Abstract
Social relationships among spouses, family members, and friends are known to affect physical and mental health. In particular, long-lasting bonds between socio-sexual partners have profound effects on cognitive, social, emotional, and physical well-being. We have previously reported that pair bonding in monogamous prairie voles (Microtus ochrogaster) is prevented by a single prolonged stress (SPS) paradigm, which causes behavioral and endocrine symptoms resembling post-traumatic stress disorder (PTSD) patients in rats (Arai et al., 2016). Since fear memory function is crucial for anxiety-related disorders such as PTSD, we investigated the effects of pair bonding on fear learning in prairie voles. We applied an SPS paradigm to male prairie voles after the cohabitation with a male (cage-mate group) or female (pair-bonded group). The cage-mate group, but not the pair-bonded group, showed enhanced fear response in a contextual fear conditioning test following the SPS treatment. Immunohistochemical analyses revealed that cFos-positive cells in the central amygdala were increased in the pair-bonded group after the contextual fear conditioning test and that oxytocin immunoreactivity in the paraventricular nucleus of the hypothalamus was significantly higher in the pair-bonded group than the cage-mate group. This pair-bonding dependent blunting of fear memory response was confirmed by a passive avoidance test, another fear-based learning test. Interestingly, intracerebroventricular injection of an oxytocin receptor antagonist 30 min before the passive avoidance test blocked the blunting effect of pair bonding on fear learning. Thus, pair bonding between socio-sexual partners results in social buffering in the absence of the partner, blunting fear learning, which may be mediated by oxytocin signaling.
Keywords: Central amygdala, Fear conditioning, Lateral ventricle, PVN, Cannulation, Pair bonding
1. Introduction
Affiliative social relationships, such as among spouses, family members, and friends, are essential for human well-being and affect psychological, physiological, and behavioral functions. Positive social relationships reduce health problems including cardiovascular diseases, asthma, and infection, whereas the disruption of social bonds has negative effects on physical and mental health (Lieberwirth and Wang, 2014). In particular, enduring bonds between socio-sexual partners appear to have profound impacts on the cognitive, social, emotional, and physical well-being (Lieberwirth and Wang, 2014). However, the neurobiological mechanisms underlying the health promoting effects of positive social bonds are unknown.
We have used a socially monogamous rodent, the prairie vole (Microtus ochrogaster), to investigate relationships between social bonding and symptoms relevant to neuropsychiatric disorders. Prairie voles show pair bonding between mates and biparental care of off-spring. Pair bonding is assessed in the laboratory using a partner preference test, in which a bonded male prairie vole typically spends significantly longer time huddling with the partner female than with a novel female vole (Aragona and Wang, 2004). Using pharmacological, molecular biological, and immunohistochemical techniques combined with this test, the neuropeptides vasopressin and oxytocin, as well as dopamine have been shown to have critical roles in the formation and maintenance of pair bonds in prairie voles (Lieberwirth and Wang, 2016; Walum and Young, 2018). Recently, we have shown that exposure to a single prolonged stress (SPS) paradigm disturbs the formation of pair bond in prairie voles (Arai et al., 2016). After male prairie voles were subjected to a SPS paradigm, which is composed of restraint in a narrow tube for 2 h, forced swimming, and anesthesia until unconsciousness (Liberzon and Young, 1997), they were housed without any handling for 7 days to recover from the SPS paradigm. Then, SPS-treated male voles were cohabited with a female for 7 days, a duration which is known to be long enough for pair bonding, however, they did not prefer to huddle with their partner females. SPS-treated rats mimic pathophysiological abnormalities and behavioral characteristics of patients of post-traumatic stress disorder (PTSD), including enhanced negative feedback of the hypothalamic–pituitary–adrenal (HPA) axis and enhanced anxiety-like behaviors (Yamamoto et al., 2009; Lisieski et al., 2018).
Our previous report indicates that stressful stimuli such as the SPS paradigm negatively affect pair bonding of prairie voles. In the current study, we aimed to clarify the effect of pair bonding on fear learning and memory. Fear learning and memory functions appear to have crucial roles in the pathogenesis, symptomatology, and treatment of fear and anxiety-related disorders, including PTSD, phobias, and anxiety disorders (de Quervain et al., 2017; Javanbakht, 2019). Numerous studies have shown that higher social support is associated with reduced PTSD symptom severity in combat veterans, disaster survivors, and victims of sexual assault (Pietrzak et al., 2009; Paul et al., 2015; Borja et al., 2006). In romantic relationships, more anxiously and avoidantly attached individuals experience heightened psychological and physiological responses to stressful situations (Stanton and Campbell, 2014). In anticipation of a public-speaking in front of an audience, men receiving support from their romantic partner show attenuation of cortisol responses compared with ones receiving support from an opposite-sex stranger (Kirschbaum et al., 1995).
Here, we show that cohabitation with a female and pair-bonding in monogamous male prairie voles blunted fear learning responses using contextual fear conditioning and passive avoidance tests. The number of cFos-positive cells was increased in the central amygdala (CeA) after the memory test of the contextual fear conditioning. Finally, the intracerebroventricular administration of oxytocin receptor antagonist recovered fear learning despite pair bonding. Together with the fact that oxytocin immunoreactivity was increased in the paraventricular nucleus (PVN) of pair bonded voles, our results suggest that cohabitation with an opposite sex partner with pair bonding inhibits fear learning through oxytocin signaling.
2. Methods
2.1. Animals
All animals were maintained and bred in the Bioresource Center of Gunma University Graduate School of Medicine. Four–seven prairie voles (Microtus ochrogaster) were housed with same sex individuals in a polycarbonate standard cage (24 × 40 × 15 cm) with wood chips bedding (white flake, Oriental Yeast Co., Ltd., Tokyo, Japan) after weaning at 3–4 week-old. They were provided food (standard rabbit chow LRC4, Oriental Yeast Co., Ltd.) and water ad libitum. The housing and experimental rooms were maintained on standard laboratory conditions (a 14:10 h light/dark cycle (lights on at 8:00), 23 °C). Adult male prairie voles (115.15 ± 27.44 days-old) were used as experimental subjects. All animal experiments conformed to the Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan, and approved by the Animal Experiment Committee at Gunma University.
2.2. Partner preference test
Partner preference tests were performed according to our previous report (Arai et al., 2016). In brief, the apparatus consisted of three transparent acrylic chambers of the same size (20 × 25 × 20 cm) with wood chip bedding. Two chambers were placed in front of a center chamber and were connected to the center chamber with two hollow tubes (6 cm diameter × 10 cm), so that male subjects were able to move freely to any chamber. A female prairie vole which was previously cohabited with the subject prairie vole (called the “partner”) and a novel “stranger” female were tethered to the right or left chamber. Each female was used twice, once as the partner in her own pair and again as the stranger in another pair, so that both partner and stranger had the same sociosexual experience prior to testing. The position of partner and stranger female voles was counterbalanced among tests. The male subject was placed in the center chamber and was allowed to freely investigate to all chambers for 3 h. The apparatus was thoroughly cleaned with 70% ethyl alcohol between every test. The duration of huddling behavior (close physical contact with side-by-side positioning) with each female was quantified. The partner preference index was calculated using the following formula; the partner preference index = (time huddling with the partner female − the time huddling with a stranger female) / (the sum of the time huddling with both the partner and stranger females).
For subjects in the cage-mate group, two stranger female voles were presented for 3 h as above in order to unify the condition of inter-sexual interaction during the experimental period. The partner preference index was not calculated for this group since both females were novel.
2.3. Single prolonged stress (SPS)
An SPS paradigm with a modification for prairie voles was performed according to our previous report (Arai et al., 2016). Subjects were restrained in a 50 ml conical tube for 2 h and then subjected to forced swimming in water at 23 °C for 5 min. They recuperated for 15 min in their home cages and then were anesthetized using diethyl ether until they lost consciousness. Sham-treated animals experienced only handling. Subjects were individually housed without disturbance for 6 days after the SPS procedure.
2.4. Contextual fear conditioning test
A chamber with gray-colored acrylic walls (18.5 × 18.5 × 16 cm, MSK-001, Muromachi Kikai Co., LTD., Tokyo, Japan) was connected with a shock generator scrambler (SGS-003DX; Muromachi Kikai Co., Ltd.) and placed under 400 lx of illumination. Behaviors were recorded by a video camera (GZ-MG575, JVC Kenwood Co., Yokohama, Japan). The apparatus was cleaned with 70% ethyl alcohol after every test.
In Experiment 1, each prairie vole was placed in the light chamber of the apparatus (pre-conditioning). Two electric shocks (0.4 mA, 2 s) were delivered at 3 min and 4 min after the subject was placed in the chamber (conditioning). The subject was returned to the home cage 1 min after the last electric shock. On the following day, the subject vole was again placed into the apparatus for 5 min without electric shocks (post-conditioning, Fig. 1A). The duration of freezing behavior during the first 3 min was manually quantified by an observer blind to the experimental group of the subject. Freezing was defined as the total absence of body movement except for movements associated with breathing.
Fig. 1.
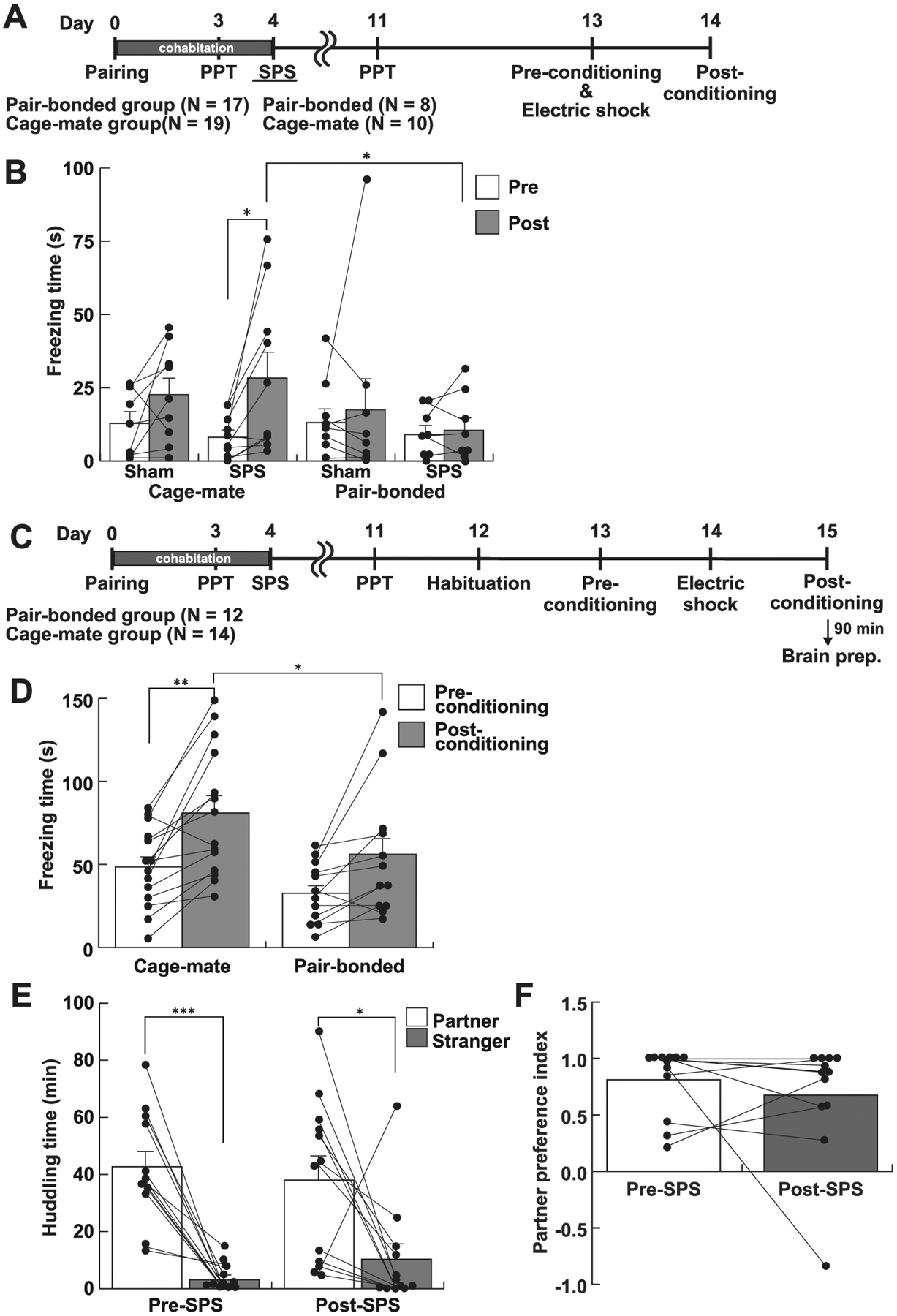
Pair bonding blunted fear learning. A, The experimental schedule of Experiment 1. Subjects were cohabited with a naïve female vole or male for 4 days. A partner preference test (PPT) was performed at the Day 3. The SPS treatment was carried out at the Day 4. The contextual fear conditioning test was performed at the Day 13 and 14. B, Freezing time at the pre- and post-conditioning sessions. Only SPS – cage-mate group showed significantly longer freezing time in post-conditioning than pre-conditioning session. In SPS groups, the freezing time in the cage-mate group in the post-conditioning session was significantly longer than that in the pair-bonded group. C, The experimental schedule of Experiment 2. A contextual fear conditioning test was performed between the Day 12 and 15. D, Freezing time in the contextual fear conditioning test. The cage-mate group significantly prolonged freezing time in the post-conditioning session compared to the pre-conditioning session. In contrast, the pair-bonded group did not show prolonged freezing in the post-conditioning session. In the post-conditioning session, the pair-bonded group showed shorter freezing duration than the cage-mate group. E, Huddling time in the partner preference test before and after the SPS paradigm. SPS-treated male voles still preferred to huddle with their partner rather than a stranger female. F, The partner preference index was not changed between pre- and post- SPS phase, although one subject preferred to huddle with a stranger female than the partner. White and gray bars represent mean values + standard error. *, p < 0.05; **, p < 0.01.
In Experiment 2, we quantified freezing duration with a slight modification of the test (Fig. 1C), since subjects showed the relatively short duration of freezing (< 30 s in a 180 s session) in Experiment 1. Each prairie vole was placed in the chamber for 5 min to acclimate to the apparatus on the first day (habituation). The next day, the subject was again placed in the chamber (pre-conditioning). On the following day, electric shocks (0.4 mA, 2 s) were delivered at 3 min and 4 min. The subject was returned to the home cage at 5 min. The subject was placed in the chamber without electric shocks for 5 min 24 h after the electric shocks (post-conditioning). The duration of freezing behavior during a 5 min test period in the pre- and post- conditioning session was quantified as above.
2.5. Passive avoidance test
The passive avoidance test was performed using a Step - Through Test system (STC-001M, Muromachi Kikai Co., Ltd., a dark box, 14 × 17.5 × 15 cm, 0 lx; a light box, 9 × 11.5 × 15 cm, 400 lx). Subjects were individually placed in the light box. The sliding door was opened 30 s later, so that the subject was allowed to investigate both dark and light boxes for 4.5 min (habituation). The next day, each subject was placed in the light box again as above. When the subject entered into the dark box (pre-conditioning), the sliding door was closed, and then two electronic shocks (0.4 mA, 2 s) were delivered with an inter-stimulus interval of 1 min. Subjects were returned to the home cage 10 s after the last electronic shock. Twenty four hours later, a subject vole was placed in the apparatus for 5 min without electric shocks (memory test). To investigate the extinction of the fear memory, each subject vole was placed in the apparatus for the memory test for the following 3 consecutive days (Fig. 5A). The latency to enter the dark box was manually measured by a blind observer.
Fig. 5.

Pair bonding ameliorated freezing response in a passive avoidance paradigm. A, The experimental schedule of Experiment 3. A passive avoidance test was performed from the Day 6 to 8 followed by the extinction test (Ext). B, Partner preference test confirmed pair bonding. C, The latency to enter the dark box in the passive avoidance test. At the memory test, there was significant difference in the latency between cage-mate and pair-bonded groups. The latency in the cage-mate group was significantly reduced during consecutive 3 days of the extinction. Bars represent mean values + standard error. *, **, significant difference vs the pre-conditioning session of the same experimental group with p < 0.05 and p < 0.01, respectively. †, ††, significant difference vs the memory session of the cage-mate group with p < 0.05 and p < 0.01, respectively.
2.6. Cannula implantation and intracerebroventricular (icv) injection
According to a previous report (Bosch et al., 2009), a 22 G guide cannula (C313GS-5-SP, Plastics One; Roanoke, VA) was implanted to the right lateral ventricle (AP +0.6 mm; ML +1.3 mm; DV −3.0 mm) under isoflurane anesthesia. After surgery, all subjects were housed undisturbed for 4 days for recovery.
Intracerebroventricular (icv) injection was performed according to Wei et al. (2015). Two μl of artificial cerebrospinal fluid (aCSF, Tocris Bioscience, Minneapolis, MN) or a selective oxytocin receptor antagonist (desGly-NH2, d(CH2)5[Tyr (Me) 2, Thr4] OVT; 0.25 μg/μl in aCSF, generous gift from Dr. Manning, University of Toledo, Manning et al., 2012) was injected at a flow rate of 0.66 μl/min. The internal cannula was left in place for 1 min following injection. Then, the guide cannula was closed with a dummy cannula.
2.7. Experiment procedure
All behavioral tests were performed during the light phase (9:00–15:00). Prairie voles exhibit acyclic activity patterns unlike mice and rats which are nocturnal. Prairie voles show general locomotion, eating and drinking, sleeping, and grooming similarly during light and dark phases (Baumgardner et al., 1980; Grippo et al., 2007).
2.7.1. Experiment 1
To investigate the effect of pair bonding on fear memory in prairie voles, a contextual fear conditioning test was performed 9 days after the SPS administration (Fig. 1A). Each male subject was cohabited with a male (cage-mate group; N = 19) or an unfamiliar naïve female (pair-bonded group; N = 17) for 4 days. The formation of a pair bond was confirmed using a partner preference test on the Day 3. To make the social stimulation uniform, subjects in the cage-mate group were individually presented with two unfamiliar females in the partner preference test arena for 3 h. On the following day, prairie voles in each group were randomly divided into two groups. Subjects in one group were administrated the SPS paradigm, whereas those in the other group were given only handling as a sham treatment. Hence, subjects were randomly divided into 4 experimental groups; SPS – cage-mate group, N = 10; SPS – pair-bonded group, N = 8; sham – cage-mate group, N = 9; sham – pair-bonded group, N = 9. A contextual fear conditioning test was carried out on the Day 13 and 14.
2.7.2. Experiment 2
To confirm the blunting of fear learning by pair bonding and to investigate the effect of SPS on pair bonding, a partner preference test and a contextual fear conditioning test were performed after the SPS administration (Fig. 1C). Each male prairie vole was cohabited with a male (cage-mate group; N = 14) or an unfamiliar naïve female (pair-bonded group; N = 12) for 4 days. To confirm pair bond formation, a partner preference test was performed on the Day 3. The SPS paradigm was applied to subject voles on the Day 4. After the SPS paradigm, subjects were individually housed for 7 days undisturbed. Again, a partner preference test was performed on the Day 11. The contextual fear conditioning test was performed from the Day 12 to 15.
Ninety minutes after the post-conditioning session of the contextual fear conditioning test, all subjects were deeply anesthetized with mixed anesthetic agents (0.3 mg/kg medetomidine, 4.0 mg/kg midazolam, 5.0 mg/kg butorphanol, Kawai et al., 2011) and immediately perfused transcardially with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and 15.6% saturated picric acid. Brains were removed, soaked in the same solution at 4 °C for 18 h, and stored in 20% sucrose in 0.1 M phosphate buffer (pH 7.4) at 4 °C.
Immunohistochemical staining was performed according to our previous report (Arai et al., 2016). Briefly, brains were sectioned at 30 μm using a cryostat (CM3050S, Leica Biosystems GmbH, Nussloch, Germany), were collected at every 180 μm, and were stored in cryoprotectant (0.1 M phosphate buffer, pH 7.4, 0.9% NaCl, 30% sucrose, 1% polyvinylpyrrolidone, 30% ethylene glycol) at −20 °C until use. After incubating in PBS containing 0.7% H2O2 and 40% methanol for 15 min, sections were washed with PBS containing 0.3% Triton X-100 (PBS-T) for 10 min three times and incubated with rabbit anti- cFos antibody (SC-52, Santa Cruz Biotechnology Inc., Dallas, TX, 1:1000 dilution) or rabbit anti-oxytocin antibody (#20068, ImmunoStar Inc., Hudson, WI, 1: 10,000 dilution) in PBS-T containing 1% bovine serum albumin for 18 h at room temperature. After washing, sections were incubated with biotinylated anti-rabbit IgG antibody (1:1000 dilution, Vector Laboratories Inc., Burlingame, CA) for 2 h. Then, sections were incubated with a complex of biotinylated peroxidase and avidin (1:1000 dilution, PK- 6100, Vector Laboratories Inc.) for 2 h. Immunoreactivity was detected by incubating in 0.25 mg/ml diaminobenzidine and 0.01% H2O2 in Tris-HCl (pH 7.5). All sections were mounted on glass slides (S8441, Matsunami Glass Ind., Ltd., Japan). Tissues were photographed using a microscope (ECLIPS 80i, Nikon Instruments Inc., Tokyo, Japan) combined with a CCD camera (DFC290, Leica Biosystems GmbH). Using Image J software (NIH), the number of cFos-positive cells in indicated brain regions was bilaterally counted (PVN, CeA, BLA, 4 sections; dBNST, vBNST, 2 sections), and the intensity of oxytocin-immunoreactivity in the paraventricular nucleus of the hypothalamus (PVN) on 3 sections was measured after black and white values were inverted.
2.7.3. Experiment 3
To verify the blunting effect of pair bonding on fear learning, a passive avoidance test, which is another behavioral test of fear-based learning, was performed (Fig. 5A). A separate cohort of male subjects were cohabited with a male (cage-mate group; N = 12) or an unfamiliar naïve female (pair-bonded group; N = 12) for 5 days. A partner preference test was performed on the Day 5. A passive avoidance test was carried out from the Day 6 to 8, which was followed by an extinction assay on the following three consecutive days.
One vole in the pair-bonded group and three in the cage-mate group were excluded from analysis because they showed outlier values for the latency to enter the dark box in the pre-conditioning session, so that we analyzed 11 and 9 subjects of pair-bonded and cage-mate groups, respectively. Outlier values were defined as values that were beyond the range between the value of the lower quantile minus 1.5 times the interquartile range and the value of the higher quantile plus 1.5 times the interquartile range.
2.7.4. Experiment 4
To investigate the involvement of oxytocin signaling in the pair bond-induced blunting of fear memory, an oxytocin receptor antagonist was injected icv before conditioning in a passive avoidance test (Fig. 6A). Each male vole was cohabited with an unfamiliar naïve female for 5 days. A partner preference test was performed on the Day 5 to confirm a partner preference. On the following day, 18 subjects were implanted a guide cannula. After surgery, voles were individually housed for 5 days. A passive avoidance test was performed daily from the Day 11 to 13. Two μl of oxytocin receptor antagonist, desGly-NH2, d(CH2)5[Tyr (Me) 2, Thr4] OVT dissolved in aCSF (N = 8) or aCSF (N = 6) was injected 30 min before conditioning. The icv injection was not applied to 4 subjects because the implanted cannula dropped off.
Fig. 6.
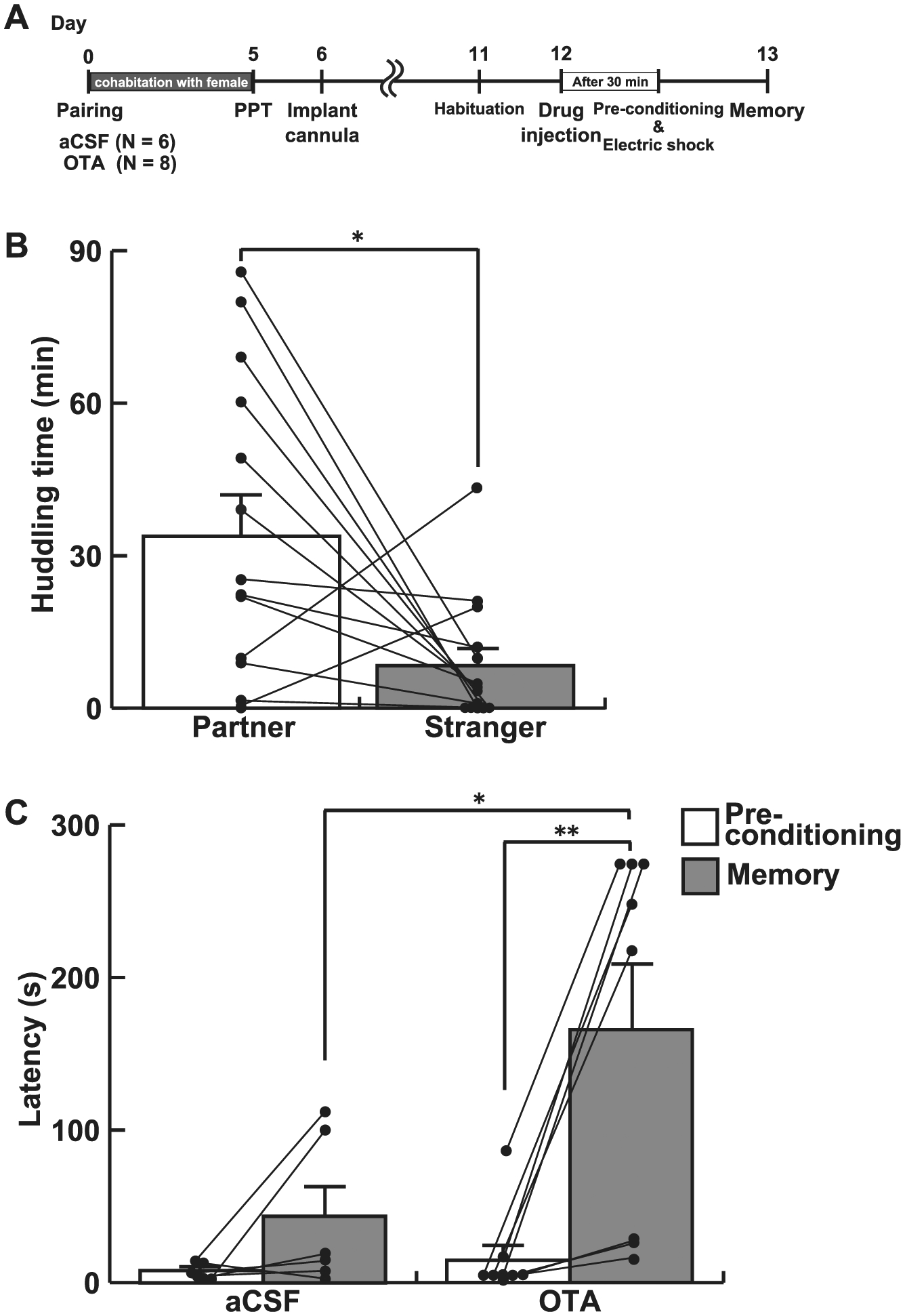
Icv infusion of an oxytocin receptor antagonist diminished the effect of pair-bonding on fear learning. A, The experimental schedule of Experiment 4. An oxytocin receptor antagonist was infused icv to pair-bonded male voles 30 min before the conditioning. B, Partner preference test confirmed pair bonding between subjects and their partners. C, The latency to enter the dark box. The latency was significantly longer in voles infused with an oxytocin receptor antagonist (OTA) in the memory test, compared with the pre-conditioning session. In contrast, a significant difference in latency was not detected between the pre-session and the memory test in aCSF-injected voles. Further, the latency in the memory test was significantly longer in voles injected an oxytocin receptor antagonist than aCSF-injected voles. Bars represent mean values + standard error. *, p < 0.05; **, p < 0.01.
2.8. Statistical analysis
Statistical analyses were performed using IBM SPSS statistics software (Version 24, IBM Corp., Armonk, NY). Data of the partner preference test and immunohistochemical staining was analyzed with Student’s t-tests. Contextual fear conditioning and passive avoidance tests were analyzed with two or three-way repeated measures analysis of variance (ANOVA) with Bonferroni’s post hoc tests for pairwise comparison. The data are presented as mean ± standard error. All tests were set with the significance level at p < 0.05.
3. Results
3.1. Pair bonding blunted fear learning in a contextual fear conditioning paradigm
In Experiment 1, both pair-bonded and cage-mate groups were subjected to the SPS paradigm 9 days before the contextual fear conditioning was performed (Fig. 1A). Statistical analysis using a three-way repeated measures of ANOVA showed a significant main effect of timing (Fig. 1B, pre-conditioning vs post-conditioning, F1, 32 = 7.70, p = 0.009, ηp2 = 0.194) without any other significant main effects or interaction (treatment, F1, 32 = 0.25, p = 0.624, ηp2 = 0.008; partner, F1, 32 = 1.15, p = 0.291, ηp2 = 0.035; the interaction between treatment and partner, F1, 32 = 0.35, p = 0.560, ηp2 = 0.011; timing and partner, F1, 32 = 3.51, p = 0.070, ηp2 = 0.099; timing and treatment, F1, 32 = 0.34, p = 0.562, ηp2 = 0.011). In the cage-mate group, only SPS-treated voles showed significantly prolonged freezing time at the post-conditioning session, compared to the pre-conditioning session (p = 0.015), whereas sham treated voles did not show a significant increase in freezing time at the post-conditioning (p = 0.252). Importantly, freezing duration was not increased in the pair-bonded group at the post-conditioning session regardless of the SPS-treatment (sham, p = 0.613; SPS, p = 0.869). Paired t-test also indicated that the pair-bonded group did not exhibit an increase in freezing duration the post-conditioning session (sham, T8 = 0.51, p = 0.626, Cohen’s d = 0.18; SPS, T7 = 0.57, p = 0.589, Cohen’s d = 0.15).
In Experiment 2, the quantification of fear response was more attentively performed for a 5 min session with larger sample sizes, since freezing duration in Experiment 1 was shorter than expected. A two-way repeated measures ANOVA revealed a significant main effect of timing (F1, 24 = 23.11, p < 0.001, ηp2 = 0.491), but not partner (F1, 24 = 3.80, p = 0.063, ηp2 = 0.137) or the interaction between timing and partner (F1, 25 = 0.79, p = 0.384, ηp2 = 0.032) in the contextual fear conditioning test (Fig. 1D). Post hoc analysis indicated that the cage-mate group showed significantly prolonged freezing in the post-conditioning session compared to the pre-conditioning session (p = 0.009) whereas the pair-bonded group showed only trend to increase freezing duration in the post-conditioning session (p = 0.089). Further, the pair-bonded group showed significantly shorter freezing behavior than the cage-mate group in the post-conditioning session (Fig. 1D, p = 0.038). When the difference of the latency between preand post- conditioning was analyzed by a paired t-test, the latency in the pair-bonded group in the post-conditioning session was significantly increased compared with that in the pre-conditioning session (T11 = 2.65, p = 0.023, Cohen’s d = 0.73), suggesting that the pair-bonded group still had the capacity for fear learning but that they showed a blunted fear response relative to the cage-mate group.
3.2. The pair bond formed during cohabitation was not disrupted by the subsequent SPS
After cohabitation for 5 days, male subject voles spent significantly longer time huddling with their partner females than with stranger females (T11 = 6.23, p < 0.001, Cohen’s d = 2.79, Fig. 1E left). Following the SPS-treatment they still showed the partner preference (T11 = 2.53, p = 0.028, Cohen’s d = 1.14, Fig. 1E right). The partner preference index showed that only 1 vole out of 12 subjects preferred to huddle with a stranger female rather than the partner female post-SPS (Fig. 1F).
3.3. Pair bonding affected the number of cFos-positive cells in the central amygdala as well as oxytocin immunoreactivity in the PVN
Although we detected significant differences between cage-mate and pair-bonded groups in the contextual fear conditioning test, the difference was not marked. Hence, we used immunohistochemistry in an attempt to identify potential neural differences between the groups in brain regions implicated in fear responses, such as the central and basolateral amygdala (CeA and BLA, respectively), dorsal and ventral bed nucleus of stria terminalis (dBNST and vBNST, respectively), and the PVN (Fig. 2, Hostinar et al., 2014; Martinon and Dabrowska, 2018; Maddox et al., 2019). The number of cFos-positive cells in the CeA was significantly increased in the pair-bonded group compared to the cage-mate group following the contextual fear conditioning test (Fig. 3, T23 = 3.18, p = 0.004, Cohen’s d = 1.27). However, there were no differences in the number of cFos-positive cells between two groups in the other regions (Fig. 3 right, BLA, T16 = 1.62, p = 0.124, Cohen’s d = 0.77; PVN, T24 = 1.80, p = 0.085, Cohen’s d = 0.71; dBNST, T15 = 0.73, p = 0.476, Cohen’s d = 0.36; vBNST, T16 = 0.13, p = 0.900, Cohen’s d = 0.06).
Fig. 2.
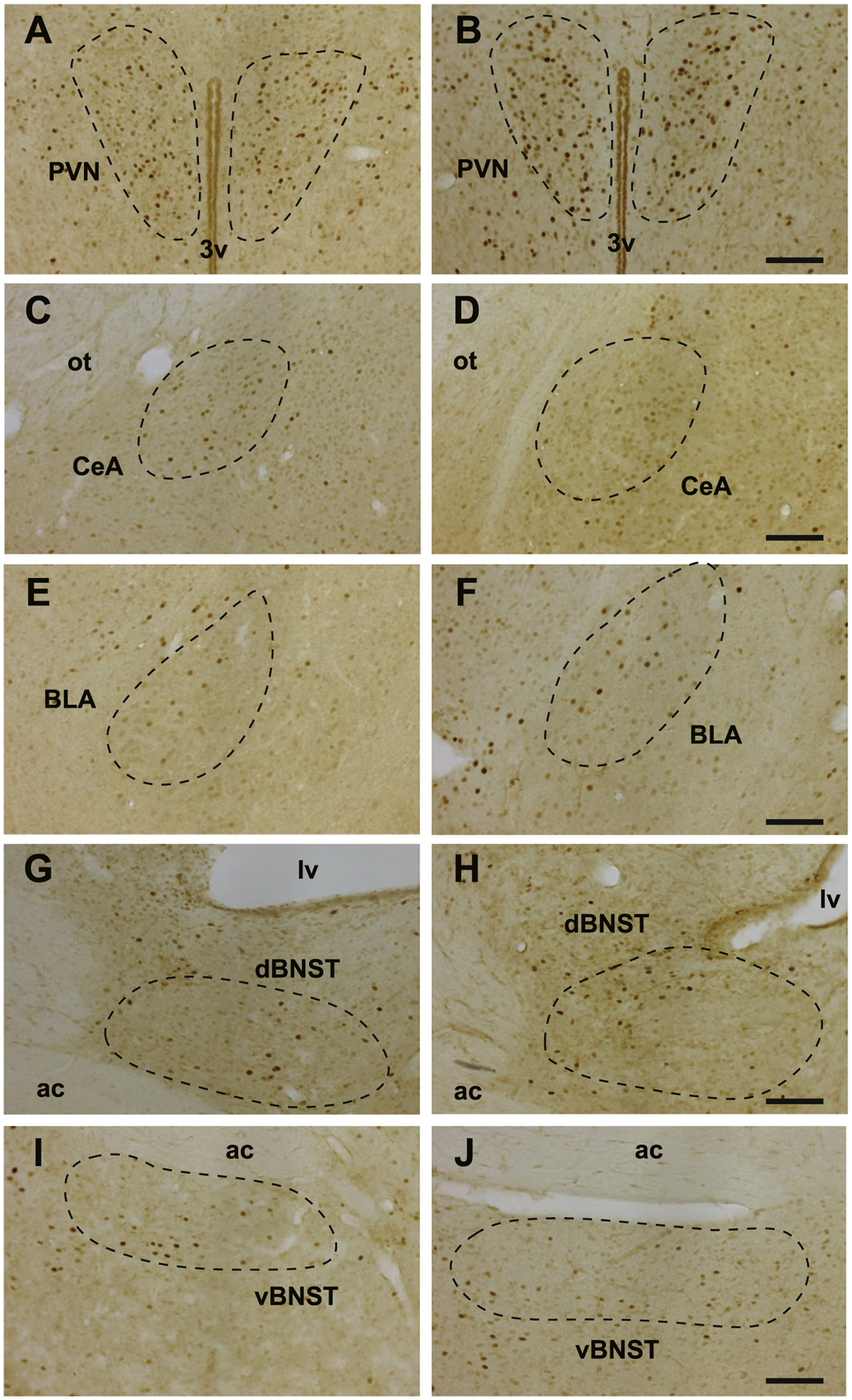
cFos immunoreactivity in various brain regions. The regions of interest are circled with dashed line. A, PVN of the cage-mate group. B, PVN of the pair-bonded group. C, CeA of the cage-mate group. D, CeA of the pair-bonded group. E, BLA of the cage-mate group. F, BLA of the pair-bonded group. G, dBNST of the cage-mate group. H, dBNST of the pair-bonded group. I, vBNST of the cage-mate group. J, vBNST of the pair-bonded group. 3v, the 3rd ventricle. lv, the lateral ventricle. ot, optic tract. ac, anterior commissure. Bars, 100 μm.
Fig. 3.
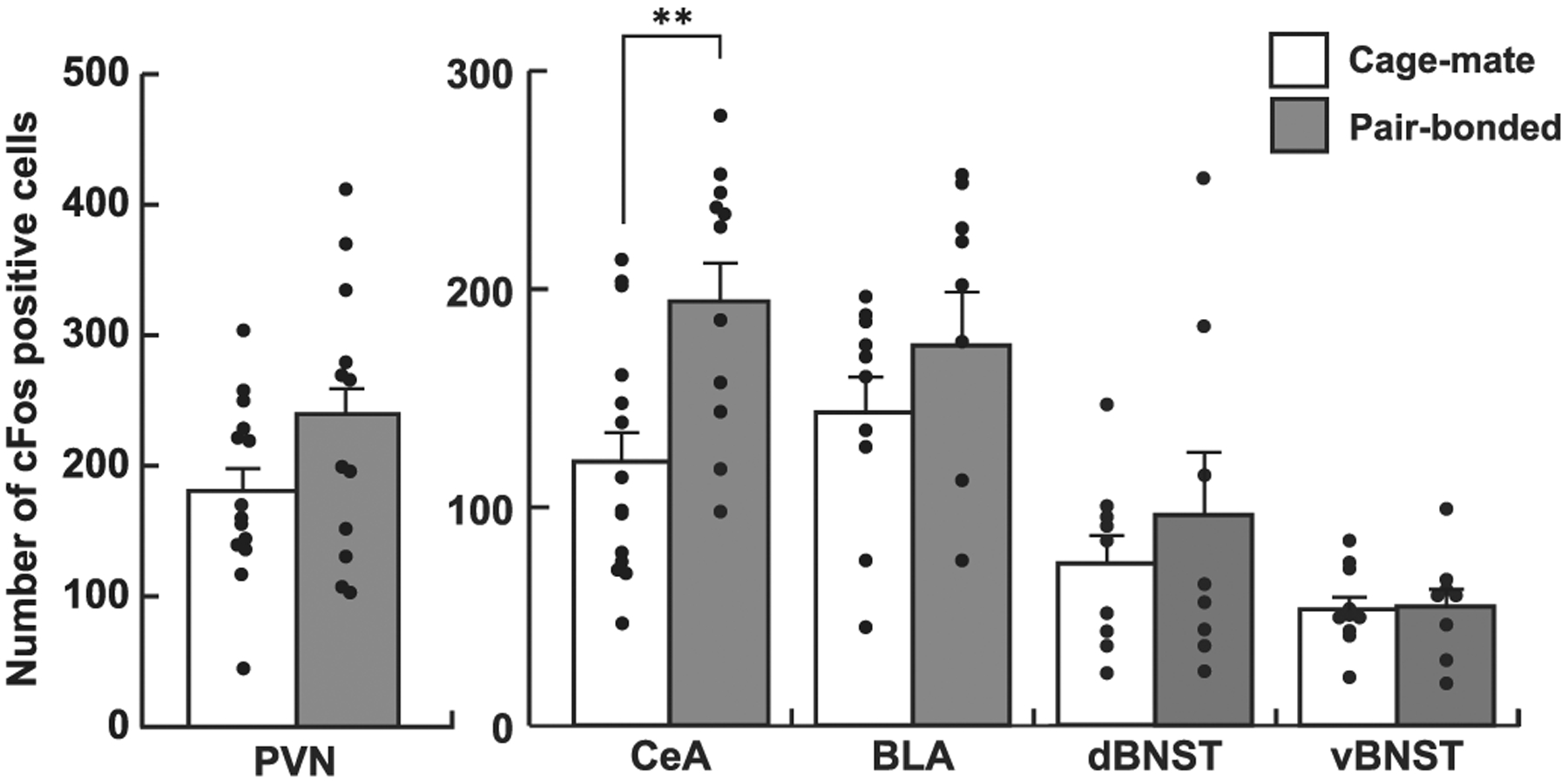
The number of cFos-positive cells in various brain regions. The number of cFos-positive cells was significantly increased in the CeA of the pair-bonded group, compared with the cage-mate group. There was no significant difference in other brain regions. Bars represent mean values + standard error. **, p < 0.01.
The intensity of oxytocin immunoreactivity at the PVN was significantly higher in the pair-bonded group than the cage-mate group (Fig. 4, T24 = 2.28, p = 0.032, Cohen’s d = 0.90).
Fig. 4.
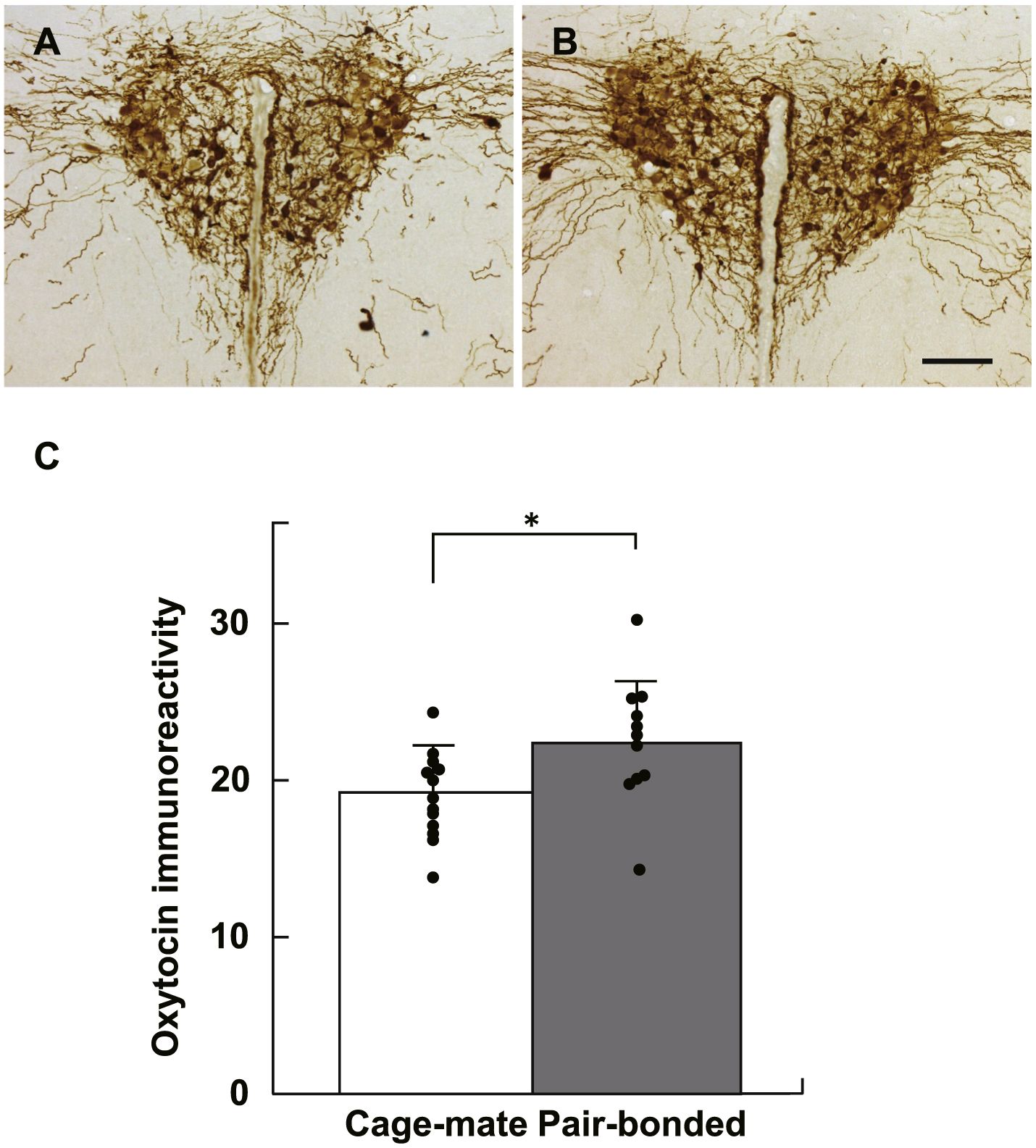
Immunohistochemical analysis of oxytocin immunoreactivity in the PVN. A, Cage-mate group. B, Pair-bonded group. C, The immunoreactivity was significantly higher in the cage-mate group than the pair-bonded group. Bars represent mean values + standard error. *, p < 0.05.
3.4. The pair bond blunted fear learning in a passive avoidance paradigm
Prairie voles appear to show less freezing reaction than traditional experimental animals such as rats and mice. In the present study, SPS-treated prairie voles showed 80 s on average of freezing duration in a 5 min session (Fig. 1B, D), whereas numerous reports using a contextual fear conditioning paradigm indicate over 120 s of freezing in mice and rats in a 5 min session (e. g. Pugh et al., 1999; Balogh et al., 2002; Olsen et al., 2012). Although our contextual fear conditioning test appeared to indicate a blunting effect of pair bonding on fear learning, the difference of freezing time between pair-bonded and cage-mate groups was subtle. Hence, after confirming the formation of a pair bond (Fig. 5B), we investigated the pair bonding-dependent blunting effect on the expression of fear learning using another fear-based learning paradigm, a passive avoidance test (Fig. 5). A two-way repeated measures ANOVA of the latency to enter the dark box showed a significant main effect of timing (Fig. 5C, F4, 72 = 14.40, p < 0.001, ηp2 = 0.445), but not group (F1, 18 = 3.04, p = 0.098, ηp2 = 0.145) or the interaction of timing and group (F4, 72 = 1.16, p = 0.337, ηp2 = 0.060). Post hoc analyses revealed that both groups significantly delayed the latency to enter the dark box in the memory test compared with the pre-conditioning session (cage-mate group, p < 0.001; pair-bonded group, p = 0.047). When the difference of the latency between pre-conditioning and memory test was analyzed by a paired t-test, both groups showed delayed latency at the memory test (cage-mate group, T8 = 3.93, p = 0.004, Cohen’s d = 1.88; pair-bonded group, T10 = 2.63, p = 0.025, Cohen’s d = 1.10). However, the pair-bonded group showed a significantly shorter latency than the cage-mate group in the memory test (Fig. 5C, p = 0.024). The cage-mate group decreased the latency during the extinction sessions (Fig. 5C, memory test vs extinction 2, p = 0.044; vs extinction 3, p = 0.01).
3.5. The administration of oxytocin receptor antagonist reversed the blunting effect of pair bonding on fear learning response
To test the potential role of oxytocin receptor signaling in blunting fear learning, we paired experimental males with female partners for 5 days and infused oxytocin receptor antagonist (or aCSF) icv. Thirty min after the icv infusion of the oxytocin receptor antagonist, the passive avoidance paradigm was performed (Fig. 6A). A two-way repeated measures ANOVA showed a significant main effect of drug (F1, 12 = 4.80, p = 0.049, ηp2 = 0.286), timing (F1, 12 = 14.16, p = 0.003, ηp2 = 0.541) as well as the interaction between drug and timing (Fig. 6C, F1, 12 = 5.48, p = 0.037, ηp2 = 0.314). Pair-bonded voles administrated with an oxytocin receptor antagonist displayed a significantly delayed latency to enter the dark box in the memory test compared with the pre-conditioning session (p < 0.001), while the subjects administrated with aCSF did not (p = 0.121). Similar results were obtained when paired t-tests were used (oxytocin receptor antagonist, T6 = 3.79, p = 0.007, Cohen’s d = 1.71; aCSF, T5 = 1.79, p = 0.134, Cohen’s d = 1.01). The latency in the memory test by voles administrated the oxytocin receptor antagonist was significantly prolonged compared to those receiving aCSFs (p = 0.039). These results suggest that oxytocin signaling blunts fear leaning in this paradigm and that this mechanism may contribute to the shorter latency in the memory test.
4. Discussion
In the current study, male prairie voles cohabitated with a female showed a decreased freezing duration compared with those cohabitated with a same-sex cage-mate, suggesting a buffering effect of sociosexual experience and/or pair bonding, when they were individually subjected to a contextual fear conditioning test (Fig. 1). An immunohistochemical analysis revealed a significant increase in cFos-positive cells in the CeA and oxytocin immunoreactivity in the PVN in the pair-bonded group, compared with the cage-mate group (Figs. 2–4). The blunting effect of pair bonding on fear learning was confirmed using a passive avoidance test (Fig. 5). Importantly, icv injection of an oxytocin receptor antagonist 30 min before the conditioning prevented the blunting effect of pair bonding (Fig. 6).
An SPS paradigm is known to induce PTSD-like behaviors, including impaired fear extinction, impaired social and novel object recognition memory, and increased anxiety-like behaviors, in rats (Imanaka et al., 2006; Yamamoto et al., 2008; Knox et al., 2012; Eagle et al., 2013). However, depression-like behaviors were not detected in prairie voles after the SPS paradigm (Arai et al., 2016). In the current study, a contextual fear conditioning test showed that SPS treatment enhanced fear learning and memory in prairie voles, especially in unmated males (Fig. 1A–D). Although this phenomenon is similar to that reported in SPS-treated rats and mice, freezing behavior in prairie voles is not as robust as reported in rats and mice. Freezing behavior may be less adaptive in grasslands, the natural habitat of prairie voles, than in forests and manmade structures where mice and rats prefer to reside. Wild rats trapped in the field freeze when a human approaches them at a distance of 1–5 m (Blanchard et al., 1986a, 1986b). Fourth generation wild mice, which were originally trapped in the field, then bred and kept in the laboratory, showed longer freezing to a recently killed hand-held rat than SWISS CD-1 laboratory mice (Blanchard et al., 1998). These reports support the above hypothesis regarding species differences in freezing, although there are no reports investigating fear-based learning using wild rats/mice.
In this study, the SPS paradigm did not disrupt the pair bond once it had formed (Fig. 1E, F), whereas our previous study indicates that the administration of the SPS paradigm to sexually naïve male vole prevents pair bond formation (Arai et al., 2016). This discrepancy may be derived from plastic changes in the neurocircuitry involved in pair bonding (https://www.biorxiv.org/content/10.1101/752345v1). Pair bonding raises the density of oxytocin and dopamine D1 receptors in the nucleus accumbens (NAcc), and vasopressin V1a receptor in the anterior hypothalamus (Gobrogge and Wang, 2016). A hypothetical neural mechanism of pair bond formation has been proposed (Numan and Young, 2016). In this model, oxytocin released from the PVN strengthens synapses in the amygdala to promote social memory. Then, oxytocin action on oxytocin receptors and dopamine action on dopamine D2 receptors in the NAcc disinhibit the ventral pallidum (VP) by depressing the activity of GABAergic medium spiny neuron inputs from NAcc to VP. This leads to strong activation of the VP to the olfactory and other stimuli from the partner over time, creating an enduring and selective pair bond. The amygdala may be susceptible to stressors from an SPS paradigm before synaptic strengthening by oxytocin in the amygdala, while strengthened synapses in this brain region may be tolerant to an SPS paradigm.
Using a passive avoidance test as well as a contextual fear conditioning test, we showed that pair-bonding blunted fear learning, or the expression of a fear response (Figs. 1, 5). The presence of affiliative conspecifics during or after the exposure to a stressful event attenuates fear responses (Liu and Yuan, 2016; Ishii et al., 2016). This phenomenon is called “social buffering”. When female prairie voles are exposed to a 1 h immobilization stress and recover with their bonded partner, anxiety-like behaviors and circulating levels of corticosterone, a stress hormone, are reduced compared to subjects recovering alone (Smith and Wang, 2014). The presence of a bonded partner during 1 h immobilization also prevents anxiety-like behavior in an elevated plus maze test (Donovan et al., 2018). Prairie voles show oxytocin-dependent consolation behaviors such as increased grooming towards partner conspecifics that have experienced an unobserved stressor, providing social buffering (Burkett et al., 2016). In contrast to these reports, it is important to note that the blunting of fear learning in the current study did not require the presence of the partner vole during or after fear learning. Prairie voles cohabited with an opposite sex conspecific display lower durations of passive behavioral responses in forced swimming and tail suspension tests, compared to both sexes housed individually (Bosch et al., 2009; Bosch et al., 2016; McNeal et al., 2017). We compared pair-bonded and cage-mate groups with regard to diminished long-lasting effects of social isolation stress, although we did not investigate whether pair bonding affects the threshold of pain suffered from electric foot shock. Our previous report indicates that separation for 4 days from the bonded partner female exacerbates pain behaviors and reduces the threshold of thermal stimulus on the plantar surface (Osako et al., 2018). Subjects in both experimental groups were cohabited with a conspecific until the day before the 3-day session of a passive avoidance testing (Fig. 5). Short-term isolation may act as a stressor, which generally causes stress coping reactions with psychological and endocrine consequences. Following only 4 days of separation from the partner female, male prairie voles display increased passive stress-coping (Bosch et al., 2009). Short-term separation from a bonded female partner markedly reduces the expression of oxytocin mRNA in the PVN and decreases the release of oxytocin in the NAcc through corticotropin releasing factor receptor 2 signaling (Bosch et al., 2016). Hence, pair bonding is likely to interfere with fear learning or expression, which phenomenon may represent an atypical sustained social buffering which occurs even in the absence of the partner.
The fact that an administration of an oxytocin receptor antagonist diminished or reversed the blunting effect of pair bonding on fear learning (Fig. 6) suggests the involvement of oxytocin signaling in this phenomenon. This speculation is consistent with previous reports showing that oxytocin reduces fear responses and anxiety-like behaviors (Ring et al., 2006; Toth et al., 2012; Ellenbogen et al., 2014). Microinjection of oxytocin receptor agonists into the CeA before the conditioning reduces fear responses (Viviani et al., 2011; Lahoud and Maroun, 2013). Pair bonding induces neurotransmitter receptor plasticity in prairie voles, such that mating and pair bonding enhances the density of oxytocin receptor in the NAcc (Gobrogge and Wang, 2016). Pair bonding-dependent increases of oxytocin receptor in some brain regions may mediate the attenuation of fear learning. Using an opto-genetic technique, Knobloch et al. (2012) demonstrated that activation of oxytocinergic neurons projecting to CeA activates local GABAergic circuit in this brain region, which decreases freezing response in fear-conditioned rats. Further, a recent report reveals that hypothalamic oxytocinergic neurons represent a fear memory engram in rats (Hasan et al., 2019). According to a hypothesis presented by the authors, a contextual fear experience activates a small subpopulation of parvo-cellular oxytocinergic neurons in the PVN, which innervate magnocellular oxytocinergic neurons at the SON. Magnocellular neurons in the SON release oxytocin from the neurohypophysis into the blood to regulate various physiological demands to cope with stress responses, while oxytocinergic engram cells in the SON release glutamate into the CeA instead of oxytocin in a context-dependent manner, which attenuates context fear-induced freezing. Form these viewpoints, it is interesting that the number of cFos positive cells in the CeA and oxytocin-immunoreactivity in the PVN was increased in the pair-bonded group in our study (Figs. 3, 4). It should be necessary to clarify in a future study whether the cFos positive cells were GABAergic or not.
Pair bonding prevented the increase in freezing duration or the latency to enter the dark box in Experiments 1 and 4, respectively (Figs. 1B, 6C), while it only blunted the increases in Experiments 2 and 3 (Figs. 1D, 5C). Recently, Kiyokawa et al. reported that using a cued fear conditioning, the freezing duration to a conditioned stimulus in conditioned rats increases as the intensity of foot shock increases even in the presence of an unfamiliar unconditioned conspecific, suggesting that such residual stress responses after receiving social buffering may be resistant to social buffering (Kiyokawa et al., 2018). Different schedules among our experiments possibly delivered inconsistent perceived shock intensity to subjects regardless of the constant mechanical condition (0.4 mA, 2 s). Such inconsistency of the shock intensity may contribute to various fear responses among our experiments. The buffering intensity by pair bonding may be enough to prevent fear response to the low intensity of stressors.
The current study has some limitations. It is not clear that the observed effects are truly due to changes in fear learning or reduced sensitivity of perceived pain from the shocks. Although pair bonded animals showed a reduced fear learning response, the effects were not statistically significant using ANOVA. It is possible that larger sample sizes would reveal fear learning, which is an adaptive response, but our results are consistent with a blunted fear learning response in pair bonded animals. Another limitation is that our subjects were only males, since it is well-known that circulating estrogen, which varied according to an estrus cycle, affects fear learning and memory (Cover et al., 2014; Garcia et al., 2018). It is possible that females would show a different response, although female voles do not cycle as rats and mice do. Future studies should include both males and females. It is important to note that our pair-bonded group differed from the cage-mate group not only in terms of pair bonding, but also in sociosexual experience. Upon introduction to a novel male, female prairie voles experience an activation of the hypothalamic-pituitary-gonadal (HPG) axis (Dluzen et al., 1981; Roberts et al., 1999), although effects of cohabitation on the HGP axis in male voles is unknown. Thus in addition to establishing a bond, the pair bonded group males may have also experience elevated steroid hormones, which could contribute to the effects that we observed. Further, introduction to a novel female causes a significant decline in serum corticosterone levels of male prairie voles (DeVries et al., 1997). These reports suggest that the degree of difference between the pair-bonded and cage-mate groups may be affected by factors other than just social bonding. The relative contributions of social bonding, exposure to female stimuli and mating cannot be dis-associated in this present study. Future studies should be performed using groups in which bonding occurred without mating and where mating occurred without bonding. It is not possible to conclude definitely that oxytocin signaling is the mechanism leading to the blunted fear response in pair bonded animals, but our data together are consistent with this hypothesis. It is possible that oxytocin receptor antagonist would also increase the fear response in non-pair bonded animals as well. Nevertheless, our studies demonstrate that pair bonding and oxytocin signaling both contribute to the fear learning response in the passive avoidance paradigm and in the context of the SPS paradigm.
5. Conclusions
In the current study, we showed that an SPS paradigm did not affect partner preference between bonded sexual partners, although this paradigm prevented the formation of pair bonds in our previous work. SPS-treated male prairie voles cohabited with a male conspecific for 4 days showed enhanced fear response in the contextual fear conditioning test, whereas subjects cohabited with a female conspecific did not show a significant fear response regardless of the SPS treatment. The number of cFos positive cells in the CeA was significantly increased in the pair-bonded group compared with the cage-mate group after the contextual fear conditioning test. This phenomenon was clearly confirmed by the passive avoidance test, suggesting that pair bonding interferes with fear learning or expression even though conspecifics were not presented during or after the stressors. Interestingly, icv administration of an oxytocin receptor antagonist diminished the blunting effect of pair bonding. We hypothesize that pair bonding blunts fear learning via enhanced oxytocin signaling, possibly in the CeA. This phenomenon may represent an atypical sustained social buffering by bonding between socio-sexual partners.
Acknowledgement
We gratefully thank Dr. Maurice Manning, College of Medicine and Life Sciences, The University of Toledo, for generously providing of the oxytocin antagonist used in this study.
Funding
This research was supported by JSPS KAKENHI 17K10290 to SM, NIH grant P50MH100023 to LJY, P51OD011132 to YNPRC, and Research Grant for Public Health Science to YH.
References
- Aragona BJ, Wang Z, 2004. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR J. 45, 35–45. 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Arai A, Hirota Y, Miyase N, Miyata S, Young LJ, Osako Y, Yuri K, Mitsui S, 2016. A single prolonged stress paradigm produces enduring impairments in social bonding in monogamous prairie voles. Behav. Brain Res 315, 83–93. 10.1016/j.bbr.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh SA, Radcliffe RA, Logue SF, Wehner JM, 2002. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav. Neurosci 116, 947–957. 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- Baumgardner DJ, Ward SE, Dewsbury DA, 1980. Diurnal patterning of eight activities in 14 species of muroid rodents. Anim. Learn. Behav 8, 322–330. 10.3758/BF03199612. [DOI] [Google Scholar]
- Blanchard RJ, Blanchard DC, Flannelly KJ, Hori K, 1986a. Ethanol changes patterns of defensive behavior in wild rats. Physiol. Behav 38, 645–650. 10.1016/0031-9384(86)90258-1. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flannelly KJ, Blanchard DC, 1986b. Defensive behavior of laboratory and wild Rattus norvegicus. J. Comp. Psychol 100, 101–107. 10.1037//0735-7036.100.2.101. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hebert MA, Ferrari P, Palanza P, Figueira R, Blanchard DC, Parmigiani S, 1998. Defensive behaviors in wild and laboratory (Swiss) mice: the mouse defense test battery. Phisol. Behav 65, 201–209. 10.1016/S0031-9384(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Borja SE, Callahan JL, Long P, 2006. Positive and negative adjustment and social support of sexual assault survivors. J. Trauma. Stress 19, 905–914. 10.1002/jts.20169. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ, 2009. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology 34, 1406–1415. 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ, 2016. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78. 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, Young LJ, 2016. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover KK, Maeng LY, Lebrón-Milad K, Milad MR, 2014. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl. Psychiatry 4, e422 10.1038/tp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Carter CS, 1997. Social modulation of corticosteroid responses in male prairie voles. Ann. N. Y. Acad. Sci 807, 494–497. 10.1111/j.1749-6632.1997.tb51949.x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD, Carter CS, Getz LL, 1981. Male vole urine changes luteinizing hormone – releasing hormone and norepinephrine in female olfactory bulb. Science 212, 573–575. 10.1126/science.7010608. [DOI] [PubMed] [Google Scholar]
- Donovan M, Liu Y, Wang Z, 2018. Anxiety-like behavior and neuropeptide receptor expression in male and female prairie voles: the effects of stress and social buffering. Behav. Brain Res 342, 70–78. 10.1016/j.bbr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Fitzpatrick CJ, Perrine SA, 2013. Single prolonged stress impairs social and object novelty recognition in rats. Behav. Brain Res 256, 591–597. 10.1016/j.bbr.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen A, Cardoso C, Joober R, 2014. Intranasal oxytocin attenuates the human acoustic startle response independent of emotional modulation. Psychophysiol. 51, 1169–1177. 10.1111/psyp.12263. [DOI] [PubMed] [Google Scholar]
- Garcia NM, Walker RS, Zoellner LA, 2018. Estrogen, progesterone, and the menstrual cycle: a systematic review of fear learning, intrusive memories, and PTSD. Clin. Psychol. Rev 66, 80–96. 10.1016/j.cpr.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Gobrogge K, Wang Z, 2016. The ties that bond: neurochemistry of attachment in voles. Curr. Opin. Neurobiol 38, 80–88. 10.1016/j.conb.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW, 2007. Cardiac regulation in the socially monogamous prairie vole. Physiol. Behav 90, 386–393. 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MT, Althammer F, da Gouveia MS, Goyon S, Eliava M, Lefevre A, Kerspern D, Schimmer J, Raftogianni A, Wahis J, Knobloch-Bollmann HS, Tang Y, Liu X, Jain A, Chavant V, Goumon Y, Weislogel JM, Hurlemann R, Herpertz SC, Pitzer C, Darbon P, Dogbevia GK, Bertocchi I, Larkum ME, Sprengel R, Bading H, Charlet A, Grinevich V, 2019. A fear memory engram and its plasticity in the hypothalamic oxytocin system. Neuron 103, 133–146. 10.1016/j.neuron.2019.04.029. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan R, Gunnar MR, 2014. Psychobiological mechanisms underlying the social buffering of the HPA axis: a review of animal models and human studies across development. Psychol. Bull 140, 256–282. 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka A, Morinobu S, Toki S, Yamawaki S, 2006. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav. Brain Res 173, 129–137. 10.1016/j.bbr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Ishii A, Kiyokawa Y, Takeuchi Y, Mori Y, 2016. Social buffering ameliorates conditioned fear responses in female rats. Horm. Behav 81, 53–58. 10.1016/j.yhbeh.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Javanbakht A, 2019. A theory of everything: overlapping neurobiological mechanisms of psychotherapies of fear and anxiety related disorders. Front. Behav. Neurosci 12, 328 10.3389/fnbeh.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S, Takagi Y, Kaneko S, Kurosawa T, 2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim 60, 481–487. 10.1538/expanim.60.481. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH, 1995. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med 57, 23–31. 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kawai K, Takeuchi Y, 2018. The benefits of social buffering are maintained regardless of the stress level of the subject rat and enhanced by more conspecifics. Physiol. Behav 194, 177–183. 10.1016/j.physbeh.2018.05.027. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, CHarlet A, Hoffman LC, Elisava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V, 2012. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566. 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I, 2012. Single prolonged stress disrupts retention of extinguished fear in rats. Learn. Mem 19, 43–49. 10.1101/lm.024356.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud N, Maroun M, 2013. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology 38, 2184–2195. 10.1016/j.psyneuen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Young EA, 1997. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology 6, 411–422. 10.1016/S0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z, 2014. Social bonding: regulation by neuropeptides. Front. Neurosci 8, 171 10.3389/fnins.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z, 2016. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr. Opin. Neurobiol 40, 8–13. 10.1016/j.conb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisieski MJ, Eagle AL, Conti AC, Liberzon I, Perrine SA, 2018. Single-prolonged stress: a review of two decades of progress in a rodent model of post-traumatic stress disorder. Front. Psychiatry 15, 196 10.3389/fpsyt.2018.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yuan TF, 2016. Physical interaction is required in social buffering induced by a familiar conspecific. Sci. Rep 6, 39788 10.1038/srep39788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Hartmann J, Ross RA, Ressier KJ, 2019. Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron 102, 60–74. 10.1016/j.neuron.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankkowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G, 2012. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuro-Oncol 24, 609–628. 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon D, Dabrowska J, 2018. Corticotropin-releasing factor receptors modulate oxytocin release in the dorsolateral bed nucleus of the stria terminalis (BNST) in male rats. Front. Neurosci 12, 183 10.3389/fnins.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Appleton KM, Johnson AK, Scotti ML, Wardwell J, Murphy R, Bishop C, Knecht A, Grippo AJ, 2017. The protective effects of social bonding on behavioral and pituitary-adrenal axis reactivity to chronic mild stress in prairie voles. Stress 20, 175–182. 10.1080/10253890.2017.1295444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Young LJ, 2016. Neural mechanisms of mother–infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav 77, 98–112. 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RHJ, Agam M, Davis MJ, Raber J, 2012. ApoE isoform-dependent deficits in extinction of contextual fear conditioning. Genes Brain Behav. 11, 806–812. 10.1111/j.1601-183X.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osako Y, Nobuhara R, Arai YP, Tanaka K, Young LJ, Nishihara M, Mitsui S, Yuri K, 2018. Partner loss in monogamous rodents: modulation of pain and emotional behavior in male prairie voles. Psychosom. Med 80, 62–68. 10.1097/PSY.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LA, Felton JW, Adams ZW, Welsh K, Miller S, Ruggiero KJ, 2015. Mental health among adolescents exposed to a tornado: the influence of social support and its interactions with sociodemographic characteristics and disaster exposure. J. Trauma. Stress 28, 232–239. 10.1002/jts.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Southwick SM, 2009. Psychological resilience and postdeployment social support protect against traumatic stress and depressive symptoms in soldiers returning from Operations Enduring Freedom and Iraqi Freedom. Depress. Anxiety 26, 745–751. 10.1002/da.20558. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Watkins LR, Maier SF, Rudy JW, 1999. Role of interleukin-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav. Brain Res 106, 109–118. 10.1016/S0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- de Quervain D, Schwabe L, Roozendaal B, 2017. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat. Rev. Neurosci 18, 7–19. 10.1038/nrn.2016.155. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S, 2006. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology 185, 218–225. 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Wolf KN, Sprangel E, Rall WF, Wildt DE, 1999. Prolonged mating in prairie voles (Microtus ochrogaster) increases likelihood of ovulation and embryo number. Biol. Reprod 60, 756–762. 10.1095/biolreprod60.3.756. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z, 2014. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76, 281–288. 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SCE, Campbell L, 2014. Psychological and physiological predictors of health in romantic relationships: an attachment perspective. J. Pers 82, 528–538. 10.1111/jopy.12056. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID, Slattery DA, 2012. Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology 223, 149–158. 10.1007/s00213-012-2702-4. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R, 2011. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107. 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Walum H, Young LJ, 2018. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci 19, 643–654. 10.1038/s41583-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Lee DaY, Cox CD, Karsten CA, Peñagarikono O, Geschwind DH, Gall CM, Piomelli D, 2015. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc. Natl. Acad. Sci 112, 14084–14089. 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S, 2008. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology 33, 2108–2116. 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I, 2009. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress. Anxiety 26, 1110–1117. 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]


