Abstract
Psychological distress persisting for weeks or more promotes pro-inflammatory immune dysregulation, a risk factor for a range of chronic diseases. We have recently shown that mindfulness training reduces distress among university students. Here we present an exploratory trial to study immune dysregulation in a cohort of students who were exposed to progressively greater stress as the exam period approached, and to explore whether mindfulness training mitigated this dysregulation. Healthy University of Cambridge students were randomised to join an 8-week mindfulness course (N = 27), or to mental health support as usual (N = 27). Psychological distress, immune cell proportions, cytokines, CRP and serum cortisol were measured at baseline and during the exam period. Increased distress was associated with statistically significant increases in the proportion of B cells, regardless of trial arm (*p = 0.027). There were no other associations between any of the measured parameters, distress or mindfulness. Our finding that the proportion of B cells increases with psychological distress supports the findings of other studies. However, we found no evidence that mindfulness training is able to buffer the effects of psychological distress on healthy participants’ immune system. In order to detect these effects, should they exist, larger randomised trials will be required.
Subject terms: Immunology, Psychology
Introduction
The human mind, brain and immune systems have much in common. They are complex, self-organising economies that recognise self from non-self, interact with the environment and possess ability to store information. The immune system alone is a highly distributed network of effectors, regulators and signals, which depend on dynamic balances to determine the best configuration for each environmental challenge.
Prolonged systemic inflammation leads to increased risk of chronic diseases such as cancer, cardiovascular disease, diabetes, dementia and depression1, the most important global causes of morbidity and mortality. Thus, interventions that restore or maintain normal immune function in healthy and at risk populations are useful in preventing a range of mental and physical disorders2,3. Even small improvements at a population level could lead to large reductions in morbidity and mortality4.
The immune, endocrine and nervous systems are known to interact with one another to provide a co-ordinated response to external stimuli, whether it be a physical insult such as wounding or an emotional stressor such as perceived stress (reviewed in Glaser et al.)5. Acute stress promotes a pro-inflammatory state along with the “fight or flight” response; if stress persists for weeks or more (chronic stress) this pro-inflammatory state fails to resolve adequately, resulting in damage6.
The effects of stress on immune function are thought to be mediated in part by the steroid hormone cortisol which may increase during psychological distress. Modulation of immune function by cortisol and other hormones could be mediated by direct interaction with immune cells or sympathetic nervous system fibres7, or indirectly by dysregulating the production of cytokines5,8,9. A popular model that could explain the pattern of chronic disease empirically linked with chronic stress hypothesizes that cortisol and other stress factors suppress cellular (Th1) adaptive immunity, while humoral (Th2) adaptive immunity is overstimulated10. Indeed, several studies have demonstrated shifts in favour of Th2 cytokine imbalance in response to stress11,12.
A comprehensive meta-analysis of non-clinical examination stress studies found support for this model via a decrease in interferon-γ (IFN- γ) and increases in interleukin-6 (IL-6) and IL-108. However, pro- and anti- inflammatory cytokines are produced by varied immune cell subsets and it is difficult to interpret changes in peripheral blood protein levels without an understanding of the cellular response to stress, both within and beyond the T cell compartment. Furthermore, chronic stress leads to glucocorticoid resistance and desensitisation leading to the failure of cortisol to suppress inflammation independently of its blood level13.
A meta-analysis performed by Segerstrom and Miller found that NK cell activity and proportions of certain lymphocyte subsets were increased, but lymphocyte proliferation overall decreased following public speaking, an acute stressor8. Given that most immune cell subsets have receptors that recognise and can respond to neurotransmitters and hormones associated with stress, it is plausible that these changes in immune cell numbers, proliferative capacity and function could be in response to stress hormones and sympathetic innervation of lymphoid organs5. Over the last several decades many advances have been made towards understanding how the immune, endocrine and nervous systems interact and may become dysregulated during times of psychological distress. The studies mentioned above have contributed to our knowledge, but we still lack precise mechanistic understanding of how specific components of the immune system contribute to psychological distress and vice versa.
Healthy, young but stressed individuals may greatly benefit from learning lifelong skills to manage their stress and therefore prevent immune dysregulation which, if maintained throughout life, could lead to chronic disease. Teaching those skills to students could not only help to support their wellbeing and mental health when they are at university but potentially for the rest of their lives. At a time when increasing number of young people are pursuing university education, this could have huge health improvement applications.
Mindfulness meditation is a way of training the attention and its regulation for the purpose of promoting mental health. Within mindfulness-based programmes, mindfulness practice has been frequently defined as the awareness of paying attention to the present moment internally and externally, with an attitude of non-judgemental kindness and curiosity14. Mindfulness-based programmes may be described as trans-diagnostic interventions, associated with improvements on various health outcomes within both clinical and non-clinical settings15. There is evidence for the effectiveness of mindfulness-based programmes to prevent psychological distress16, and help to mitigate the negative symptoms of common mental disorders such as depression and anxiety17. Several theoretical models of mindfulness have been proposed depicting specific psychological mechanisms of action underlying these effects. The most commonly-cited mechanisms include decentering, the regulation of attention and/or self, self-compassion, and acceptance18–24.
Heightened attention and/or self-regulation emerging from mindfulness practice may reduce the extent to which potential social stressors are cognitively evaluated as threats 15,25,26. This may in turn reduce sympathetic activation and consequently pro-inflammatory immune response. A small but growing number of studies are assessing whether mindfulness training can influence the immune system. A recent meta-analysis combining randomised trials of meditation techniques in clinical and non-clinical samples found reductions in blood cortisol, C-reactive protein (CRP) and tumour necrosis factor-α (TNF-α) but no change in IL-6 compared with active controls27. The only consistent findings of a systematic review of randomised trials looking at mindfulness training in different populations were reductions in CRP, and increases in CD4+ T cell count among HIV-diagnosed individuals28. Null findings or a lack of replicated effects were found for antibodies, interleukins IL-1, IL-6, IL-8 and IL-10, IFN-γ, TNF-α, and various measures of immune cell proportion and functionality. Studies performed by Linda Witek-Janusek and colleagues demonstrated that women recently diagnosed with breast cancer had reductions in peripheral blood NK cell activity and IFN-g production, accompanied by increased IL-4, IL-6 and IL-1029,30. Interestingly, following mindfulness-based stress reduction cortisol levels were reduced and normal NK cell function re-established.
Thus, the effects of mindfulness training programmes on immunological biomarkers are less clear within non-clinical than clinical populations31. Few basic immunological outcomes have been tested in randomised trials of community samples32–40. We aimed to conduct a well-controlled pre-post exploration of how mindfulness buffers the effects of stress on the normal immune system in a typically healthy but psychologically distressed population of university students41.
Among undergraduates at the University of Cambridge stress peaks during the exam term - two months of revision and examinations which determine the outcome of their entire academic year; grades can play a crucial role in defining their career paths. In 2015 the University of Cambridge funded the Mindful Student Study: a randomised, waiting-list controlled trial of an 8-week manualised mindfulness program adapted to students42. In this trial we showed that mindfulness training reduces psychological distress among university students, particularly during the exam revision period, improving resilience to stress. We conducted a small exploratory trial alongside the Mindful Student Study to study immune dysregulation and inflammation stemming from this cohort of students who were exposed to progressively greater stress as the exam period approached, and to explore whether mindfulness training could help mitigate this dysregulation.
Results
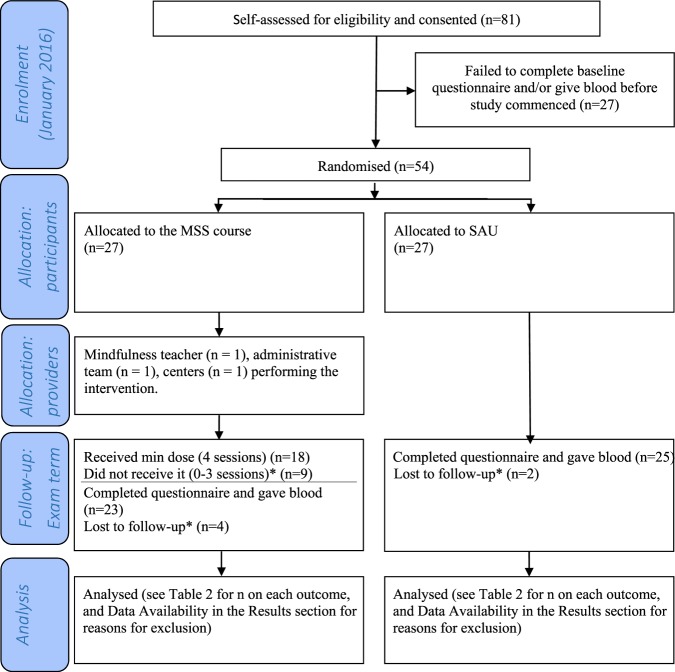
Figure 1 describes the flow of participants. The trial ran and ended as planned. Participants randomised to the intervention attended a median of seven sessions (inter-quartile range 2–7) including one person who attended no sessions at all, and six students who attended all the sessions. Eighteen participants (67% of those randomised to the intervention) attended at least half of the mindfulness course sessions, our pre-specified “minimum dose”42 also used in previous studies43, and shown to produce meaningful change44. Three participants provided reasons for abandoning their mindfulness course; these were schedule conflicts or being too busy. Six participants (11%) failed to provide outcome data. No participants had adverse reactions related to self-harm, suicidality, or harm to others16.
Figure 1.
CONSORT 2010 flow diagram. *Reasons explained in text. Abbreviations: min = minimum, MSS = Mindfulness Skills for Students; SAU = support as usual.
Table 1 shows participants’ characteristics, and Table 2 shows outcome values at all time points. In small trials randomisation can still produce imbalanced groups by chance; in our sample control group participants had a larger body mass index, a plausible confounder, therefore we have controlled for it in sensitivity analyses. However, none of the baseline random imbalances were “statistically significant”.
Table 1.
Participants’ characteristics.
| Control | Intervention | Total | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | Females | 20 | 74.07% | 18 | 66.67% | 38 | 70.37% |
| Age | 17–21 | 11 | 40.74% | 13 | 48.15% | 24 | 44.44% |
| 22–30 | 12 | 44.44% | 12 | 44.44% | 24 | 44.44% | |
| 31+ | 4 | 14.81% | 2 | 7.41% | 6 | 11.11% | |
| Nationality | UK or European Union | 16 | 59.26% | 20 | 74.07% | 36 | 66.67% |
| Ethnicity | Asian | 6 | 23.08% | 5 | 20.00% | 11 | 21.57% |
| Black | 1 | 3.85% | 0 | 0.00% | 1 | 1.96% | |
| Mixed | 2 | 7.69% | 0 | 0.00% | 2 | 3.92% | |
| Other | 0 | 0.00% | 3 | 12.00% | 3 | 5.88% | |
| White | 17 | 65.38% | 17 | 68.00% | 34 | 66.67% | |
| Disability | Yes | 1 | 3.70% | 4 | 14.81% | 5 | 9.26% |
| Degree | Undergraduate | 12 | 44.44% | 12 | 44.44% | 24 | 44.44% |
| Masters | 1 | 3.70% | 3 | 11.11% | 4 | 7.41% | |
| MPhil | 5 | 18.52% | 5 | 18.52% | 10 | 18.52% | |
| PhD | 9 | 33.33% | 7 | 25.93% | 16 | 29.63% | |
| Last Year | Yes | 14 | 51.85% | 13 | 48.15% | 27 | 50.00% |
| BMI | Underweight | 0 | 0% | 2 | 9% | 2 | 4% |
| Healthy weight | 14 | 56% | 15 | 65% | 29 | 60% | |
| Overweight | 7 | 28% | 5 | 22% | 12 | 25% | |
| Obese | 4 | 16% | 1 | 4% | 5 | 10% | |
Table 2.
Outcome values for all time points and groups, and statistical tests for pre-post changes on the total sample.
| Baseline | Exam term | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | Range | N | Mean | SD | Median | Range | ||
| CORE-OM | MSS | 26 | 0.93 | 0.49 | 0.87 | 0.18 to 2.06 | 25 | 1.01 | 0.61 | 0.85 | 0.09 to 2.29 |
| Control | 27 | 0.9 | 0.54 | 0.76 | 0.03 to 0.2.32 | 27 | 1.05 | 0.52 | 1 | 0.06 to 2.06 | |
| Total | 53 | 0.91 | 0.51 | 0.82 | 0.03 to 2.32 | 52 | 1.03 | 0.56 | 0.96 | 0.06 to 2.29 | |
| Cortisol | MSS | 26 | 313.65 | 123.48 | 325 | 109 to 518 | 22 | 324.55 | 201.82 | 300 | 121 to 992 |
| Control | 27 | 353.96 | 199.15 | 303 | 118 to 896 | 25 | 289.32 | 168.97 | 205 | 119 to 781 | |
| Total | 53 | 334.19 | 166.06 | 316 | 109 to 896 | 47 | 305.81 | 183.87 | 228 | 119 to 992 | |
| IL-8 | MSS | 26 | 8.43 | 3.29 | 7.9 | 3.9 to 17.6 | 22 | 9.35 | 3.86 | 8.15 | 5.1 to 21.4 |
| Control | 27 | 7.24 | 2.54 | 6.6 | 3 to 14.4 | 25 | 8.85 | 3.67 | 8.2 | 4.3 to 21.6 | |
| Total* | 53 | 7.82 | 2.97 | 7 | 3 to 17.6 | 47 | 9.08 | 3.72 | 8.2 | 4.3 to 21.6 | |
| TNF-α | MSS | 26 | 1.78 | 0.43 | 1.75 | 1 to 2.7 | 22 | 2.12 | 0.7 | 1.85 | 1.4 to 4.2 |
| Control | 27 | 1.74 | 0.39 | 1.7 | 1.2 to 3.1 | 25 | 1.97 | 0.43 | 1.8 | 1.5 to 3.5 | |
| Total** | 53 | 1.76 | 0.4 | 1.7 | 1 to 3.1 | 47 | 2.04 | 0.57 | 1.8 | 1.4 to 4.2 | |
| CRP | MSS | 26 | 1.84 | 2.16 | 1.3 | 0.5 to 11.7 | 22 | 1.32 | 1.23 | 0.75 | 0.5 to 4.4 |
| Control | 27 | 2.15 | 1.89 | 1.4 | 0.9 to 8.8 | 25 | 1.78 | 2.24 | 1 | 0.5 to 9.4 | |
| Total* | 53 | 2 | 2.01 | 1.4 | 0.5 to 11.7 | 47 | 1.57 | 1.83 | 1 | 0.5 to 9.4 | |
| % CD4+ T Lymphocytes | MSS | 24 | 0.62 | 0.1 | 0.64 | 0.33 to 0.75 | 20 | 0.6 | 0.09 | 0.62 | 0.45 to 0.73 |
| Control | 26 | 0.65 | 0.11 | 0.66 | 0.39 to 0.82 | 25 | 0.59 | 0.1 | 0.59 | 0.33 to 0.76 | |
| Total* | 50 | 0.63 | 0.1 | 0.65 | 0.33 to 0.82 | 45 | 0.6 | 0.1 | 0.62 | 0.33 to 0.76 | |
| % CD8+ T Lymphocytes | MSS | 24 | 0.23 | 0.1 | 0.21 | 0.14 to 0.58 | 20 | 0.28 | 0.08 | 0.26 | 0.18 to 0.47 |
| Control | 26 | 0.19 | 0.08 | 0.17 | 0.09 to 0.5 | 25 | 0.29 | 0.08 | 0.29 | 0.15 to 0.5 | |
| Total*** | 50 | 0.21 | 0.09 | 0.18 | 0.09 to 0.58 | 45 | 0.29 | 0.08 | 0.28 | 0.15 to 0.5 | |
| % CD19+ B Lymphocytes | MSS | 24 | 0.15 | 0.09 | 0.14 | 0.03 to 0.37 | 19 | 0.22 | 0.08 | 0.21 | 0.05 to 0.34 |
| Control | 26 | 0.16 | 0.07 | 0.16 | 0.05 to 0.29 | 25 | 0.25 | 0.09 | 0.23 | 0.08 to 0.4 | |
| Total*** | 50 | 0.16 | 0.08 | 0.16 | 0.03 to 0.37 | 44 | 0.23 | 0.08 | 0.23 | 0.05 to 0.4 | |
| % CD14+ Monocytes | MSS | 24 | 0.35 | 0.12 | 0.33 | 0.12 to 0.6 | 18 | 0.42 | 0.1 | 0.4 | 0.22 to 0.61 |
| Control | 26 | 0.38 | 0.1 | 0.37 | 0.16 to 0.58 | 24 | 0.45 | 0.12 | 0.47 | 0.22 to 0.61 | |
| Total** | 50 | 0.37 | 0.11 | 0.36 | 0.12 to 0.6 | 42 | 0.43 | 0.11 | 0.42 | 0.22 to 0.66 | |
| % NK cells | MSS | 24 | 0.25 | 0.13 | 0.21 | 0.09 to 0.63 | 18 | 0.29 | 0.13 | 0.29 | 0.11 to 0.49 |
| Control | 26 | 0.18 | 0.09 | 0.15 | 0.06 to 0.44 | 24 | 0.24 | 0.11 | 0.22 | 0.08 to 0.54 | |
| Total** | 50 | 0.21 | 0.11 | 0.2 | 0.06 to s0.63 | 42 | 0.26 | 0.12 | 0.23 | 0.08 to 0.54 | |
*p < 0.05; **p < 0.01; ***p < 0.001. All p values are adjusted for multiple comparisons. Abbreviations: MSS: Mindfulness Skills for Students course; NK: natural killer.
Data from two participants did not pass quality controls and were removed from further analysis. Only four blood biomarkers had levels that were above the limit of quantification for the majority of participants: cortisol, CRP, IL-8, and TNF-α. Cortisol is a hormone that is thought to mediate the effects of chronic stress on the immune system through complex interplays; CRP, IL-8, and TNF-α are pro-inflammatory proteins secreted by cells during the course of a normal pro-inflammatory immune response8.
We performed a PLS analysis to determine the combinations of cell classes (components) which best explained variation in distress levels measured during the exam period. Predictor variables were the 154 cell classes and the response variable was the CORE-OM score. Permutation testing of the model was not significant (p = 0.738), precluding inference about the contribution of specific cell classes to the model (Supplementary figure S2). We therefore manually chose cell classes based on prior evidence within the scientific literature.
Immune responses to stress
As a preliminary check, we performed a full-sample pre-post comparison of outcome values to assess the effects of the stressful exam revision period on the immune system. Several factors related to inflammation were significantly increased the second time participants were sampled, which was during the exam period: IL-8 (*p < 0.05), TNF-α (**p < 0.01), CD8+ T cells (***p > 0.001), B cells (***p < 0.001), monocytes (**p < 0.01), and NK cells (**p < 0.01). Conversely, CRP (*p < 0.05), CD4+ T cells (*p < 0.05) were significantly decreased (after correction for multiple comparison testing) when sampled during the exam period (Table 2).
Table 3 summarises the analyses assessing how changes in perceived distress levels correlated with changes in blood biomarkers and cell proportions. The proportion of B lymphocytes significantly increased by 0.05 with every one-point increase in the distress scale (p adjusted for multiple comparisons = 0.027); as a sensitivity analysis we adjusted this model for body mass index and the result was maintained. The effect is visually described in Fig. 2 (similar figures for all outcomes are shown in supplementary figures S3 and S4). There were no other significant relationships between biomarkers and distress levels.
Table 3.
Summary of random intercept models looking at fixed effects for change in distress.
| Outcome (unit) | Regression coefficienta | Standard error | P-valueb |
|---|---|---|---|
| Cortisol (nmol.L) | −45.67 | 34.69 | 0.95 |
| CRP (mg.L) | 0.52 | 0.33 | 0.77 |
| IL-8 (pg.ml) | 0.48 | 0.63 | 1 |
| TNFa (pg.ml) | −0.07 | 0.10 | 1 |
| % CD4+ T Lymphocytes | 0.04 | 0.02 | 0.40 |
| % CD8+ T Lymphocytes | −0.03 | 0.02 | 0.77 |
| % CD19+ B Lymphocytes | 0.05 | 0.02 | 0.027 |
| % CD14+ Monocytes | 0.03 | 0.02 | 0.95 |
| % NK cells | −0.002 | 0.02 | 1 |
aAdjusted mean difference. bAll p values are adjusted for multiple comparisons. Abbreviations: NK: natural killer.
Figure 2.
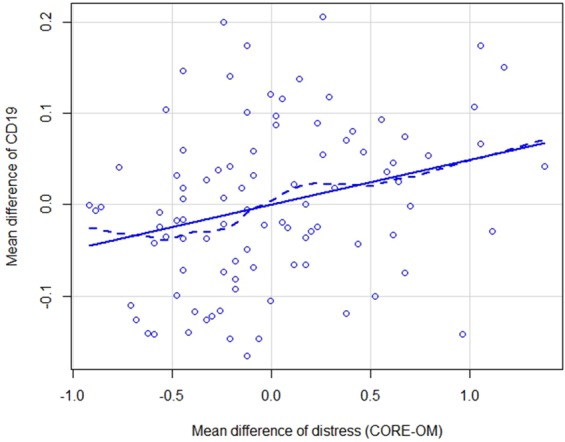
Relationship between mean differences of CORE-OM and mean differences of CD19. To obtain the mean differences, the distances of each CORE and CD19 data point from the mean CORE and CD19 at their respective time points were calculated.
Effects of mindfulness training
Table 4 summarises the analyses assessing the effects of mindfulness training on blood biomarkers and immune cells. There were no significant effects on any of the parameters (unadjusted p = 0.03 for the effect on NK cells). Results were similar in the per-protocol analyses.
Table 4.
Summary of random intercept models looking at fixed effects for trial arm (ITT analysis).
| Outcome (unit) | Regression coefficienta | Standard error | P-valueb |
|---|---|---|---|
| Cortisol (nmol.L) | −2.34 | 40.92 | 1 |
| CRP (mg.L) | −0.40 | 0.49 | 1 |
| IL-8 (pg.ml) | 0.94 | 0.84 | 1 |
| TNFa (pg.ml) | 0.08 | 0.11 | 1 |
| %CD4+ T Lymphocytes | −0.01 | 0.03 | 1 |
| %CD8+ T Lymphocytes | 0.02 | 0.02 | 1 |
| %CD19+ B Lymphocytes | −0.02 | 0.02 | 1 |
| %CD14+ Monocytes | −0.03 | 0.03 | 1 |
| %NK cells | 0.07 | 0.03 | 0.27 |
aAdjusted mean difference. bAll p values are adjusted for multiple comparisons. Abbreviations: ITT: intention-to-treat; NK: natural killer.
Discussion
In our opportunistic study within a larger trial, healthy university students were exposed to an objectively stressful insult – exam revision – persisting for several weeks, but their subjective experience of psychological distress varied. We set out to test whether participants’ changes in subjective experience were linked to stress-related pro-inflammatory immune change, and whether mindfulness training would buffer these effects.
Regardless of whether the students underwent mindfulness training or not, several immune parameters were significantly increased across the whole cohort during the exam period when compared with the baseline measurements. A potential confounding factor lies in the season of sampling; all baseline measurements were collected in January (winter) whereas the exam period measurements were collected in June (spring). Relatively little is known regarding seasonal changes in immune cell populations. One study found that seasonal gene expression changes in peripheral blood mononuclear cells (PBMCs) strongly correlated with the expression levels of genes known to mark different immune cell types45, indirectly suggesting that the cellular composition of peripheral blood changes with the season. A second study found that complete blood count was subject to seasonal variation, however this study did not address changes to specific immune cell subsets such as B cells and NK cells46. It is possible that these changes across the entire cohort in our study may represent, in part, seasonal effects. Regardless, for all but the CD19+ B cells, these effects were not related to measures of perceived psychological distress.
A distinctive feature of B cells is that they produce antibodies; an increase in their proportion may indicate elevated immune activity that may in turn increase chronic tissue inflammation47,48. Our finding that the proportion of B cells increases with increased psychological distress (Table 3 and Fig. 2) is in line with previous research on exam stress in university samples. In a recent cohort study, students B cells increased significantly with exam stress compared to community controls while awakening cortisol response flattened49. B cells however decreased when controlling for awakening cortisol response. It is possible that this could be due to negation of the mediating influence of decreased cortisol on upregulation of B cell production. Further supporting our findings, Maes et al. assessed students the day before examinations and found several changes, among them an increase in the absolute numbers of B cells50. Very little is known about the precise role of B cells in psychological distress. Only a handful of studies have demonstrated alterations in the proportion or activity of B cells as a result of psychological distress, and to the best of our knowledge no mechanism has yet been proposed.
Regarding our null findings in other parameters, Segerstrom et al. also did not find evidence of TNF-α being effected by exam stress in a meta-analysis of a hundred healthy individuals8. In other studies, academic stress did not elevate cortisol levels51, and there were no correlations between plasma cortisol levels and type-1/type-2 cytokine production52. The effect size coefficients suggest there could be an inverse relationship between cortisol and distress. This was not statistically significant, but this could be due to lack of statistical power, as suggested by the large dispersion measures.
In healthy individuals immune parameters are relatively stable within a range53. Distress may need to surpass a threshold for the immune system to be triggered. The immune system is remarkably flexible and capable of substantial change without compromising an otherwise healthy host8. A comprehensive but somewhat dated meta-analysis of examination stress studies did not find evidence for this type of stress, either objectively or subjectively experienced, markedly affecting the number or percentage of cells in peripheral blood8. An alternative explanation for these null findings is some immunological changes related to stress may be relatively restricted to the brain and meningeal immune compartments, and not reflected (or less easily detected) in peripheral blood cells. The immunophenotyping used in our study is far more comprehensive than any previously published studies to our knowledge, but we still detected few meaningful cell percentage changes. Cell changes among clinical populations may be easier to detect, e.g. asthmatic students54.
Our controlled pre-post analysis represents significant progress compared to evidence coming from cross-sectional samples. We applied multiple comparison corrections and leveraged multivariate techniques to ensure a robust analysis of the high-dimensional data generated by this study. However, given the small sample size, we were unable to adjust findings for plausible confounders such as body mass index (for which we only have controlled one sensitivity analysis), age or gender. There is a need for adjusted replication in larger samples.
Loss to follow up (11%) may have biased our sample in ways that are hard to predict, although such figures are to be expected in trials testing non-clinical behavioural interventions55. In addition, many cytokine analyses were limited by sample levels being below reliable quantification ranges in our analyses. Indeed, cytokine levels are generally low in otherwise healthy individuals, so quantification methods may need higher sensitivity to measure these reliably.
With respect to the effects of mindfulness training, we know from the Mindful Student Study, conducted on a larger sample of 616 students, that it significantly reduces self-reported psychological distress with a moderate effect size16. The present study could not clearly detect how this reduction in distress generated by mindfulness training translates into changes in immune parameters related to inflammation. However, based on our data we cannot discard a biological linkage between mindfulness and distress. There was an initial indication that the proportion of NK cells may increase with mindfulness training, but statistical significance did not survive multiple comparison correction. However, a previous non-randomised trial suggested that mindfulness training increases NK cell activity among women recently diagnosed with breast cancer30, and lower levels of NK activity were detected among healthy individuals who were more distressed than their peers8,56.
NK cells play a major role in the rejection of both tumour and virally infected cells; higher levels may indicate a stronger defence against these threats but the functional implications for chronic tissue inflammation are still unclear57,58. In any case, despite the methodological strengths of our randomised design (mainly the minimization of confounding effects), a larger trial will be required to clarify the effect of mindfulness training on the proportion of NK cells. Alternatively, this could be clarified by increasing the number of repeated measures within a shorter period and more proximal to the stressor. It is plausible that whilst there is only a marginal change in the proportion of NK cells, there may be alterations to their cytolytic activity or gene expression profiles which were not investigated in the current study.
More generally, Black et al. did not find evidence that mindfulness training affects IL-8, TNF-α or immune cell counts28. Other randomised trials of community samples also failed to find effects on CRP35,59 and cell counts60, although mindfulness meditation significantly influenced TNF-α levels in healthy individuals when individual studies were combined in a meta-analysis. Trials on clinically anxious or depressed populations found significant effects on TNF-α with samples of 60–70 participants61,62; however a recent actively controlled trial with HIV patients failed to find any effects63,27.
In line with our trial, mindfulness training has repeatedly failed to show evidence of reducing salivary cortisol reactivity to acute stress in randomised trials of community samples32,36,38,59. In a trial conducted in Germany with 313 volunteers from the community, after 3-month mindfulness-based attention training, self-reported stress reactivity to the Trier Social Stress Test (TSST) was reduced but salivary cortisol stress response was increased (non-significantly) relative to no training40. Three-day mindfulness meditation training in a trial assessing university students in the United States also reduced self-reported stress reactivity but significantly increased salivary cortisol reactivity, relative to the cognitive training controls39. The latter result might be explained by a subconscious increase in distress during the early stages of mastering emotion regulation mindfulness skills, when decentering and interoceptive awareness have not been well developed yet. However, this post-hoc explanation would need to be tested in a purposively designed prospective study.
This small exploratory study contributed to the existing literature by strengthening the evidence on some promising directions regarding stress, mindfulness and immune dysregulation. Our finding that the proportion of B cells increases with increased psychological distress is robust and confirms previous research, but needs well-adjusted replication. If mindfulness training is to buffer the effects of psychological distress on the immune system of healthy populations under stressful situations, randomised trials with larger samples, more measurement time points, and perhaps more intense training, will be required to detect it. A larger sample size would additionally allow the researcher to investigate the effects of the ‘dose’ of mindfulness-based interventions on immune cell parameters. In order to avoid multiple testing, future trials could focus their efforts on NK cells and B cells which our study has shown may be promising directions.
Methods
The Cambridge Psychology Research Ethics Committee approved this study on 16/12/2015 (PRE.2015.098). Informed consent was obtained from all participants, and volunteer participants followed all the steps of the Mindful Student Study randomised controlled trial in accordance with guidelines and regulations16,42. The trial was registered with the Australia and New Zealand Clinical Trials Registry on 30/10/2015, number ACTRN12615001160527.
In January 2016 University of Cambridge students without severe mental illness or crisis self-enrolled online and were randomly allocated (1:1), automatically via remote survey software using computer-generated random numbers, to join an 8-week mindfulness course adapted for university students plus mental health support as usual (intervention group), or to mental health support as usual alone (control group). Mental health support as usual consisted of the opportunity to access centralised support at the University of Cambridge Counselling Service (UCS) in addition to pastoral support at university college level (Cambridge is a collegiate University comprising 31 Colleges which admit, accommodate, tutor and teach students), and public health services external to the University. Those allocated to the control group were offered a mindfulness course one year later, providing they were still students at the University.
The primary outcome was self-reported psychological distress during the main examination period in the University academic calendar (May-June 2016). Please see trial publications for more details on procedures such as randomisation, questionnaire data collection, adverse event monitoring, et cetera16,42.
The inclusion criteria for participation in the sub-study reported here were: (a) Mindful Student Study participant recruited in January 2016; (b) having an exam, viva, 1st year PhD report, or dissertation deadline between 15 May & 15 July 2016 (self-reported). The exclusion criteria, all self-reported, were: (a) personal or family history of autoimmune disorders, severe allergy or asthma; (b) being on regular steroid medication; (c) illegal drug or alcohol dependence (addiction); (d) having meditated for more than 10 hours in total in the past or having done a mindfulness 8-week course. Sample size for this sub-study was determined based on resource availability given the costs of immunophenotyping. Hence the pilot nature of this sub-study.
The intervention, called Mindfulness Skills for Students, consisted of a secular, face-to-face, group-based skills training programme based on the course book ‘Mindfulness: A Practical Guide to Finding Peace in a Frantic World’64, and adapted for university students. Adaptations were focused on permeating every session with elements of flexibility, self-discovery, self-compassion and empowerment, aimed at generating a natural transfer of skills developed in meditation to study, decision-making and relationships. This course aimed to optimise wellbeing and resilience across a range of students and was not specifically developed for those students in the clinical range. An experienced and certified mindfulness teacher delivered seven, parallel courses between late January and March 2016 (i.e., all the participants randomised to the intervention did the course at the same time); each course accommodated up to 30 students and comprised eight, weekly sessions lasting 75–90 minutes.
Measures and procedures
Psychological distress was measured using the Clinical Outcomes in Routine Evaluation Outcome Measure (CORE-OM), a 34-item generic questionnaire which has been widely used with UK university students65. A higher score indicates increased distress. The total mean score (range 0–4) is obtained by dividing the total score by the number of completed items (as long as no more than three items have been missed)66. CORE-OM has good convergent validity, internal and test-retest reliability and sensitivity to change67.
Participants in the present study consented to donate two blood samples on top of completing the Mindful Student Study CORE-OM questionnaire. One sample was taken before participants were randomised (baseline measurement) and the second sample approximately 3–4 months after randomisation (exam period measurement). At both time points, the CORE-OM questionnaires were collected within a week of blood sampling.
We used state-of-the-art immunophenotyping to examine the proportions of over 150 different key peripheral immune cell subsets to explore the activation patterns that emerge from exposure to chronic stress, and how mindfulness training modifies these patterns. Methods, staining procedure, antibody combinations, instrument configuration and gating strategies are described in the supplementary information. Immunophenotyping by multi-colour flow cytometry is the most powerful technology available for the analysis of population dynamics, cellular phenotype and function in the immune system68. It systematically evaluates the unique repertoires of cell subsets and activation markers. In addition, we measured key molecules associated with inflammatory processes: cortisol, pro- and anti-inflammatory cytokines (IL-2, IL-4, IL-6, IL-8, IL-10, IFN-γ, TNF-α, IL-1b, IL-12p70, IL-13), Epstein Barr virus and cytomegalovirus antibodies, and CRP.
All participants provided two samples of up to 22.5 mL venous blood. Blood were drawn from a butterfly placed in the forearm while participants were lying quietly on an examination couch in a clinical research facility at Addenbrookes Hospital (Cambridge, UK) with medical supervision. Their height and weight were measured using standard scales. Participants were also asked a few standard medical questions by a member of the research team (e.g., “Do you feel sick today?”) to collect information on possible recent/ongoing inflammatory processes that could explain any abnormal results in their blood on that day. As a token of appreciation, £20 were offered to each participant per sampling session in the form of Amazon vouchers.
Samples for each individual were given a unique, anonymised code and immediately subdivided for the different analyses. The researchers processing the samples were unaware of the identities of the individuals who gave the samples and had no access (blind) to questionnaire and trial arm data.
Cytokines were analysed in duplicate using the MesoScale Discovery multiplexed panel as per manufacturer’s instructions. Only cortisol, CRP, IL-8, and TNF-α were reliably within the range of detection of the assay. Immunophenotyping was performed as described in the supplementary information (SI) using the antibody panel described in SI Table 1 and SI Table 2. Leukocyte populations were expressed as relative proportions of the parent population.
Analysis
This was an exploratory study to generate biological hypotheses for further testing. However, previous research suggests the following pre-specified hypotheses:
The increase in perceived distress as a result of exams approaching generates a dysregulated pro-inflammatory state, characterised by increased proportions of monocytes and activated lymphocytes, accompanied by increases in pro-inflammatory humoral immune factors6,69. To test this hypothesis, we used random intercept models including each cell-type/humoral marker and psychological distress. The small sample size precluded further adjusting.
Individuals trained in mindfulness will show less pro-inflammatory dysregulation35,62. To test this hypothesis, we used random intercept models including each cell-type/humoral and arm data. The analyses followed the intention-to-treat principle, but per-protocol analyses were also conducted as sensitivity analyses to explore the impact of adherence to the intervention. In the per-protocol analyses only those intervention participants who received our pre-specified “minimum dose” (i.e. attended at least half of the mindfulness course sessions) were included.
The number of cell classes returned by this type of immunophenotyping is very large, therefore in order to pre-select the most promising cell classes and thus reduce the number of predictors we used two strategies: data driven, and theory driven. For the data driven strategy we conducted a partial least squares (PLS) analysis using all of the 154 cell classes that were enumerated by the immunophenotyping analysis. PLS is a multivariate technique used to identify associations between a response variable, in this case psychological distress, and a set of predictors, here the relative immune cell counts. Permutation test and bootstrap were used to assess accuracy and confidence intervals, respectively (method described in full in Fernandez-Egea, E. et al.70). For the theory-driven strategy we looked at those cell classes implicated in previous distress and/or mindfulness studies: CD4+ and CD8+ T cells28, B cells49, NK cells30 and CD14 monocytes50.
To further avoid spurious results due to multiple testing, we corrected the p values using the Holm adjustment71. Mixed linear models were used for the analyses, which make efficient use of all available data in small samples72. Analyses were conducted at an alpha level of p = 0.05 (two-sided) using ‘R‘ 3.4.4. The current sample was independent of that included in the other Mindful Student Study analyses.
Supplementary information
Acknowledgements
We are very grateful to Elizabeth English, Sarah Ayerst, Caroline Ford, Fiona Sutton, and the study participants. We also wish to thank Natalia Savinykh-Yarkoni and Alex Hatton for their advice and support in flow cytometry, and Golam Khandaker and Mary-Ellen Lynall for their helpful comments on an earlier draft of the manuscript. This article presents independent research funded by the Mindful Trust, the University of Cambridge Vice-Chancellor’s Endowment Fund, the University Counselling Service and the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) East of England, at the Cambridgeshire and Peterborough NHS Foundation Trust. This research was supported by the Cambridge NIHR Biomedical Research Centre Cell Phenotyping Hub. L.T. is supported by the NIHR Cambridge BRC.
Author contributions
L.T., J.G. and P.B.J. conceived and planned the study; J.G. applied for funding; J.G., G.D., M.V. and P.B.J. were involved in participant recruitment and trial execution; L.T., J.G. and M.V. collected and processed the data; the analysis was done by L.T., J.G. and J.S.; L.T. and J.G. wrote the first draft; all authors reviewed and contributed to the manuscript.
Data availability
Anonymised data available on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lorinda Turner and Julieta Galante.
Supplementary information
is available for this paper at 10.1038/s41598-020-62274-7.
References
- 1.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA psychiatry. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Molecular psychiatry. 2016 doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huppert FA. A new approach to reducing disorder and improving well-being. Perspectives on Psychological Science. 2009;4:108–111. doi: 10.1111/j.1745-6924.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- 5.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature reviews. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 6.Morey JN, Boggero IA, Scott AB, Segerstrom SC. Current Directions in Stress and Human Immune Function. Current opinion in psychology. 2015;5:13–17. doi: 10.1016/j.copsyc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lill-Elghanian D, Schwartz K, King L, Fraker P. Glucocorticoid-induced apoptosis in early B cells from human bone marrow. Experimental biology and medicine (Maywood, N. J.) 2002;227:763–770. doi: 10.1177/153537020222700907. [DOI] [PubMed] [Google Scholar]
- 8.Segerstrom SC, Miller GE. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychological bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhabhar FS. Enhancing versus Suppressive Effects of Stress on Immune Function: Implications for Immunoprotection versus Immunopathology. Allergy, Asthma, and Clinical Immunology. 2008;4:2–11. doi: 10.2310/7480.2008.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Annals of the New York Academy of Sciences. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 11.Maes M, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biological psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 12.Witek-Janusek L, Gabram S, Mathews HL. Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology. 2007;32:22–35. doi: 10.1016/j.psyneuen.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological psychiatry. 2001;49:391–404. doi: 10.1016/S0006-3223(00)01088-X. [DOI] [PubMed] [Google Scholar]
- 14.Mindfulness All-Party Parliamentary Group. Mindful Nation UK Report, http://themindfulnessinitiative.org.uk/images/reports/Mindfulness-APPG-Report_Mindful-Nation-UK_Oct2015.pdf (2015).
- 15.Alsubaie M, et al. Mechanisms of action in mindfulness-based cognitive therapy (MBCT) and mindfulness-based stress reduction (MBSR) in people with physical and/or psychological conditions: A systematic review. Clinical psychology review. 2017;55:74–91. doi: 10.1016/j.cpr.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Galante J, et al. A mindfulness-based intervention to increase resilience to stress in university students (the Mindful Student Study): a pragmatic randomised controlled trial. The Lancet Public Health. 2018;3:e72–e81. doi: 10.1016/s2468-2667(17)30231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal M, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA internal medicine. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baer RA. Mindfulness Training as a Clinical Intervention: A Conceptual and Empirical Review. Clinical Psychology: Science and Practice. 2003;10:125–143. doi: 10.1093/clipsy.bpg015. [DOI] [Google Scholar]
- 19.Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological inquiry. 2007;18:211–237. doi: 10.1080/10478400701598298. [DOI] [Google Scholar]
- 20.Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. Journal Of Clinical Psychology. 2006;62:373–386. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- 21.Grabovac AD, Lau MA, Willett BR. Mechanisms of Mindfulness: A Buddhist Psychological Model. Mindfulness. 2011;2:154–166. doi: 10.1007/s12671-011-0054-5. [DOI] [Google Scholar]
- 22.Holzel BK, et al. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspectives on psychological science: a journal of the Association for Psychological Science. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 23.Vago DR, David SA. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Frontiers In Human Neuroscience. 2012;6:296. doi: 10.3389/fnhum.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuyken W, et al. How does mindfulness-based cognitive therapy work? Behaviour research and therapy. 2010;48:1105–1112. doi: 10.1016/j.brat.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Black DS, Christodoulou G, Cole S. Mindfulness meditation and gene expression: a hypothesis-generating framework. Current opinion in psychology. 2019;28:302–306. doi: 10.1016/j.copsyc.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu J, Strauss C, Bond R, Cavanagh K. Corrigendum to “How do Mindfulness-Based Cognitive Therapy and Mindfulness-Based Stress Reduction Improve Mental Health and Wellbeing? A Systematic Review and Meta-Analysis of Mediation Studies” Clinical Psychology Review 37 (2015) 1–12. Clinical psychology review. 2016;49:119. doi: 10.1016/j.cpr.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Pascoe MC, Thompson DR, Jenkins ZM, Ski CF. Mindfulness mediates the physiological markers of stress: Systematic review and meta-analysis. Journal of psychiatric research. 2017;95:156–178. doi: 10.1016/j.jpsychires.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Black, D. S. & Slavich, G. M. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Annals of the New York Academy of Sciences, 10.1111/nyas.12998 (2016). [DOI] [PMC free article] [PubMed]
- 29.Witek Janusek L, Tell D, Mathews HL. Mindfulness based stress reduction provides psychological benefit and restores immune function of women newly diagnosed with breast cancer: A randomized trial with active control. Brain Behav Immun. 2019;80:358–373. doi: 10.1016/j.bbi.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Witek-Janusek L, et al. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanada K, et al. Effects of mindfulness-based interventions on biomarkers in healthy and cancer populations: a systematic review. BMC complementary and alternative medicine. 2017;17:125. doi: 10.1186/s12906-017-1638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenkranz MA, et al. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun. 2013;27:174–184. doi: 10.1016/j.bbi.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson RJ, et al. Alterations in Brain and Immune Function Produced by Mindfulness Meditation. Psychosomatic Medicine. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 34.Barrett B, et al. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Annals of family medicine. 2012;10:337–346. doi: 10.1370/afm.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Creswell JD, et al. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyklicek I, Mommersteeg PM, Van Beugen S, Ramakers C, Van Boxtel GJ. Mindfulness-based stress reduction and physiological activity during acute stress: a randomized controlled trial. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2013;32:1110–1113. doi: 10.1037/a0032200. [DOI] [PubMed] [Google Scholar]
- 37.Creswell, J. D. et al. Alterations in resting state functional connectivity link mindfulness meditation with reduced interleukin-6: a randomized controlled trial. Biological psychiatry, 10.1016/j.biopsych.2016.01.008 (2016). [DOI] [PubMed]
- 38.Flook, L., Goldberg, S. B., Pinger, L., Bonus, K. & Davidson, R. J. Mindfulness for Teachers: A Pilot Study to Assess Effects on Stress, Burnout, and Teaching Efficacy. Mind Brain Educ7, 10.1111/mbe.12026. (2013). [DOI] [PMC free article] [PubMed]
- 39.Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Engert V, Kok BE, Papassotiriou I, Chrousos GP, Singer T. Specific reduction in cortisol stress reactivity after social but not attention-based mental training. Sci Adv. 2017;3:e1700495. doi: 10.1126/sciadv.1700495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macaskill A. The mental health of university students in the United Kingdom. British Journal of Guidance & Counselling. 2013;41:426–441. doi: 10.1080/03069885.2012.743110. [DOI] [Google Scholar]
- 42.Galante, J. et al. Protocol for the Mindful Student Study: a randomised controlled trial of the provision of a mindfulness intervention to support university students’ well-being and resilience to stress. BMJ open610.1136/bmjopen-2016-012300. (2016). [DOI] [PMC free article] [PubMed]
- 43.Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clinical psychology review. 2015;37C:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Demarzo M, et al. Efficacy of 8- and 4-Session Mindfulness-Based Interventions in a Non-clinical Population: A Controlled Study. Frontiers in psychology. 2017;8:1343. doi: 10.3389/fpsyg.2017.01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dopico XC, et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nature communications. 2015;6:7000. doi: 10.1038/ncomms8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Taioli E. Seasonal Variations of Complete Blood Count and Inflammatory Biomarkers in the US Population - Analysis of NHANES Data. PloS one. 2015;10:e0142382. doi: 10.1371/journal.pone.0142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 48.DeFuria J, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGregor BA, Murphy KM, Albano DL, Ceballos RM. Stress, cortisol, and B lymphocytes: a novel approach to understanding academic stress and immune function. Stress. 2016;19:185–191. doi: 10.3109/10253890.2015.1127913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maes M, et al. The effects of psychological stress on leukocyte subset distribution in humans: evidence of immune activation. Neuropsychobiology. 1999;39:1–9. doi: 10.1159/000026552. [DOI] [PubMed] [Google Scholar]
- 51.Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19:765–772. doi: 10.1016/0306-4530(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 52.Marshall GD, Jr., et al. Cytokine Dysregulation Associated with Exam Stress in Healthy Medical Students. Brain, Behavior, and Immunity. 1998;2:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- 53.Brodin P, Davis MM. Human immune system variation. Nature reviews. Immunology. 2017;17:21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang D-H, Coe CL, Mccarthy DO. Academic Examinations Significantly Impact Immune Responses, but Not Lung Function, in Healthy and Well-Managed Asthmatic Adolescents. Brain, Behavior, and Immunity. 1996;10:164–181. doi: 10.1006/brbi.1996.0015. [DOI] [PubMed] [Google Scholar]
- 55.Eysenbach G. The law of attrition. Journal of medical Internet research. 2005;7:e11. doi: 10.2196/jmir.7.1.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiecolt-Glaser JK, et al. Psychosocial Modifiers of Immunocompetence in Medical Students. Psychosomatic Medicine. 1984;46:7–14. doi: 10.1097/00006842-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Parisi L, et al. Natural Killer Cells in the Orchestration of Chronic Inflammatory Diseases. Journal of immunology research. 2017;2017:4218254–4218254. doi: 10.1155/2017/4218254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tosello-Trampont A, Surette FA, Ewald SE, Hahn YS. Immunoregulatory Role of NK Cells in Tissue Inflammation and Regeneration. Frontiers in immunology. 2017;8:301–301. doi: 10.3389/fimmu.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: a randomized trial. Brain Behav Immun. 2013;27:145–154. doi: 10.1016/j.bbi.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arefnasab, Z. et al. Mindfulness-based Stress Reduction (MBSR) and Its Effects on Psychoimmunological Factors of Chemically Pulmonary Injured Veterans. Iran J. Allergy Asthma Immunol (2016). [PubMed]
- 61.Hoge EA, et al. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry research. 2018;262:328–332. doi: 10.1016/j.psychres.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh E, Eisenlohr-Moul T, Baer R. Brief mindfulness training reduces salivary IL-6 and TNF-alpha in young women with depressive symptomatology. J. Consult Clin Psychol. 2016;84:887–897. doi: 10.1037/ccp0000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hecht, F. M. et al. A randomized, controlled trial of mindfulness-based stress reduction in HIV infection. Brain Behav Immun, 10.1016/j.bbi.2018.05.017 (2018). [DOI] [PMC free article] [PubMed]
- 64.Williams, M. & Penman, D. Mindfulness: a practical guide to finding peace in a frantic world. (Hachette UK, 2011).
- 65.Connell J, Barkham M, Mellor-Clark J. The effectiveness of UK student counselling services: an analysis using the CORE System. British Journal of Guidance & Counselling. 2008;36:1–18. doi: 10.1080/03069880701715655. [DOI] [Google Scholar]
- 66.Core System Group. CORE system user manual, http://www.coreims.co.uk/index.html.
- 67.Evans C, et al. Towards a standardised brief outcome measure: psychometric properties and utility of the CORE-OM. The British journal of psychiatry: the journal of mental science. 2002;180:51–60. doi: 10.1192/bjp.180.1.51. [DOI] [PubMed] [Google Scholar]
- 68.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nature reviews. Immunology. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnaba V, Paroli M, Piconese S. The ambiguity in immunology. Frontiers in immunology. 2012;3:18. doi: 10.3389/fimmu.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez-Egea E, et al. Peripheral Immune Cell Populations Associated with Cognitive Deficits and Negative Symptoms of Treatment-Resistant Schizophrenia. PloS one. 2016;11:e0155631. doi: 10.1371/journal.pone.0155631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. American Journal of Public Health. 1996;86:726–728. doi: 10.2105/AJPH.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O Connell, N. S. et al. Methods for Analysis of Pre-Post Data in Clinical Research: A Comparison of Five Common Methods. Journal of Biometrics & Biostatistics08, 10.4172/2155-6180.1000334 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised data available on request.