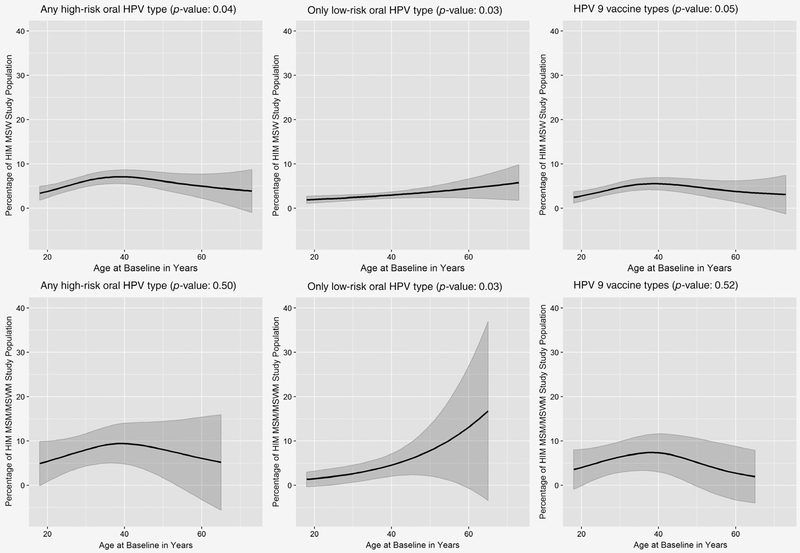
Figure 2.
Oral HPV prevalence by age and sexual orientation among MSW (men having sex with women) and MSM (men having sex with men)/MSWM (men having sex with women and men) in the HIM study oral cohort. Interaction between smoothed age and sexual orientation was not statistically significant for high-risk, low-risk or the nine-vaccine HPV types.
Data availability
Data available on request due to privacy/ethical restrictions.
Results
Of 3,166 men included in our study, 3,098 (97.9%) had oral gargle specimens that were adequate for HPV DNA analyses (1,198 from Brazil, 1,005 from Mexico and 895 from the USA). The distribution of sociodemographic and sexual behavior characteristics (Table 1) varied significantly by country (p < 0.01). Among USA participants, the majority (61.5%) were between 18 and 30 years compared to 36.6% of Brazilian and 35.7% of Mexican participants (Table 1). Approximately 19% of Brazilian participants reported having sex with men (MSM) or men and women (MSMW) compared to 5% of Mexican and USA participants. The distribution of lifetime number of sex partners differed significantly by country, with 35.7, 9.7 and 22.7% of the participants in Brazil, Mexico and USA, respectively, reporting more than 19 sexual partners during their lifetime.
Overall, oral HPV prevalence was low and did not significantly differ by country (Table 2). However, variations in oral HPV prevalence were observed with high-risk oral HPV prevalence highest among Mexican (7.3%) and lowest among the Brazilian (5.3%) participants. Apart from this, the prevalence of any HPV type, low-risk HPV, 4vHPV types, 9vHPV types and grouped HPV 16,18, 31, 33, 45, 52 and 58 types was highest among Mexican participants (10.0, 3.4, 4.0, 5.5 and 2.8%, respectively) and lowest among USA participants (7.6, 2.6, 2.2, 3.7 and 2.2%, respectively). Approximately 1.0% (Brazil and USA) to 1.5% (Mexico) of participants had multiple oral HPV type infections. Only 1 of 20 HPV 16 positive subjects (5.0%) among Brazilian men was positive for other types whereas 7 of 18 (38.9%) HPV 16 positive men from Mexico and 2 of 14 (14.3%) HPV 16 positive men from the USA had concurrent infections with other HPV types. Approximately 0.3–0.7% of the study population in each country had infections with multiple HPV types, or with both high-risk and low-risk types.
HPV 16 was the most commonly detected HPV with no significant difference between countries; prevalence ranged between 1.6 and 1.8% (Table 3). The only HPV types that differed significantly by country were HPV types 33, 39, 11 and 54; however, the prevalence of these types was low (≤1.5% in any country). HPV types 34, 40 and 42 were not detected among study participants.
The age-specific oral HPV prevalence by country is presented in Figure 1. High-risk HPV prevalence peaked at ages 41–50 years among Brazilian (9.2%) and Mexican (6.0%) participants and at ages 31–40 years among US participants (11.0%). 9vHPV prevalence peaked at ages 31–40 years among Brazilian participants (5.2%) and at ages 41–50 years among Mexican (6.8%) and US (8.1%) participants. 4vHPV prevalence peaked at ages 18–40 years for Brazilian men (4.3%), at ages 51–73 years among Mexican men, and at ages 41–50 years among US men (5.1%). Oral HPV 16 prevalence peaked at ages 18–30 years among Brazilian participants (2.5%), at ages 18–30 years and 41–50 years among Mexican participants (1.9%) and at ages 41–50 years among the US participants (4.0%). Interaction between age group and country of residence was significant for high-risk HPV at 31–40 years and 9 vaccine HPV types, 4 vaccine HPV types and HPV 16 at 41–50 years age group.
Overall, no significant difference in oral high-risk, low-risk, of 9vHPV prevalence was observed by sexual orientation. Among MSW participants, age was significantly associated with any high-risk and only low-risk HPV while among MSM/MSWM participants, age was significantly associated with only low-risk HPV (Figure 2). The peak high-risk HPV prevalence was 7.1 and 9.4% among MSW and MSM/MSWM participants, respectively, both at approximately 40 years of age. The peak low-risk HPV prevalence was 5.8 and 16.7% among MSW and MSM/MSWM participants respectively, both at the oldest age groups. The peak 9vHPV prevalence was 5.5 and 7.4% among MSW and MSM/MSWM participants, respectively, both at approximately 38 years of age. Overall, the age-patterning of high-risk, low-risk and 9vHPV was similar between MSW and MSM/MSWM participants and the association between age and high-risk, low-risk and 9vHPV did not differ significantly between MSW and MSM/MSWM participants.
Discussion
The HIM study is the first study to compare oral HPV prevalence among healthy men residing in three countries using data and specimens collected and analyzed with a single protocol. Overall, any oral HPV prevalence was ~10% among participants from Brazil, Mexico, and the USA and did not differ by country. Oral HPV prevalence age-patterning did not differ by sexual orientation although MSM/MSWM had higher peak-prevalence of low-risk HPV than MSW. The peak oral HPV prevalence among USA HIM Study participants preceded the age of peak US OPC incidence rate19 by approximately 20 years, similar to the time course observed for the natural history of cervical HPV and cervical cancer incidence.
Oral HPV prevalence was low with a range of 7.6–10.0% which is in sharp contrast to a prevalence of approximately 60% detected at the external genitals20 and a prevalence of approximately 15% at the anal canal23 in the HIM study. Any oral HPV prevalence among USA participants in our study (7.6%) was also lower than the 10.1% estimate reported from NHANES 2009–2010.11 Differences in estimates of oral HPV among US men may be due to differences in the age distribution of the HIM study and the NHANES population: 61.3% of the USA participants in the HIM study were aged between 18 and 30 years, an age group with low oral HPV prevalence, compared to 26.3% of the NHANES participants.
The importance of estimating age-specific oral HPV prevalence, as we presented here, is highlighted in the comparison of oral high-risk HPV prevalence in male participants of NHANES (2009–2010 and 2011–2012 cycles) and the HIM Study. In NHANES,19 oral high-risk HPV prevalence peaked at 25–30 years and again at 55 years with a maximum prevalence of approximately 6%. In the US HIM cohort, high-risk HPV prevalence peaked in the 31–40 years age group where the prevalence exceeded 11%. Differences in the age-specific oral HPV estimates across studies may be due to greater inclusion of high risk men in the HIM Study than NHANES or use of more sensitive SPF10 PCR-DEIA-LiPA25 system for HPV genotyping in the HIM Study compared to the Linear Array assay utilized in NHANES (Manuscript undergoing peer review).24
Several oral HPV meta-analyses have been recently published,25–27 some of which examined the association between age and oral HPV prevalence. However, these analyses utilized data collected using different HPV DNA genotyping protocols, different underlying age structures, did not carefully assess same-sex behavior, and were prone to ecologic bias. In the current study, we observed oral HPV prevalence to vary by age, HPV type and country using individual-level data collected with a single protocol.
This is the first report assessing the age-patterning of oral HPV prevalence separately among MSW and MSM/MSWM using data collected with a single protocol and oral samples evaluated using the same procedure. The peak high-risk HPV prevalence was 7.1 and 9.4% among MSW and MSM/MSWM participants respectively, both at approximately 40 years of age. Previous studies measuring high-risk oral HPV among MSM reported prevalence estimates of 8.8% among HIV uninfected and 24.8% among HIV infected men in the Netherlands28 and 5.9% among HIV uninfected men in London, UK.29 The peak prevalence among MSM/MSWM participants from our study was higher (~10%) than the estimates among HIV uninfected MSM participants in these studies. However, the age-specific prevalence was not examined in these publications; therefore, it is difficult to compare estimates from our current study to theirs. Given the findings from our study, more studies with larger sample sizes are needed to examine age-oral HPV prevalence associations among individuals with different sexual orientations.
A major strength of our study is the collection of demographic, behavioral and oral gargle data from men from three countries, belonging to a broad range of ages, using the same protocol. Our study design reduced the potential for measurement error and enabled comparison of oral HPV prevalence among men from these countries. Although our study may lack generalizability to the underlying target population, the race and ethnicity distribution in our individual study site populations resembled the male populations of the countries in which the sites were situated.30 One limitation of our study is the limited sample size of MSM/MSWM which gave rise to large standard errors in the penalized regression spline models, and hence, the results should be interpreted with caution.
It is clear that age-specific oral HPV prevalence varies widely by country and types of HPV examined. This highlights the need for additional studies of oral HPV that include a broad age range utilizing sensitive methods for HPV genotyping, across multiple countries. More studies need to be conducted regarding acquisition and transmission of oral HPV infections, especially in light of increasing incidence of OPCs attributable to HPV, which has already surpassed cervical cancer in some countries such as the USA: OPC incidence rate among men (8.3 per 100,000) is higher than cervical cancer incidence among women (7.2 per 100,000).5 There is a lack of information regarding mode of transmission and whether this varies by age group in different regions of the world. Given the increasing global incidence of HPV-attributable OPCs, information regarding oral HPV natural history across multiple global regions is needed to inform effective preventive interventions.
What’s new?
This study reports the prevalence of oral human papillomavirus among 3,098 HPV Infection in Men (HIM) Study participants residing in Brazil, Mexico and the United States. In this study, we show that although sexual behaviors varied widely across countries, oral HPV prevalence did not significantly differ by country.
Footnotes
Conflict of interest: A.R.G. is a member of the Merck Advisory Board and her institution has received funding for research through a Merck Investigator-initiated studies program. L.L.V. is a consultant to Merck for the HPV prophylactic vaccine and provides occasional consultancy to BD, Roche and Qiagen concerning HPV tests.
References
- 1.Zur Hausen H Condylomata acuminata and human genital cancer. Cancer Res 1976;36:794. [PubMed] [Google Scholar]
- 2.IARC. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France: IARC, 2004. [Google Scholar]
- 3.Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol 2005;6:204. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine 2006;24:S11–25. [DOI] [PubMed] [Google Scholar]
- 5.Van Dyne EA, Henley SJ, Saraiya M, et al. Trends in human papillomavirus–associated cancers—United States, 1999–2015. Morb Mortal Wkly Rep 2018; 67:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 2009;125:362–6. [DOI] [PubMed] [Google Scholar]
- 7.Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003;95:1772–83. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viens LJ. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep 2016;65:661–6. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012;307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickard RK, Xiao W, Broutian TR, et al. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sex Transm Dis 2012;39:559–66. [DOI] [PubMed] [Google Scholar]
- 13.Anaya-Saavedra G, Ramírez-Amador V, Irigoyen-Camacho ME, et al. High association of human papillomavirus infection with oral cancer: a case-control study. Arch Med Res 2008;39:189–97. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Losa MR, Barrera ES, Herrera-Pech V, et al. Epidemiology of oral HPV in the oral mucosa in women without signs of oral disease from Yucatan, Mexico. Braz J Microbiol 2015;46: 301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matos LL, Miranda GA, Cernea CR. Prevalence of oral and oropharyngeal human papillomavirus infection in Brazilian population studies: a systematic review. Braz J Otorhinolaryngol 2015;81:554–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer 2017;140:1968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betiol JC, Sichero L, Costa HOO, et al. Prevalence of human papillomavirus types and variants and p16(INK4a) expression in head and neck squamous cells carcinomas in Sao Paulo, Brazil. Infect Agent Cancer 2016;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro KB, Levi JE, Pawlita M, et al. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol 2011;40: 489–502. [DOI] [PubMed] [Google Scholar]
- 19.Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev 2008;17:2036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodology 2011;73:3–36. [Google Scholar]
- 22.Rose NL, Yang H, Turner SD, et al. An assessment of the mechanisms for the transfer of lead and mercury from atmospherically contaminated organic soils to lake sediments with particular reference to Scotland, UK. Geochim Cosmochim Acta 2012;82:113–35. [Google Scholar]
- 23.Sudenga SL, Nyitray AG, Torres BN, et al. Comparison of anal HPV natural history among men by country of residence: Brazil, Mexico, and the United States. J Infect 2017;75:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettampadi D, Sirak BA, Fulp WJ, et al. Oral HPV prevalence assessment by Linear Array vs. SPF10 PCR-DEIA-LiPA25 system in the Human Papillomavirus Infection in Men (HIM) study. J Clin Microbiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mena M, Taberna M, Monfil L, et al. Might oral human papillomavirus (HPV) infection in healthy individuals explain differences in HPV-attributable fractions in oropharyngeal cancer? A systematic review and meta-analysis. J Infect Dis 2018;219:1574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shigeishi H, Sugiyama M. Risk factors for oral human papillomavirus infection in healthy individuals: a systematic review and meta-analysis. J Clin Med Res 2016;8:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tam S, Fu S, Xu L, et al. The epidemiology of oral human papillomavirus infection in healthy populations: a systematic review and meta-analysis. Oral Oncol 2018; 82:91–9. [DOI] [PubMed] [Google Scholar]
- 28.Mooij SH, Boot HJ, Speksnijder AG, et al. Oral human papillomavirus infection in HIV-negative and HIV-infected MSM. AIDS (London, England) 2013;27:2117–28. [DOI] [PubMed] [Google Scholar]
- 29.King EM, Gilson R, Beddows S, et al. Oral human papillomavirus (HPV) infection in men who have sex with men: prevalence and lack of anogenital concordance. Sex Transm Infect 2015; 91:284–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreimer AR, Villa A, Nyitray AG, et al. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomarkers Prev 2011;20:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.