Abstract
Detailed crystallographic characterization of a tri-aspartate metal-binding site previously identified on the three-fold symmetry axis of a hexameric enzyme, LarE from Lactobacillus plantarum, was conducted. By screening an array of monovalent, divalent, and trivalent metal ions, we demonstrated that this metal binding site stoichiometrically binds Ca2+, Mn2+, Fe2+/Fe3+, Co2+, Ni2+, Cu2+, Zn2+, and Cd2+, but not monovalent metal ions, Cr3+, Mg2+, Y3+, Sr2+ or Ba2+. Extensive database searches resulted in only 13 similar metal binding sites in other proteins, indicative of the rareness of tri-aspartate architectures, which allows for engineering such a selective multivalent metal ion binding site into target macromolecules for structural and biophysical characterization.
Subject terms: Biochemistry, Structural biology
Introduction
Metals are essential components of many biological macromolecules, especially proteins. About a third1,2 of the protein structures in the PDB3 contain one or more metal atoms. The atomic structures of metal-containing cofactors and other metal-binding sites often provide insights into molecular mechanisms and/or reveal critical structural roles. Ten metal cations Mg2+, K+, Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+/+, and Zn2+ are commonly associated with proteins4, but other elements also may play important roles in catalysis or are associated with toxic effects1. Because of their unique spectroscopic characteristics, some transition metals have been used to study structural and dynamics properties of macromolecules5–7.
In this study, we present a crystallographic analysis of a recently discovered8 tri-Asp metal-binding site on the three-fold symmetry axis of the hexameric LarE protein from Lactobacillus plantarum. LarE8,9, a member of the PP-loop ATP pyrophosphatase family10, in conjunction with LarB11,12 and LarC13, participates in the synthesis of the nickel-pincer nucleotide cofactor of lactate racemase14–17. Our crystallographic studies indicated binding specificity towards certain divalent or trivalent metal ions. An extensive database search for similar metal binding sites in other proteins resulted in only 13 hits and structural comparison among these sites revealed the uniqueness of the tri-Asp site in LarE. Given its rarity in protein structures, we propose that such a metal binding site can be engineered into macromolecules18,19 of interest to facilitate structural and biophysical characterization.
Materials and methods
Crystallization optimization of LarE
LarE from L. plantarum (Table S1) initially was overexpressed and purified from Lactococcus lactis cells containing pGIR0728,11. Broad screening for crystallization at 21 °C resulted in small diamond-shaped crystals (Table S2) in space groups R3 or C212121, both from the same crystallization conditions of 0.1 M imidazole, pH 7.0, 0.15 M malic acid, pH 7.0, and 22% poly(ethylene glycol) monomethyl ether 550. Both crystal forms revealed an overall hexamer and both contained a metal bound at low occupancy, but with a strong anomalous signal at a wavelength of 0.979 Å (R3) and 0.999 Å (C212121), to residue Asp231 of each of three LarE chains; we termed this site the tri-Asp metal-binding site of LarE. We added no metal during enzyme purification or crystallization, so the metal identity was unclear.
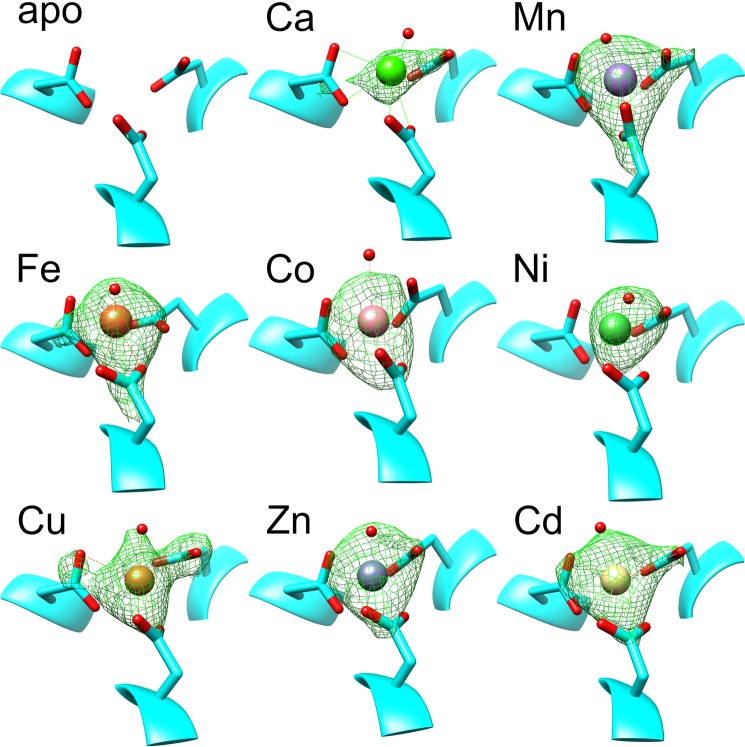
To enhance protein yield for optimizing crystallization, we switched to an Escherichia coli expression system (Table S1) using construct pGIR0768. Broad screening at 21 °C revealed several crystallization hits that were further optimized (Table S2). We previously reported two different apoprotein species (5UDQ, 5UNM), three substrate-bound forms (5UDR, 5UDS, 5UDT), and four different tri-Asp metal-bound (Mn, Fe, Ni, and Zn) structures (5UDU, 5UDV, 5UDW, and 5UDX)8. We present here four additional tri-Asp metal-bound (Ca, Co, Cu, and Cd) structures (6UTP, 6UTQ, 6UTR, and 6UTT). Table S3 summarizes all LarE structures.
Analysis of LarE metal binding by crystallographic methods
Soaking experiments
If not stated otherwise all soaking solutions contained a metal dissolved in 50 mM ammonium sulfate, 50 mM Bis-Tris, pH 6.5, and 30.0% v/v pentaerythritol ethoxylate (15/4 EO/OH). The analyzed datasets ranged between 2.3 and 3.6 Å resolution, with the majority around 2.6 Å. After molecular replacement and one round of refinement using default parameters in Phenix20, the presence of a bound metal was assessed. In all cases, datasets of crystals that were not soaked also were determined in parallel to confirm the absence of a metal. Characterization of the metal-binding site by crystallography included several variations of conditions. We altered the timespans of crystal soaking with a metal solution (0.5, 5, 30, 60 min, and 22 h) using 3.8 mM FeSO4. As datasets from all time points showed similar metal electron density, all reported soaking experiments were performed for one hour, if not stated otherwise. We examined a wide range of metal ions to investigate the metal specificity of the tri-Asp binding site at metal concentrations of 3.8 mM, unless stated otherwise.
Crystallization, data collection and structure determination of Ca, Co, Cu, and Cd bound structures
For crystallization, 5 μL (0.6 μL for Co) of ~25 mg/ml LarE (100 mM Tris-HCl, pH 7.5, 300 mM NaCl) were mixed with 5 μL (1.2 μL for Co) of reservoir solution. The hanging drop reservoir contained 100 μL of 30% (25% for Co) v/v pentaerythritol ethoxylate (15/4 EO/OH), 50 mM Bis-Tris pH 6.5 (50 mM MOPS pH 7.0 for Co), and 100 mM ammonium sulfate. The formed crystals were soaked 60 min in 3.8 mM calcium chloride or copper(II) sulfate or cadmium chloride dissolved in 30% v/v pentaerythritol ethoxylate (15/4 EO/OH), 50 mM Bis-Tris, pH 6.5, and 50 mM ammonium sulfate. The cobalt sulfate soaking time was reduced to 5 min as it appeared to damage the crystals rapidly; its final concentration may differ from 3.8 mM due to the low solubility of the metal salt.
Data sets were collected at the Advanced Photon Source LS-CAT beamlines (21-ID-D). As the Co soaked crystal diffracted to lower resolution than the other crystals, data collection was done at the Co K-edge at 1.600 Å to optimize the anomalous signal. The other datasets were collected at the default wavelengths at the respective beamlines (0.979 Å and 1.127 Å), which still give reasonable anomalous signal for these elements. Datasets were processed with xds21, with merging and scaling done using aimless22. Phenix Phaser molecular replacement20 used the wild-type apoprotein model 5UDQ. Model building and refinement were conducted in Coot23 and Phenix20. Simulated annealing composite-omit maps for the entire molecule were created in Phenix. The original experimental data created by aimless and the final pdb file without further refinement were used as input files. Crystallographic statistics are listed in Tables 1 and 2. UCSF Chimera24 was used to create structure figures.
Table 1.
Data collection and processing.
| Calcium bound | Cobalt bound | Copper bound | Cadmium bound | |
|---|---|---|---|---|
| Diffraction source | APS 21-ID-D | APS 21-ID-D | APS 21-ID-D | APS 21-ID-D |
| Wavelength (Å) | 0.979 | 1.600 | 1.127 | 1.127 |
| Detector | DECTRIS EIGER X 9 M | DECTRIS EIGER X 9 M | DECTRIS EIGER X 9 M | DECTRIS EIGER X 9 M |
| Space group | P 41 2 2 | P 41 2 2 | P 41 2 2 | P 41 2 2 |
| a, b, c (Å) | 107.7, 107.7, 320.4 | 106.2, 106.2, 313.6 | 109.0, 109.0, 323.7 | 107.8, 107.8, 319.6 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 |
| Resolution range (Å) | 48.15–2.49 (2.55–2.49) | 87.93–3.55 (3.83–3.55) | 48.74–2.41 (2.46–2.41) | 48.98–2.39 (2.43–2.39) |
| Total No. of reflections | 555563 (31312) | 212999 (20329) | 1163885 (75724) | 742009 (75461) |
| No. of unique reflections | 66185 (3834) | 43827 (3838) | 60947 (4095) | 41119 (4302) |
| Completeness (%) | 98.5 (86.6) | 89.6 (84.4) | 99.5 (93.1) | 99.3 (97.5) |
| Redundancy | 8.4 (8.2) | 10.5 (11.4) | 15.4 (14.9) | 9.8 (9.6) |
| 〈 I/σ(I)〉 | 18.2 (2.0) | 9.0 (3.2) | 15.6 (1.9) | 17.8 (2.1) |
| CC1/2 | 0.999 (0.758) | 0.994 (0.914) | 0.999 (0.705) | 0.999 (0.682) |
| Rmerge. | 0.069 (0.866) | 0.218 (0.818) | 0.102 (1.245) | 0.069 (1.188) |
| Rp.i.m. | 0.036 (0.452) | 0.091 (0.322) | 0.038 (0.467) | 0.033 (0.573) |
Values for the outer shell are given in parentheses.
Table 2.
Structure solution and refinement.
| Calcium bound | Cobalt bound | Copper bound | Cadmium bound | |
|---|---|---|---|---|
| Resolution range (Å) | 38.49–2.49 (2.52–2.49) | 87.93–3.55 (3.64–3.55) | 48.35–2.41 (2.41–2.44) | 37.88–2.39 (2.41–2.39) |
| Final Rcryst | 0.202 (0.317) | 0.231 (0.283) | 0.208 (0.337) | 0.213 (0.312) |
| Final Rfree | 0.256 (0.357) | 0.298 (0.311) | 0.253 (0.380) | 0.254 (0.326) |
| Metal atoms | 2 | 6 | 2 | 7 |
| Phosphate molecules | 6 | 6 | 6 | 6 |
| Sulfate molecules | 6 | 10 | 5 | 7 |
| Water | 87 | 13 | 122 | 175 |
| R.m.s. deviations | ||||
| Bonds (Å) | 0.008 | 0.010 | 0.009 | 0.008 |
| Angles (°) | 0.999 | 1.126 | 1.107 | 1.1050 |
| Average B factors (Å2) | 66.8 | 74.4 | 65.6 | 67.8 |
| Metal | 64.8 | 62.74 | 66.2 | 91.9 |
| Phosphate | 59.4 | 71.7 | 59.6 | 64.1 |
| Sulfate | 76.6 | 74.5 | 71.1 | 75.0 |
| Ramachandran plot | ||||
| Most favoured (%) | 97.50 | 95.44 | 96.94 | 98.0 |
| Outlier (%) | 0.0 | 0.0 | 0.0 | 0.0 |
Values for the outer shell are given in parentheses.
Co-crystallization experiments
LarE was screened for co-crystallization of components in the Hampton Additive Screen (HR2-428) using two different conditions (Table S2) at the recommended concentrations (5 μL protein plus 1 μL additive plus 4 μL reservoir).
Results and Discussion
LarE contains an unusual tri-Asp metal-binding site
Crystals of E. coli-derived LarE protein were hexameric and possessed metals bound to the tri-Asp sites when they had been subjected to size-exclusion chromatography, whereas the crystals were free of metal when the protein was not subjected to the chromatography step (Fig. 1A). We reason that the tri-Asp site captures low levels of contaminating metal ions, most likely Ni, Co, or Cd, from the Superdex 200 resin used for other protein purifications in the laboratory. This result implies that the trimer is capable of binding metals with high affinity in solution. Omission of the size-exclusion step from all further LarE purifications used for crystallization experiments had no effect on crystal growth. We also observed weak binding to the tri-Asp site by unidentified metal(s) in fully-grown crystals that had been left untouched for months. These observations indicate that the tri-Asp site is able to extract low levels of metal impurities from different environments. In the following crystal soaking experiments, we excluded the crystals older than two weeks to avoid spontaneous metal enrichment at the tri-Asp site.
Figure 1.
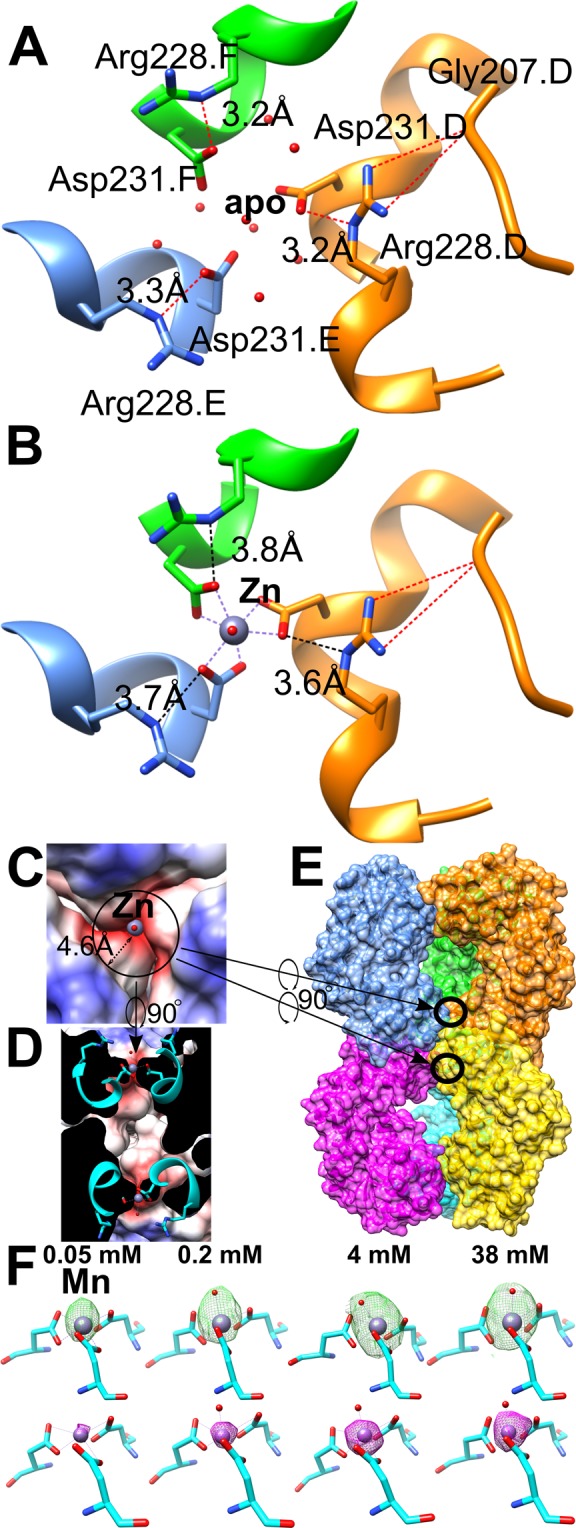
Tri-Asp metal binding site in LarE. (A) Close-up of the tri-Asp site in the apoprotein structure (PDB ID 5UDQ) filled with water molecules, illustrated as red spheres. Hydrogen bonds involving Arg228 are shown as red dashed lines, with the distances of the bonds indicated. The letter after the residue name and number indicates the protein chain. (B) Same view upon metal binding, illustrated for the Zn bound structure (PDB ID 5UDX). (C) Same top view and structure as B with electrostatic potential shown. (D) 90 degree turned cross-sectional view of the tri-Asp site within the hexamer. (E) Zoomed out view of D with chains surface colored individually. The two tri-Asp sites are circled. (F) Changes of the mFo-DFc electron density map at the tri-Asp site corresponding to different soaking concentrations of Mn. The maps are shown in green at 3 σ after one round of refining the molecular replacement solution. The models shown have been refined with one Mn and one water molecule; the Mn atom is shown in violet and the water oxygen atom in red. Below is shown the corresponding anomalous signal map in magenta at 6 σ.
In the apoprotein structure (Fig. 1A) the tri-Asp site is pre-formed to receive a metal ion by a hydrogen bond network consisting of Asp231, Arg228, Gly207, and several water molecules filling the site. Notably, the arginine residues form a positively charged ring around the tri-Asp site, limiting the metal-Asp interactions along the three-fold symmetry axis and likely responsible for only one metal ion being captured by these residues. Metal binding occurs with displacement of the water molecules (Fig. 1B). The Asp231 side chains move closer together to chelate the metal, in the process weakening the hydrogen bonds with the Arg228 side chains, which move slightly further away. Taking protonation into account, in the apoprotein the hydrated metal has to pass through a ~4.6 Å radius charged ring (Fig. 1C) introduced by the Arg residues. Arg228 itself is kept in place by hydrogen bonding to the backbone of a nearby loop containing Gly207. This interaction is nearly unchanged between the apoprotein and metal bound state (Fig. 1A,B), and overall only Asp231 and Arg228 appear to be influenced by metal binding. The charged ring may play a role in facilitating the binding of certain metals over others, as the metal has to pass by the arginine ring. This appears to be the only access towards the tri-Asp site as the head to head trimer-trimer interface blocks access from the other side in the hexamer where an inaccessible cavity is found (Fig. 1D,E). As shown in Fig. 1F, Mn2+ binds to this site in a concentration-dependent manner and the electron density of Mn reaches a maximum at 4 mM, and the same for the anomalous signals. Therefore, in the later experiments, the crystal soaking solutions contained approximately 4 mM metal ions of interest.
We previously reported that the tri-Asp site can bind Mn2+ (PDB ID code 5UDU), Fe2+ (5UDV), Ni2+ (5UDW), or Zn2+ (5UDX)8, including anomalous peak maps and element identification for Ni with datasets collected above and below the Ni K-edge. Here we examined the effect of added Ca2+ (6UTT), Co2+ (6UTP), Cu2+ (6UTR), and Cd2+ (6UTQ) (Fig. 2). In all cases, the Asp231 side chains bind the metals with bidentate coordination, with a seventh site always showing extra density that we modelled as a water molecule. Even in the case of the low resolution Co2+ bound dataset this extra density is observed. As 3.55 Å dataset resolution cannot justify placement of water alone we inferred the shown mode based on the observations in the other datasets. Simulated annealing composite-omit maps of all metal bound structures clearly indicate the presence of these metals when compared to the apoprotein structure (Fig. 2). The carboxyl-group of all Asp231 residues shows higher B-factors than the backbone in that region or than the metal atom itself, so we cannot rule out that our seven coordination model is an average of mixed monodentate and bidentate chelation, resulting in a lower coordination number. Nevertheless, the best interpretation of electron density is always a seven-coordinated metal atom consistent across all observed elements. Although seven-coordinated metals appear to be unusual, MetalPDB25 contains 1573 structures with metals having a coordination number of seven. The eight metals included in our LarE datasets account for 1239 of the seven-coordinate metal-containing protein structures.
Figure 2.
Simulated annealing composite-omit maps of tri-Asp sites in LarE structures. Shown are 2mFo-DFc maps at 3 σ within 4 Å of the metal atom (or in case of apoprotein, 2.5 Å from the Asp231 carboxyl oxygen atoms). Metals are colored using the Jmol element colors. Corresponding dataset information is listed in Table S3.
Crystallographic characterization of metal binding to the tri-Asp site
To probe the elemental preference of the metal-binding site, we soaked crystals with 3.8 mM of MgCl2, MgSO4, KCl, K2HPO4, Ca acetate, CaCl2, CrCl3, MnSO4, FeSO4, FeCl3, CoSO4, NiSO4, CuCl, CuCl2, ZnSO4, CdCl2, and CsCl. We observed binding to the tri-Asp site for Ca2+, Mn2+, both Fe2+ and Fe3+, Co2+, Ni2+, Cu2+, Zn2+, and Cd2+, whereas Mg2+, K+, Cr3+, Cu+ and Cs+ did not bind at that location (data not shown). The oxidation state of the metals throughout the text is inferred from the oxidation state of the reagents used. We cannot rule out changes of oxidation states after dissolving the reagents in the soaking solutions, exposure to air for an hour during soaking, or due to exposure to synchrotron radiation. For example, Fe2+ and Fe3+ cannot be differentiated based on electron density. Although Cu+ is readily oxidized to Cu2+ we saw a clear difference between Cu+ and Cu2+ soaked datasets. In general, soaking experiments reduced diffraction power and had a negative effect on merging statistics; especially the Cu and Co datasets were of worse quality than the apoprotein datasets or those for other metals like Ca. Several metal soaks (Co, Ni, Zn, and Cd) also resulted in binding of these metals to surface exposed residues. These partially disordered sites only appeared after metal soaks, showing a significant electron density peak and in some cases anomalous peaks. In most cases the surface site involved only a single histidine residue. This resulted in higher B-factors of these metals compared to the tri-Asp site. For example, the two Cd2+ metals (PDB ID 6UTQ) at the tri-Asp site have an average B-factor of 60.1 Å2, while the average of all modelled cadmium atoms in the structure is significantly higher (Table 2).
We found that the binding of most metal ions to the LarE tri-Asp site in crystal soaking experiments prevented crystal growth during co-crystallization studies. In additive screening, we were able to obtain datasets for LarE co-crystallized with 10 mM CrCl3, NaBr, SrCl2, YCl3, and BaCl2 or with 100 mM NaI and CsCl as well as with the organic compounds glutathione (1 mM), glycyl-glycyl-glycine (30 mM), glycine (100 mM), phenol (10 mM), and L-proline (10 mM). Cr3+ and Cs+ did not bind at the tri-Asp site in these datasets, confirming the soaking results. Similarly, Br−, Sr2+, Y3+, I−, and Ba2+, as well as the mentioned organic compounds, did not bind to LarE.
Tri-Asp metal-binding sites at three-fold symmetry axes in other proteins
We attempted to identify similar metal-binding sites in the protein databank using various webservers. MetalPDB25 (as of August 2019) contains 6304 protein (1736 unique sequences) structures with metal binding sites coordinated by at least three aspartate residues. 338 structures (218 unique sequences) contain sites with only three aspartate residues chelating; i.e. without any other protein residue being involved. We narrowed these hits to tri-Asp sites in proteins at three-fold symmetry axes and combined them with hits from other searches using MIPS26, MetalS327, and Metalmine28 resulting in 14 sites, including LarE (Fig. 3).
Figure 3.
Tri-Asp metal-binding sites in proteins at three-fold symmetry axes. For LarE the Zn2+ bound structure 5UDX is shown. The Zn2+ atom is dark purple, Mg2+ is grass green, Ca2+ is green, K+ is purple, and Cd2+ is light brown. Illustrations are scaled to show all residues within 6 Å of the metal. Sites shown for E. coli maltoporin 1AF640, S. typhimurium maltoporin 2MPR41, hydroxylamine oxidoreductase 4N4N29, enzyme IIAlactose 1E2A42, deoxyuridine triphosphatase 3ARN43, Panicum mosaic virus 4FY144, acyl-CoA hydrolase 2GVH (unpublished), PTS cellobiose specific enzyme IIA 3K1S (unpublished), carp fishelectin 4RUS45, C1q-like protein 4QQL and 4QQ246, RhCC oxygenase 4U5P47, and salmon anemia virus receptor complex 5T9648.
Beyond the tri-Asp chelation these sites do not appear to share any nearby structure features. The sites are surrounded by either no residues (2GVH, 4RUS), hydrophobic residues (3ARN, 3K1S, 5T96), positively charged (4N4N, 4U5P) similar to LarE, polar uncharged (1AF6, 2MPR), methionines (1E2A), or negatively charged residues (4FY1, 4QQL, 4QQ2). In contrast to LarE these sites are mostly occupied by lighter elements like Na and in particular Mg. The Ca sites and the single Cd site do not share any resemblance to LarE. Lastly, nearly all structures show monodentate binding, often with three additional water molecules resulting in an octahedral coordination site.
Only one match with a similar chelation geometry to LarE was identified: Nitrosomonas europaea hydroxylamine oxidoreductase (Fig. 3, PDB ID 4N4N)29. In that case, three Asp residues bind K+ via bidentate coordination, with a seventh site occupied by a water molecule. Here an Arg side chain also gates one entrance side, however as the Arg residue is adjacent to the Asp residue and not one helix turn away like in LarE the gate appears to be narrower. In hydroxylamine oxidoreductase, however, the opposite side is surface exposed, while in LarE the other trimer of the hexamer blocks accessibility (Fig. 1D). The monovalent ion was not discussed in that publication and the metal speciation of that site may differ from K+ as the interpretation of this cation was apparently based solely on the crystallization conditions. Thus, the metal-bound tri-Asp site of LarE is rare; however, we do not know whether such sites may exist in other apoprotein structures.
Compared with the potassium-bound tri-Asp site in 4N4N (and 4N4O), the unique environment of the tri-Asp site in LarE blocks the binding of alkali cations. The back-to-back trimer arrangement allows the metal to approach only through the arginine ring, which reduces electrostatic interaction and therefore effectively prevents monovalent cation binding. The stronger coulombic force between the negatively charged sink (Fig. 1C) and the di- or tri-valent cations may overcome the repulsion imposed by the arginine ring, resulting in metal binding to the tri-Asp site strictly along the three-fold axis. However, not all of the tested multivalent cations were captured by the tri-Asp site. Multiple factors may contribute to the observed metal selectivity among the studied multivalent cations. Firstly, size matters30. It is shown that the metal ions with a radius greater than that of Ca2+ (100 pm), like Sr2+ or Ba2+ cannot bind (Table S4). Secondly, Cr3+ is kinetically inert in ligand exchange, and so it is not expected to bind efficiently. Thirdly, Mg2+ is known to exhibit a lower first stability constant for replacement of water in the aqueous ion by a ligand compared to other divalent transition metal ions in the Irving-Williams series31,32. A recent MD simulation study using a polarizable force field provides insights on Mg’s low affinity towards many Ca-preferred highly charged metal binding sites33. Crystallographically, Mg2+ is much lighter than the other multivalent cations studied in this work, so Mg2+ binding with low occupancy would not be readily detected. Although we did not see any density upon MgSO4 or MgCl2 soaking, we cannot exclude the possibility that Mg2+ can actually weakly bind there. One additional putative factor would be the arginine ring. Although electrically unfavorable for metal binding, arginine residues can directly coordinate transition metal ions using the nitrogen atoms in the guanidine moiety25,34. The hypothetical interaction between the arginine residues and “soft” metals may facilitate the latter to pass through the arginine ring, whereas the same interaction is unlikely to happen for a hard cation (Mg2+, Ca2+, Ba2+, Sr2+, and Y3+ studied here). Y3+ binding to proteins appears to be rare in general with only 41 PDB entries3. Also single positively-charged metals and negatively charged elements like Br− and I− appear to be unable to bind.
Coordination number is another factor that may play an important role in metal preference at the tri-Asp site. The metal atoms in all of our structures are hexacoordinated by carboxyl groups with a seventh coordination site always showing positive density that we interpreted as a water molecule. For small molecule complexes in the Cambridge Structural Database (CSD) the most common and second most frequent coordination numbers for Mn2+, Fe2+/3+, Co2+, Ni2+, Zn2+, and Cd2+ are 6 and 4; while for Ca2+ they are 6 and 7; and for Cu2+ they are 5 and 635. Thus, the preference for hexacoordination generally fits with our binding at the tri-Asp site. A coordination number of 7 is only seen frequently in Ca2+ but does exist for the other elements in small molecule complexes within the CSD. However, the coordination number alone might not be the best predictor of binding to the LarE tri-Asp site, as the most common or second most frequent coordination number for Cr3+, Y3+ and Sr2+ is also 6 in the CSD; and for Ba2+ the most common are 7 and 8. We also investigated the coordination number of metal atoms in proteins using MetalPDB25 (Table S5). The results show a similar picture for these elements with the most frequent coordination number being 6 for Mn, Fe, Co, and Ni; 7 (closely followed by 6) for Ca; and 4 for Cu, Zn, and Cd (with 6 coordination being less frequently observed). Y, Sr, and Ba have a limited number of PDB entries with the majority having coordination numbers of 1–3, suggesting a lack of complete hydration, in agreement with the lack of visible water molecules in those protein structures. Cr only has 10 entries in MetalPDB, again illustrating its inert nature. Lastly Mg2+ in small molecules35 most frequently has a coordination number of 6, with 7 being rarely observed. In addition to the reasons already outlined that exclude Mg from binding, there appears to be a significant difference in the hexacoordination of Mg by carboxylate side chains compared to the other metals. In 96% of the cases for this subset of protein structures, Mg exhibits monodentate chelation by Asp/Glu residues36. This behavior also is illustrated for Mg atoms chelated by three Asp residues at three-fold symmetry axes (Fig. 3; PDB entries 1AF6, 1E2A, 3ARN, 3K1S, 4RUS, 4U5P, 5T96). The frequent observation of hexacoordinated Mg is achieved by the addition of three water molecules to the three amino acid side chains. In contrast to the nearly exclusive monodentate binding of Mg by carboxylates, the Ca sites in protein structures involving carboxylates are 71% monodentate and 29% bidentate36. This difference gives another strong argument why Mg2+ binding was not observed for LarE. Interestingly, when carboxyl groups are involved in chelating Zn2+ or Cd2+ in protein structures, 7 coordination (as observed in LarE) is actually the most frequently observed coordination37.
Additional investigation (such as metal binding to a variant with substituted Arg228) is warranted to determine the exact mechanism for metal specificity at the tri-Asp site in LarE. Nevertheless, the tri-Asp site in LarE represents an unusual metal binding site that selectively and stoichiometrically binds certain multivalent cations.
Implications
As a D231R variant showed the same activity as wild-type protein and a similar hexameric gel filtration profile8, we conclude that no biological role can be established for this metal binding site based on our data. However, this rare metal binding site could be of interest to those working with small molecule mimics or for targeted protein engineering to create a multivalent metal binding site. This site, able to capture trace amounts of metal ions from solution when the protein is in its soluble or crystalline state, has been demonstrated to bind Ca2+, Mn2+, Fe2+/Fe3+, Co2+, Ni2+, Cu2+, Zn2+, and Cd2+, many of which have characteristic spectroscopic properties.
By engineering such a tri-Asp site into other target proteins, the binding of selected metals, such as Cu2+ and Ni2+ 38,39, could be used to probe protein structure and dynamics. While it is known that acidic residues bind such divalent metal cations, the distinct properties of the LarE-type metal-binding site (i.e., its ability to capture trace amounts of metal ion, its broad yet defined metal ion specificity, and its apparent uniqueness in proteins) make it an intriguing site for protein engineering, particularly at trimer interfaces. The presence of a single water molecule at the metal-binding site also raises the possibility that such engineered proteins could exhibit catalytic activities involving activation of this ligand.
Supplementary information
Acknowledgements
This work was supported by a University of Otago Health Sciences Postdoctoral Fellowship [HSCDPD1703 to M.F.], the National Science Foundation [CHE1516126 and CHE1807073 to R.P.H. and J.H.], and by the National Institutes of Health [GM128959 to R.P.H. and J.H.]. We would like to thank Claudia Andreini for assistance with MetalPDB searches.
Author contributions
M.F., R.H. and J.H. conceptualization; M.F. and K.H. purification and crystallization; M.F. data collection and processing; M.F. and J.H. data curation and analysis; M.F., R.H. and J.H. writing, review and editing; M.F. visualization; R.H. and J.H. supervision; M.F., R.H., and J.H. funding acquisition; R.H. and J.H. project administration.
Data availability
The datasets generated and analysed during the current study are available in the worldwide Protein Data Bank under PDB IDs 6UTT (Ca bound), 6UTP (Co bound), 6UTR (Cu bound), and 6UTQ (Cd bound).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Robert P. Hausinger, Email: hausinge@msu.edu
Jian Hu, Email: hujian1@msu.edu.
Supplementary information
is available for this paper at 10.1038/s41598-020-62847-6.
References
- 1.Harding MM, Nowicki MW, Walkinshaw MD. Metals in protein structures: a review of their principal features. Crystallogr. Rev. 2010;16:247–302. doi: 10.1080/0889311x.2010.485616. [DOI] [Google Scholar]
- 2.Zheng H, et al. Validation of metal-binding sites in macromolecular structures with the CheckMyMetal web server. Nat. Protoc. 2014;9:156–170. doi: 10.1038/nprot.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman H, Henrick K, Nakamura H, Markley JL. The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res. 2007;35:D301–303. doi: 10.1093/nar/gkl971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva, J. J. R. F. S. D. & Williams, R. J. P. The biological chemistry of the elements: the inorganic chemistry of life. 2nd edn, (Oxford University Press, 2001).
- 5.Aron AT, Ramos-Torres KM, Cotruvo JA, Jr., Chang CJ. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 2015;48:2434–2442. doi: 10.1021/acs.accounts.5b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi W, et al. Characterization of metalloproteins by high-throughput X-ray absorption spectroscopy. Genome Res. 2011;21:898–907. doi: 10.1101/gr.115097.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maret W, Vallee BL. Cobalt as Probe and Label of Proteins. Method. Enzymol. 1993;226:52–71. doi: 10.1016/0076-6879(93)26005-T. [DOI] [PubMed] [Google Scholar]
- 8.Fellner M, Desguin B, Hausinger RP, Hu J. Structural insights into the catalytic mechanism of a sacrificial sulfur insertase of the N-type ATP pyrophosphatase family, LarE. Proc. Natl Acad. Sci. USA. 2017;114:9074–9079. doi: 10.1073/pnas.1704967114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fellner M, Rankin JA, Desguin B, Hu J, Hausinger RP. Analysis of the active site cysteine residue of the sacrificial sulfur insertase lare from Lactobacillus plantarum. Biochemistry. 2018;57:5513–5523. doi: 10.1021/acs.biochem.8b00601. [DOI] [PubMed] [Google Scholar]
- 10.Fellner M, Hausinger RP, Hu J. A structural perspective on the PP-loop ATP pyrophosphatase family. Crit. Rev. Biochem. Mol. Biol. 2018;53:607–622. doi: 10.1080/10409238.2018.1516728. [DOI] [PubMed] [Google Scholar]
- 11.Desguin B, et al. Lactate racemase is a nickel-dependent enzyme activated by a widespread maturation system. Nat. Commun. 2014;5:3615. doi: 10.1038/ncomms4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desguin B, Soumillion P, Hols P, Hausinger RP. Nickel-pincer cofactor biosynthesis involves LarB-catalyzed pyridinium carboxylation and LarE-dependent sacrificial sulfur insertion. Proc. Natl Acad. Sci. USA. 2016;113:5598–5603. doi: 10.1073/pnas.1600486113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desguin B, et al. Biosynthesis of the nickel-pincer nucleotide cofactor of lactate racemase requires a CTP-dependent cyclometallase. J. Biol. Chem. 2018;293:12303–12317. doi: 10.1074/jbc.RA118.003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rankin JA, et al. Lactate racemase nickel-pincer cofactor operates by a proton-coupled hydride transfer mechanism. Biochemistry. 2018;57:3244–3251. doi: 10.1021/acs.biochem.8b00100. [DOI] [PubMed] [Google Scholar]
- 15.Desguin B, et al. A tethered niacin-derived pincer complex with a nickel-carbon bond in lactate racemase. Science. 2015;349:66–69. doi: 10.1126/science.aab2272. [DOI] [PubMed] [Google Scholar]
- 16.Fellner, M., Rankin, J. A., Hu, J. & Hausinger, R. P. Lactate Racemase. Encyclopedia of Inorganic and Bioinorganic Chemistry, 1–8, 10.1002/9781119951438.eibc2508 (2017).
- 17.Hausinger RP, Desguin B, Fellner M, Rankin JA, Hu J. Nickel-pincer nucleotide cofactor. Curr. Opin. Chem. Biol. 2018;47:18–23. doi: 10.1016/j.cbpa.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Berry SM, Pfister TD. Engineering novel metalloproteins: Design of metal-binding sites into native protein scaffolds. Chem. Rev. 2001;101:3047–3080. doi: 10.1021/cr0000574. [DOI] [PubMed] [Google Scholar]
- 19.Podtetenieff J, Taglieber A, Bill E, Reijerse EJ, Reetz MT. An artificial metalloenzyme: creation of a designed copper binding site in a thermostable protein. Angew. Chem. Int. Ed. Engl. 2010;49:5151–5155. doi: 10.1002/anie.201002106. [DOI] [PubMed] [Google Scholar]
- 20.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabsch W. Xds. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D. Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettersen EF, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 25.Putignano V, Rosato A, Banci L, Andreini C. MetalPDB in 2018: a database of metal sites in biological macromolecular structures. Nucleic Acids Res. 2018;46:D459–D464. doi: 10.1093/nar/gkx989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemavathi K, et al. MIPS: metal interactions in protein structures. J. Appl. Crystallogr. 2010;43:196–199. doi: 10.1107/S002188980903982x. [DOI] [Google Scholar]
- 27.Valasatava Y, Rosato A, Cavallaro G, Andreini C. MetalS(3), a database-mining tool for the identification of structurally similar metal sites. J. Biol. Inorg. Chem. 2014;19:937–945. doi: 10.1007/s00775-014-1128-3. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Hirai A, Altaf-Ul-Amin M, Takahashi H. MetalMine: a database of functional metal-binding sites in proteins. Plant. Biotechnol. 2009;26:517–521. doi: 10.5511/plantbiotechnology.26.517. [DOI] [Google Scholar]
- 29.Maalcke WJ, et al. Structural basis of biological NO generation by octaheme oxidoreductases. J. Biol. Chem. 2014;289:1228–1242. doi: 10.1074/jbc.M113.525147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon RD. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A. 1976;32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 31.Martin RB. A stability ruler for metal-ion complexes. J. Chem. Educ. 1987;64:402–402. doi: 10.1021/ed064p402. [DOI] [Google Scholar]
- 32.Foster AW, Osman D, Robinson NJ. Metal preferences and metallation. J. Biol. Chem. 2014;289:28095–28103. doi: 10.1074/jbc.R114.588145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing Z, Liu C, Qi R, Ren P. Many-body effect determines the selectivity for Ca2+ and Mg2+ in proteins. Proc. Natl Acad. Sci. USA. 2018;115:E7495–E7501. doi: 10.1073/pnas.1805049115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ireland, S. M. & Martin, A. C. R. ZincBind-the database of zinc binding sites. Database (Oxford)2019, 10.1093/database/baz006 (2019). [DOI] [PMC free article] [PubMed]
- 35.Dudev M, Wang J, Dudev T, Lim C. Factors governing the metal coordination number in metal complexes from Cambridge Structural Database analyses. J. Phys. Chem. B. 2006;110:1889–1895. doi: 10.1021/jp054975n. [DOI] [PubMed] [Google Scholar]
- 36.Dudev T, Lim C. Monodentate versus bidentate carboxylate binding in magnesium and calcium proteins: What are the basic principles? J. Phys. Chem. B. 2004;108:4546–4557. doi: 10.1021/jp0310347. [DOI] [Google Scholar]
- 37.Dokmanic I, Sikic M, Tomic S. Metals in proteins: correlation between the metal-ion type, coordination number and the amino-acid residues involved in the coordination. Acta Crystallogr. D. Biol. Crystallogr. 2008;64:257–263. doi: 10.1107/S090744490706595X. [DOI] [PubMed] [Google Scholar]
- 38.Billesbolle CB, et al. Transition metal ion FRET uncovers K+ regulation of a neurotransmitter/sodium symporter. Nat. Commun. 2016;7:12755. doi: 10.1038/ncomms12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taraska JW, Puljung MC, Zagotta WN. Short-distance probes for protein backbone structure based on energy transfer between bimane and transition metal ions. P Natl Acad. Sci. USA. 2009;106:16227–16232. doi: 10.1073/pnas.0905207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YF, Dutzler R, Rizkallah PJ, Rosenbusch JP, Schirmer T. Channel specificity: structural basis for sugar discrimination and differential flux rates in maltoporin. J. Mol. Biol. 1997;272:56–63. doi: 10.1006/jmbi.1997.1224. [DOI] [PubMed] [Google Scholar]
- 41.Meyer JE, Hofnung M, Schulz GE. Structure of maltoporin from Salmonella typhimurium ligated with a nitrophenyl-maltotrioside. J. Mol. Biol. 1997;266:761–775. doi: 10.1006/jmbi.1996.0823. [DOI] [PubMed] [Google Scholar]
- 42.Sliz P, Engelmann R, Hengstenberg W, Pai EF. The structure of enzyme IIAlactose from Lactococcus lactis reveals a new fold and points to possible interactions of a multicomponent system. Structure. 1997;5:775–788. doi: 10.1016/S0969-2126(97)00232-3. [DOI] [PubMed] [Google Scholar]
- 43.Miyahara S, et al. Discovery of a novel class of potent human deoxyuridine triphosphatase inhibitors remarkably enhancing the antitumor activity of thymidylate synthase inhibitors. J. Med. Chem. 2012;55:2970–2980. doi: 10.1021/jm201628y. [DOI] [PubMed] [Google Scholar]
- 44.Makino DL, Larson SB, McPherson A. The crystallographic structure of Panicum Mosaic Virus (PMV) J. Struct. Biol. 2013;181:37–52. doi: 10.1016/j.jsb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capaldi S, et al. Three-dimensional structure and ligand-binding site of carp fishelectin (FEL) Acta Crystallogr. D. Biol. Crystallogr. 2015;71:1123–1135. doi: 10.1107/S1399004715004174. [DOI] [PubMed] [Google Scholar]
- 46.Ressl S, et al. Structures of C1q-like Proteins Reveal Unique Features among the C1q/TNF Superfamily. Structure. 2015;23:688–699. doi: 10.1016/j.str.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Baas BJ, et al. Functional and structural characterization of an unusual cofactor-independent oxygenase. Biochemistry. 2015;54:1219–1232. doi: 10.1021/bi501200j. [DOI] [PubMed] [Google Scholar]
- 48.Cook JD, Sultana A, Lee JE. Structure of the infectious salmon anemia virus receptor complex illustrates a unique binding strategy for attachment. Proc. Natl Acad. Sci. USA. 2017;114:E2929–E2936. doi: 10.1073/pnas.1617993114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the worldwide Protein Data Bank under PDB IDs 6UTT (Ca bound), 6UTP (Co bound), 6UTR (Cu bound), and 6UTQ (Cd bound).