Abstract
This study determined the composition of the monosaccharide, 3, 6-anhydrogalactose (AnGal), in red algae and explored the potential whitening activity of the extract. Using gas chromatography–mass spectrometry (GC–MS), the AnGal composition of six different species of red seaweed (Porphyra haitanensis, Gracilaria chouae, Gracilaria blodgettii, Gracilaria lemaneiformis, Eucheuma galetinae, and Gelidium amansii) was successfully analyzed, revealing molar ratios ranging from 1.0:1.0 to 1.0:3.1 of AnGal and galactose (Gal), respectively. Employing the tyrosinase inhibition assay, the skin-whitening effect of AnGal red seaweed polysaccharides was determined. Polysaccharides from P. haitanensis, G. chouae, and G. blodgettii as well as their degradation products showed higher tyrosinase inhibitory activity (inhibition rates 24.2–26.8%). These results suggest that the GC–MS approach could conveniently be used in quality control or for the quantitative determination of AnGal and Gal in red seaweed polysaccharides as well as exploring their potential application in cosmetic and functional food products. The findings here exhibited that red seaweed polysaccharides and their degradation products were potential ingredients for cosmeceutical industries.
Keywords: Red seaweed; Polysaccharide; 3,6-Anhydro-galactose; Skin-whitening
Introduction
Red seaweeds (Rhodophyta) are found in abundance in oceans and have, therefore, gained much attention and interest from scientists and industry. These organisms are exceptionally rich sources of bioactive molecules and secondary metabolites, such as amino acids, peptides, proteins, polyphenols, and polysaccharides. Red seaweeds derived polysaccharides have shown a broad spectrum of biological activities, including anti-oxidant, anti-wrinkle, anti-inflammatory, anti-microbial, and anti-cancer activities (Khan et al. 2020; Xu et al. 2017). Thus, red seaweeds are excellent natural sources for numerous bioactive molecules that could be used as functional ingredients in various industrial applications, such as functional food, cosmetic, and pharmaceutical industries (Cheong et al. 2018; Wijesekara et al. 2011).
Polysaccharides derived from red seaweed are usually composed of repeating disaccharide units of alternating 1,3-linked galactose (Gal) and 1,4-linked 3,6-anhydro-galactose (AnGal) residues. The biological properties of red seaweed polysaccharides are highly dependent on the amount of AnGal, which is a key bioactive monomeric sugar of red seaweeds (Zheng et al. 2020; Yun et al. 2015). Some recent reports have demonstrated that AnGal-containing polysaccharides or oligosaccharides may be responsible for the skin-whitening (Kim et al. 2017) and anti-melanogenic (Kim et al. 2018) activities of red seaweeds. However, the amount of AnGal in red algal polysaccharide varies significantly between species, in addition to seasonal variation related to climatic factors (Kravchenko et al. 2018). It is, therefore, necessary to establish a simple and accurate method to quantify AnGal in red seaweed polysaccharides, as red seaweed polysaccharides have important use in functional foods and cosmeceuticals.
Currently, the main method used for quantitative analysis of red seaweed polysaccharides involves hydrolysis of the polysaccharides to release monosaccharides followed by colorimetric assay or chromatographic analysis (Cheong et al. 2015; Cui et al. 2019; Sudharsan et al. 2018). Unfortunately, the AnGal residues are unstable in acidic conditions, as these are easily converted into Gal residues or 5-hydroxymethyl-furfural by common acid hydrolysis procedures. This is one of the main reasons for some studies to report or show the monosaccharide compositions of red seaweed polysaccharides without being able to detect AnGal (Sudharsan et al. 2018; Xu et al. 2018a, b; Seedevi et al. 2017).
In the colorimetric determination of total carbohydrates (reducing and nonreducing sugars), the anthrone method or phenol–sulfuric acid assay method (Yemm and Willis 1954; Zhang et al. 2020; Khan et al. 2019) is used. These assays are based on the hydrolysis of the red seaweed polysaccharide, followed by intramolecular dehydration of all monomers in acidic conditions. The resulting furfural derivatives then react with anthrone or phenol to form chromogenic products, which have maximal absorption wavelength at 640 nm and 490 nm, respectively. Where the Resorcinol reagent is chosen for the direct determination of AnGal (Yaphe and Arsenault 1965; Khan et al. 2020; Wang et al. 2012), the method involves treatment with thymol in the presence of ferric chloride and hydrochloric acid, with the released monosaccharides forming colored compounds. The difference in the rate of color formation with the anthrone reagent between Gal and AnGal has also been used to study the simultaneous determination of a mixture of these monosaccharides in red seaweed polysaccharides. For a chromatographic method to be used to quantify AnGal, the acid-labile problem must be remedied with the use of a methylmorpholine–borane complex (MMB) under acidic conditions. This method first involves the reductive hydrolysis of AnGal-containing polysaccharides, followed by acetylation of the alditols and, finally, analysis of alditol acetates by gas chromatography (Xu et al. 2019; Navarro and Stortz 2003).
The aim of the present study was to quantify the chemical composition of red algal polysaccharides from different species and to explore the biological activities of AnGal. First, the AnGal content in red seaweed polysaccharides was determined and compared using the established spectrophotometric methods and gas chromatography coupled with mass spectrometry (GC–MS). The anti-melanogenic activities of red seaweed polysaccharides from different species and their degradation products were then compared, and their potential use in the cosmetics industry was explored.
Materials and methods
Materials and chemicals
The red algae were purchased from a local market in Shantou. Samples were washed with fresh water to remove the salt, dried at 60 °C in a drying cabinet, before being ground into powder. Chromatographic acetonitrile was purchased from Aladdin Reagent Co. (Shanghai, China). Tyrosinase, methylmorpholine–borane complex (MMB), resorcinol, trifluoroacetic acid (TFA), and Levodopa (L-DOPA, or 3,4-dihydroxy phenylalanine), a catalytic substrate of tyrosinase, were all obtained from Sigma (St. Louis, MO, USA). Other reagents and chemicals used were of analytical grade.
Extraction of polysaccharides
Red algal powder was smashed (100 g) and then soaked with shaking for 24 h in methanol/dichloromethane/water (4:2:1; v/v/v) solution in a ratio of 10:1 (v/w, mL/g) to remove interfering compounds. The residue was collected by centrifuging at 4000×g for 10 min and then dried in an oven at 50 °C. Following this, samples were mixed with distilled water (1:40, w/v) at 90 °C for 2 h. The supernatant was centrifuged (3500×g, 5 min) and concentrated by rotary evaporator. The extracts were precipitated by adding three volumes of 95% ethanol (v/v) at 4 °C for 24 h. The precipitate was collected, re-dissolved in distilled water and divided into 3000 Da dialysis bag before being placed in distilled water for dialysis for 24 h, after which the liquid was collected and freeze-dried to obtain red seaweed polysaccharides for further analysis.
Spectrophotometric analysis of AnGal
Total carbohydrate content was determined by anthrone-sulfuric acid method with minor modification (Yemm and Willis 1954). Each sample solution (1 mL, 1 mg/mL) was placed in tubes and 0.2 mL distilled water added. After adding 1 mL of anthrone reagents (0.2 g anthrone was mixed with 100 mL of 80% (v/v) sulfuric acid before colorimetric) followed by homogeneous mixing, samples were allowed to react at 80 °C for 20 min. Next, tubes were cooled for 5 min in an ice water bath, before the total absorbance was measured three times at 640 nm. Total sugar level was quantified using a calibration curve plotted with Gal standards.
The content of AnGal was determined by resorcinol method with a minor modification (Yaphe and Arsenault 1965). Resorcinol reagent was prepared within 3 h before the colorimetric assay. Brief, resorcinol reagent was prepared with 9 mL resorcinol solution (1.5 mg/mL), 1 mL acetaldehyde solution (0.04%, v/v) and 100 mL concentrated hydrochloric acid. Next, 0.03 mL aliquot of the sample solution (1 mg/mL) was added to a centrifuge tube followed by the addition of 0.2 mL distilled water. After placing in an ice bath for 5 min, 1 mL of resorcinol reagent was added, mixed homogenously in ice bath, and then placed at room temperature for 2 min. The mixture was incubated for 10 min at 80 °C followed by cooling for 5 min in an ice bath. The absorbance of AnGal was measured at 555 nm and the concentration of AnGal calculated using a calibration curve with Gal standards. All samples were analyzed in triplicates, with Gal content in samples calculated by the total content of carbohydrate minus the content of AnGal.
Quantification of AnGal by GC–MS
First, samples were hydrolyzed for 30 min at 80 °C in a water bath using 100 μL in centrifuge tube plus 50 μL of 80 mg/mL MMB with TFA at a final concentration of 2.5 mol/L. After hydrolysis, the solution was cooled to room temperature followed by the addition of 50 μL of 80 mg/mL MMB and then dried with N2 gas. Next, samples were subjected to the second hydrolysis, followed by the addition of 100 μL of deionized water and TFA with a final concentration of 1.5 mol/L, and the mixture was oil bathed at 120 °C for 1 h. After cooling tubes to room temperature, 100 μL of 80 mg/mL MMB was added and samples dried with N2 gas. Finally, 500 μL of acetonitrile was added to samples and dried again with N2 gas. The dried sample was dissolved in a mixture of 0.5 mL ethyl acetate, 1.5 mL acetic anhydride and 50 μL perchloric acid. Samples were ultrasonic at room temperature for 10 min before allowing samples to stand at room temperature for 15 min. After adding 5 mL of deionized water, the water phase was extracted with 2 mL dichloromethane, and the dichloromethane fraction washed three times with 5 mL deionized water. The remaining solution was then filtered through a 0.45 μm nylon membrane for GC analysis. Samples (1 μL) or serial concentration of standard solutions were analyzed by GC using an Rtx-5MS column (30 cm × 0.25 mm i.d., film thickness 0.1 μm, Agilent Technologies, USA). The injector was set at a temperature of 280 °C prior to injection of the samples (1 μL with a split ratio of 1:10), while the temperature of the detector was maintained at 250 °C. The initial oven temperature was 60 °C for 2 min, followed by an incremental increase of 30 °C/min up to 120 °C (1 min hold), and then 25 °C/min up to 250 °C (30 min hold). Nitrogen at a flow rate of 2 mL/min was used as the carrier gas. The mass spectrometry was performed at 70 eV and 50–800 m/z scan fragments.
Method validation of colorimetric and GC–MS
Standard stock solutions of fructose and Gal, as well as AnGal were dissolved in distilled water to a final concentration of 2 mg/mL for spectrophotometric and gas chromatography analysis, respectively. The serially diluted standards solutions were used to prepare calibration curves. Stock solutions of samples were prepared by dissolving 20 mg of red algal polysaccharides in 10 mL volumetric flask. All experiments were assayed in triplicate.
The linearity of colorimetric was analyzed using series of concentrations of fructose ranging from 40 to 90 μg/mL. The linearity of GC–MS was calculated by injected serially concentration of processed standards. In brief, an equal amount of Gal and AnGal solutions were mixed. Then, distilled water was added into mixed standard to final concentrations of 50, 75, 125, 250, 500 μg/mL. Finally, the standard solutions were hydrolyzed and acetylated as described method above. The limit of detection (LOD) and limit of quantitation (LOQ) of the method were estimated by a standard linear equation in a ration of signal-to-noise (S/N) equal to 3 and 10 separately.
Inter-day variability of prepared red algal polysaccharide was measured to estimate the precision. Actually, 100 μg/mL of samples were analyzed six times by colorimetric and GC–MS methods in 3 days. The repeatability was validated using parallel samples at the concentration of 100 μg/mL. Treated samples were analyzed six replications under the described method, respectively. The stability of different methods was evaluated at six different times (0, 2, 8, 12, 24 h) using samples.
Partial degradation method
Partial degradation of the red algal polysaccharide was carried out by acid hydrolysis. Dried powders of the polysaccharides were dissolved in 0.6 mol/L HCl at 50 °C for 2 h. After hydrolysis, solutions were precipitated by adding three volumes of 95% (v/v) ethanol at 4 °C overnight. Next, the supernatant was collected by centrifuging at 4000×g for 5 min and then concentrated by rotary evaporator and lyophilized in a vacuum freeze dryer.
Tyrosinase activity assay
The inhibitory activity of red algal polysaccharides and their degradation products on mushroom tyrosinase was determined spectrophotometrically (Jesumani et al. 2019). Briefly, a solution of L-DOPA (100 μL, 5 mmol/L) was mixed with phosphate buffer (350 μL, 0.1 mol/L, pH 6.5), followed by the addition of different concentrations (150, 300, 450, 600, and 750 μg/mL) of the polysaccharides (25 μL). After 10 min of incubation, mushroom tyrosinase (25 μL, 2000 U/mL) was added to the solution, then incubated at 37 °C for 20 min. The absorbance was recorded at 490 nm using a microplate reader. The inhibition rate of tyrosinase was calculated according to the following formula:
where Acontrol is the absorbance without testing polysaccharide, Asample is the absorbance of the testing group.
Results and discussion
Effects of the hydrolysis process on AnGal and Gal quantification in red seaweed polysaccharides
There is an increased interest by the food and cosmetic industries for chemical analysis methods of the monosaccharide composition of red seaweed polysaccharides. Since the monosaccharide composition of red seaweed polysaccharides is usually measured by quantitative acid hydrolysis, the acid concentrations of TFA used in the two hydrolysis steps were evaluated.
The outcome of the first and second acid hydrolysis processes at the different TFA concentrations are shown in Fig. 1. As shown in Fig. 1a, the production of AnGal and Gal in the first hydrolysis step reached a plateau at 2.5 mol/L, at which the highest amount of AnGal and Gal was detected. These results indicate that most of the AnGal linkages were selectively hydrolyzed at a concentration of 2.5 mol/L TFA. At TFA concentration above 3.5 mol/L, the levels of both AnGal and Gal decreased gradually, which could be due to the convertion of the monosaccharides to other byproducts under high acid concentration. Generally, while an increase condition such as acid concentration, temperature, or duration, accelerates polysaccharide hydrolysis to monosaccharides, it also substantially intensifies the secondary degradation of monomeric sugars. This is particularly so for AnGal, which decreases under such conditions in the final solution. Therefore, the optimum conditions for the selective hydrolysis of AnGal bonds were found to be at TFA concentrations of 2.5 mol/L in the first hydrolysis step and 1.5 mol/L in the second hydrolysis step.
Fig. 1.
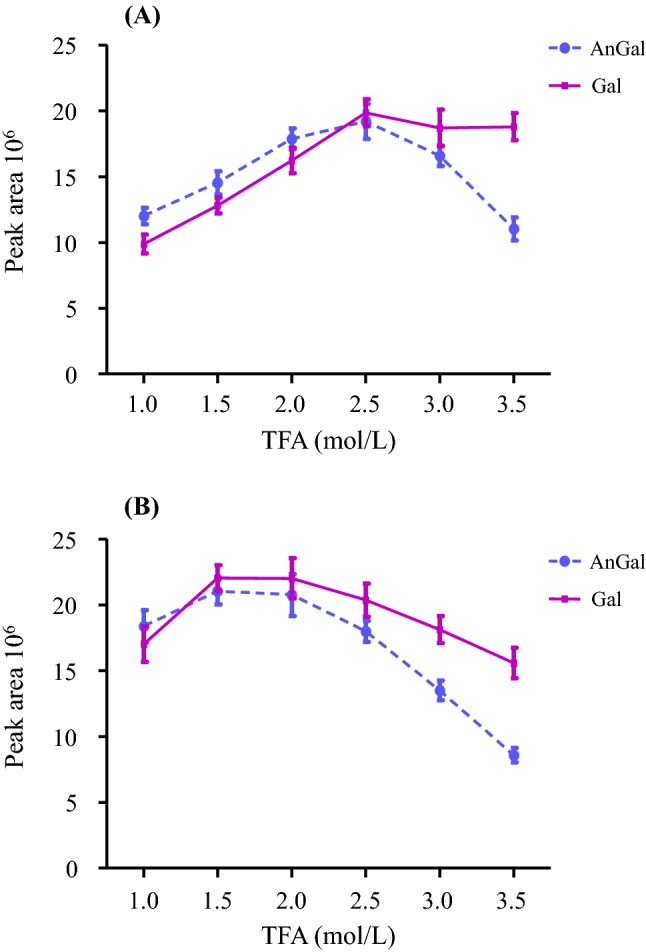
Effects of TFA concentration (1.0–3.5 mol/L) on the production of galactose and 3, 6-anhydrogalactose. The amount of galactose and 3, 6-anhydrogalactose was measured as peak areas. The first hydrolysis reaction was carried out for 30 min at 80 °C in a water bath with methylmorpholine–borane complex and different concentration of TFA (1.0-3.5 mol/L) (a). The second hydrolysis step was performed with different concentrations of TFA (1.0-3.5 mol/L) at 120 °C for 1 h (b). The results are expressed as mean ± SD (n = 3)
Validation of the proposed method
The AnGal and Gal levels in red seaweed polysaccharides were determined by colorimetric and chromatography methods. The quantitative estimation of Gal and AnGal was carried out via spectrophotometry using two colorimetric reagents, i.e., anthrone and resorcinol, while GC–MS was the chromatography method used. To ensure the reproducibility of results, samples were analyzed in triplicate dilutions in both methods. The calibration curves, linear range, LOD, and LOQ of the GC–MS and colorimetric methods are summarized in Tables 1 and 2, respectively. The GC–MS calibration curves showed good linearity (correlation coefficient > 0.99) for the 500-fold dynamic ranges for AnGal and Gal examined. The LOD [signal-to-noise ratio (S/N) > 3] and LOQ (S/N > 10) were 0.13–0.15 μg/mL and 0.43–0.52 μg/mL, respectively. From Tables 1 and 2, it is obvious that the GC–MS method has good linear range, LOD, and LOQ when compared with the colorimetric method.
Table 1.
Calibration data, limit of quantitation (LOQ), limit of detection (LOD) of the analyte determined by GC–MS
| Analyte | Regression equation | r | Linear range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| AnGal | y = 8.350 × 107x + 1.510 × 105 | 0.9939 | 0.5–250.0 | 0.13 | 0.43 |
| Gal | y = 6.963 × 107x − 2.320 × 105 | 0.9952 | 0.5–250.0 | 0.15 | 0.52 |
AnGal 3,6-anhydro-l-galactose, Gal galactose, LOD limit of detection, LOQ limit of quantification, r correlation coefficient
Table 2.
Calibration data, limit of quantitation (LOQ), and limit of detection (LOD) of two colorimetric methods
| Method | Regression equation | r | Test range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Anthrone | y = 28.04x + 0.0423 | 0.9937 | 40–90 | 3.26 | 10.88 |
| Resorcinol | y = 19.59x + 0.0489 | 0.9971 | 40–90 | 4.67 | 15.57 |
The precision, accuracy, stability, and recovery of the GC–MS and colorimetric methods are listed in Table 3. The RSDs of precision and accuracy for the GC–MS method were 1.95–2.37 and 3.17–4.31%, respectively. This data suggest that the GC–MS method used has good precision and repeatability. Meanwhile, the RSD of sample stability for the developed GC–MS method was 2.89–3.53%, which indicated that the investigated Gal and AnGal standards were stable in solution at 4 °C during the testing period. When the stability of the colored products at room temperature was examined, no significant changes in absorbance were observed within 60 min after the first measurement. The low RSD and relative error percentages (less than 4%) indicated high precision and good accuracy of the developed methods for the estimation of AnGal and Gal contents. The percentage recovery of the analytes determined by the GC–MS method varied from 104.90 to 109.21%, while that of the colorimetric methods varied from 89.37 to 105.27%. Apparently, the proposed GC–MS method was able to produce better parameters when compared with the proposed colorimetric method.
Table 3.
Precision, accuracy, stability, and recovery of the analytes determined by GC–MS and colorimetric methods
| Method | Analyte/reagent | Precision RSD % | Accuracy RSD % | Stability RSD % | Recovery % |
|---|---|---|---|---|---|
| GC–MS | AnGal | 2.37 | 3.17 | 3.53 | 104.90 |
| Gal | 1.95 | 4.31 | 2.89 | 109.21 | |
| Colorimetric | Anthrone | 4.87 | 4.08 | 2.13 | 105.28 |
| Resorcinol | 3.26 | 4.01 | 3.72 | 89.37 |
The validation results showed that both the colorimetric and GC–MS methods exhibited satisfactory accuracy and precision, as indicated by the recovery values and RSD percentages. It is evident from these results that the proposed methods are applicable for assaying AnGal and Gal in red seaweed polysaccharides with a high level of accuracy and precision.
Comparison of AnGal and Gal levels in red seaweed quantified by colorimetric and GC–MS methods
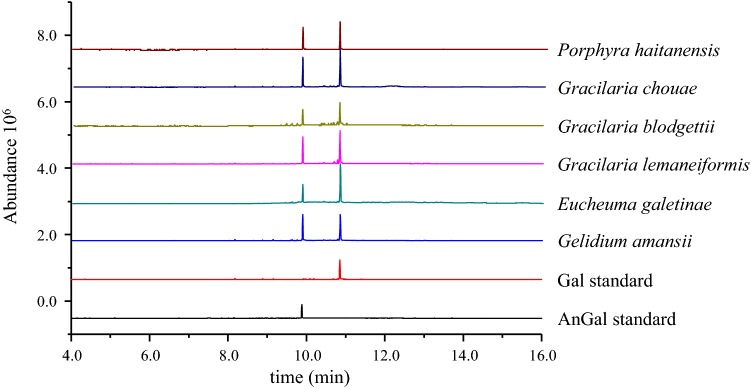
The proposed colorimetric and GC–MS methods were applied for the quantification of AnGal and Gal in red seaweed polysaccharides. In the GC–MS determination, the reduced polysaccharides were further converted into the corresponding alditol acetates and subjected to GC–MS, which then gave the total molar ratio of monosaccharides. As shown in the red seaweed polysaccharides chromatogram of Fig. 2, there were two major peaks corresponding to AnGal and Gal. These results indicated that all the polysaccharides derived from the different species of red seaweed contain AnGal and Gal in different molar ratios. The total carbohydrate and AnGal contents were obtained using the anthrone-sulfuric acid and resorcinol methods, respectively. The Gal content was estimated as the total carbohydrate content minus the AnGal content. The molar ratios of AnGal and Gal determined by the colorimetric method are summarized in Table 4. The ratio of AnGal and Gal calculated from the colorimetric method was approximately close to the corresponding values derived from the GC–MS analysis (Table 4). Although the colorimetric method is simple and rapid, it suffers from strong interference by other sugars when they are present in appreciable amounts.
Fig. 2.
Typical GC chromatograms corresponding to 3,6-anhydrogalactose standard, galactose standard, and monosaccharide composition of polysaccharides from different species of red seaweed. The chromatography was carried out at following conditions: Rtx-5MS column (30 cm × 0.25 mm i.d., film thickness 0.1 μm); initial oven temperature was 60 °C for 2 min, followed by an increment of 30 °C/min up to 120 °C (1 min hold), and then 25 °C/min up to 250 °C (30 min hold)
Table 4.
The monosaccharide composition of red algal samples determined by GC–MS and colorimetric methods
| Red algal species | GC–MS | Colorimetric |
|---|---|---|
| AnGal: Gal | ||
| Porphyra haitanensis | 1.0:1.4 | 1.0:1.2 |
| Gracilaria chouae | 1.0:1.6 | 1.0:1.7 |
| Gracilaria blodgettii | 1.0:1.5 | 1.0:1.3 |
| Gracilaria lemaneiformis | 1.0:3.1 | 1.0:2.5 |
| Eucheuma galetinae | 1.0:3.0 | 1.0:2.3 |
| Gelidium amansii | 1.0:1.0 | 1.0:0.8 |
As shown in Table 4, the monosaccharide compositions of polysaccharides derived from Porphyra haitanensis, Gracilaria chouae, and Gracilaria blodgettii consisted of AnGal and Gal with a molar ratios ranging from 1.0:1.4 to 1.6. These results are similar to previous reports. For instance, Yu et al. reported that polysaccharides derived from Gloiopeltis furcate and G. furcate were composed of AnGal and Gal in molar ratios of 1.0:1.2 and 1.0:1.6, respectively (Yu et al. 2010). Souza et al. revealed that Gracilaria birdiae polysaccharides had AnGal and Gal in a molar ratio of 1.0:2.6 and that these had significant anti-oxidant activity (Souza et al. 2012). Similarly, Bangia fusco-purpurea polysaccharide consists mainly of Gal with a small amount of AnGal, and have been shown to inhibit α-amylase and α-glucosidase in a concentration-dependent manner (Jiang et al. 2019). These previous reports and the present study all support the conclusion that the differences in biological activities between different red seaweed polysaccharides can be attributed to the differences in their monosaccharide composition.
When the AnGal/Gal molar ratios of different species of red seaweed polysaccharides were compared in this study, an apparent correlation in the structure of red seaweed polysaccharides with their monosaccharide composition and AnGal content was established, which goes to buttress an unequivocal structural relationship. These results also indicate that the structural moiety of the red seaweed polysaccharides is responsible for their biological activities. These data also proves the useful prospective application of this approach in the quality control of functional foods or cosmeceutical products.
Skin-whitening activities of red seaweed polysaccharides and their degradation products
Melanin is the key pigment that contributes to skin and hair color in humans and plays an important role in preventing skin damage induced by ultraviolet radiation. It is the end-product formed by the multiple-step transformation of l-tyrosine. However, excessive or abnormal synthesis of melanin in the human body could induce various hyperpigmentation disorders such as melisma, freckles, and skin cancer (Hridya et al. 2015). A safe and effective tyrosinase inhibitor or regulator may, therefore, be a good candidate molecule to treat hyperpigmentation. Some polysaccharides or oligosaccharides derived from seaweeds have promising application as cosmeceutical material for new melanogenesis inhibitors due to their skin-whitening potential (Thomas and Kim 2013; Ruocco et al. 2016). Therefore, the current study explored the skin-whitening effects of red seaweed polysaccharides and their degradation products using tyrosinase inhibition activity and melanin production testing.
As shown in Fig. 3, the red seaweed polysaccharides (200 μg/mL) produced 0.5–5.1% inhibition of tyrosinase, while their degradation products (200 μg/mL) exhibited 8.2–26.3% inhibition. These results indicated that the degradation products were much better inhibitors of tyrosinase compared with the native polysaccharides. As shown in Fig. 4, both P. haitanensis polysaccharides and the degradation products exhibited tyrosinase inhibition dose-dependently. For instance, at 200 μg/mL of P. haitanensis polysaccharide and its degradation products, the tyrosinase inhibition were 4.8% and 25.6%, respectively. Moreover, the degradation products of P. haitanensis, G. chouae, and G. blodgettii polysaccharides displayed higher tyrosinase inhibition compared with those of the other red seaweed species. These results suggest that P. haitanensis, G. chouae, and G. blodgettii polysaccharides and their degradation products possess potential skin-whitening activity as they have tyrosinase inhibition activity.
Fig. 3.
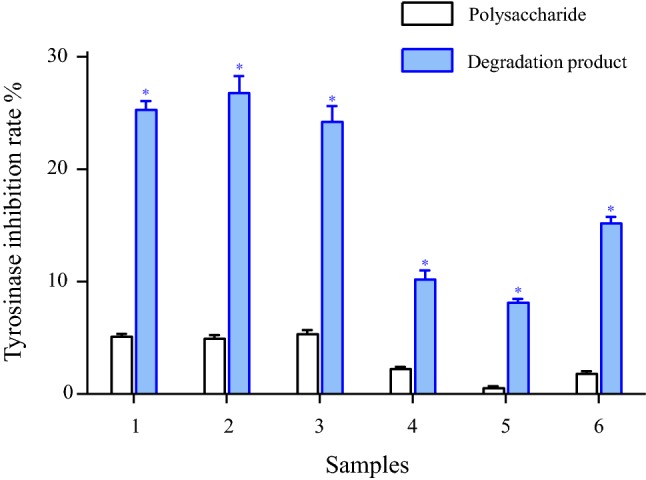
Tyrosinase inhibition rate of red seaweed polysaccharides and its degradation products at a concentration of 200 μg/mL. Solutions were incubated with mushroom tyrosinase at 37 °C for 20 min with L-DOPA and substrates. 1: Porphyra haitanensis; 2: Gracilaria chouae; 3: Gracilaria blodgettii; 4: Gracilaria lemaneiformis; 5: Eucheuma galetinae; 6: Gelidium amansii. The results were expressed as mean ± SD (n = 3) and statistical significance between degradation products and polysaccharides samples considered at p < 0.01 (*p < 0.01)
Fig. 4.
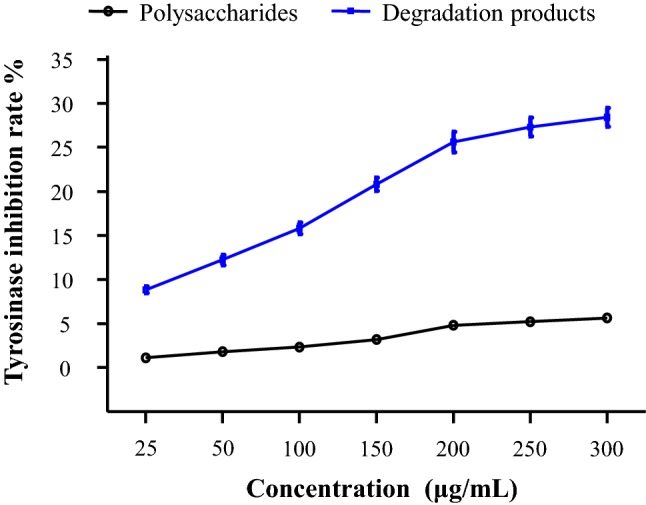
Inhibitory effect of Porphyra haitanensis polysaccharide and its degradation products on tyrosinase activity at different concentration (25–300 μg/mL). Solutions were incubated with mushroom tyrosinase at 37 °C for 20 min with L-DOPA as substrate. The results are expressed as mean ± SD (n = 3)
Advancements in marine biotechnology have greatly helped studies on aging and skin degradation linked to free radicals. In one of the recent studies, Gelidum amansii polysaccharide was shown to possess significant anti-oxidant activity and could effectively scavenge the free radicals 2,2-diphenyl-1-picrylhydrazyl and 2,2-azino-bis (3-ethylbenzothiazoline sulfonic acid), while the degradation products of β-agarase showed better radical scavenging activities than the native polysaccharide (Xu et al. 2018a, b). In addition, Chen et al. previously showed that degradation products of polysaccharides possessed higher in vitro immune-stimulatory activity than the native polysaccharides, couple with the fact that these had much stronger activation of macrophages to secrete pro-inflammatory cytokines (Chen et al. 2014). A wide spectrum of skin protection potential by polysaccharides obtained from Chnoospora minima and Sargassum polycystum have also recently been reported, including their anti-oxidant, skin-whitening, anti-inflammatory, and anti-wrinkle activities (Shanura Fernando et al. 2018).
Conclusion
The GC–MS method presented herein offers significant advantages in terms of simplicity, linearity, sensitivity, precision, accuracy, and specificity. The GC–MS method was completely validated, showing satisfactory results for all the validation parameters tested. Thus, this GC–MS method can be conveniently used in quality control research for the quantitative determination of AnGal and Gal in red seaweed polysaccharides for potential use in cosmetic and functional food products. In addition, P. haitanensis, G. chouae, and G. blodgettii polysaccharides and their degradation products were shown to possess potential skin-whitening activity through their ability to inhibit tyrosinase activity. Red seaweed polysaccharides have multiple human health application or use without side effects, suggesting that the degradation of red seaweed polysaccharides has the potential for extensive application in the food and cosmetic industries.
Acknowledgements
We are grateful to Jude Juventus Aweya for his guidance, corrections and contributions to the grammar and sentence structure of the paper This research was financially supported by grants from the National Natural Science Foundation of China (31901692); Natural Science Foundation of Guangdong Province, China (2018A030310151); Department of Education of Guangdong Province with the Teaching Reform Project (2018JGXM44).
Author contributions
KLC and XQC conceived and designed the experiments. XTX, XZ, and XTX performed the experiment and wrote the manuscript. All authors revised the paper and contributed reagents/materials/analysis tools. All authors reviewed and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
Contributor Information
Xian-Qiang Chen, Email: xianqiangchen@yeah.net.
Kit-Leong Cheong, Email: klcheong@stu.edu.cn.
References
- Chen H, Wang F, Mao H, Yan X. Degraded λ-carrageenan activates NF-κB and AP-1 pathways in macrophages and enhances LPS-induced TNF-α secretion through AP-1. Biochim Biophys Acta 1840. 2014;7:2162–2170. doi: 10.1016/j.bbagen.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Cheong KL, Wu DT, Zhao J, Li SP. A rapid and accurate method for the quantitative estimation of natural polysaccharides and their fractions using high performance size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detector. J Chromatogr A. 2015;1400:98–106. doi: 10.1016/j.chroma.2015.04.054. [DOI] [PubMed] [Google Scholar]
- Cheong KL, Qiu HM, Du H, Liu Y, Khan BM. Oligosaccharides derived m red seaweed: production, properties, and potential health and cosmetic applications. Molecules. 2018;23(10):2451. doi: 10.3390/molecules23102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Wu J, Wang S, Shu H, Zhang M, Liu K, Liu K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int J Biol Macromol. 2019;129:377–385. doi: 10.1016/j.ijbiomac.2019.02.043. [DOI] [PubMed] [Google Scholar]
- Hridya H, Amrita A, Sankari M, George Priya Doss C, Gopalakrishnan M, Gopalakrishnan C, Siva R. Inhibitory effect of brazilein on tyrosinase and melanin synthesis: kinetics and in silico approach. Int J Biol Macromol. 2015;81:228–234. doi: 10.1016/j.ijbiomac.2015.07.064. [DOI] [PubMed] [Google Scholar]
- Jesumani V, Du H, Pei P, Zheng C, Cheong KL, Huang N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int J Biol Macromol. 2019;140:216–224. doi: 10.1016/j.ijbiomac.2019.08.027. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Yu G, Liang Y, Song T, Zhu Y, Ni H, Yamaguchi K, Oda T. Inhibitory effects of a sulfated polysaccharide isolated from edible red alga Bangia fusco-purpurea on α-amylase and α-glucosidase. Biosci Biotechnol Biochem. 2019;83(11):2065–2074. doi: 10.1080/09168451.2019.1634515. [DOI] [PubMed] [Google Scholar]
- Khan BM, Qiu HM, Wang XF, Liu ZY, Zhang JY, Guo YJ, Chen WZ, Liu Y, Cheong KL. Physicochemical characterization of Gracilaria chouae sulfated polysaccharides and their antioxidant potential. Int J Biol Macromol. 2019;134:255–261. doi: 10.1016/j.ijbiomac.2019.05.055. [DOI] [PubMed] [Google Scholar]
- Khan BM, Qiu HM, Xu SY, Liu Y, Cheong KL. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int J Biol Macromol. 2020;145:1155–1161. doi: 10.1016/j.ijbiomac.2019.10.040. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yun EJ, Yu S, Kim KH, Kang NJ. Different levels of skin whitening activity among 3,6-anhydro-L-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar Drugs. 2017;15(10):321. doi: 10.3390/md15100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee JE, Kim KH, Kang NJ. Beneficial effects of marine algal-derived carbohydrates for skin health. Mar Drugs. 2018;16(11):459. doi: 10.3390/md16110459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko AO, Byankina Barabanova AO, Glazunov VP, Yakovleva IM, Yermak IM. Seasonal variations in a polysaccharide composition of Far Eastern red seaweed Ahnfeltiopsis flabelliformis (Phyllophoraceae) J Appl Phycol. 2018;30(1):535–545. doi: 10.1007/s10811-017-1262-8. [DOI] [Google Scholar]
- Navarro DA, Stortz CA. Determination of the configuration of 3,6-anhydrogalactose and cyclizable α-galactose 6-sulfate units in red seaweed galactans. Carbohydr Res. 2003;338(20):2111–2118. doi: 10.1016/S0008-6215(03)00345-8. [DOI] [PubMed] [Google Scholar]
- Ruocco N, Costantini S, Guariniello S, Costantini M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules. 2016;21(5):551. doi: 10.3390/molecules21050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedevi P, Moovendhan M, Viramani S, Shanmugam A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata) Carbohydr Polym. 2017;155:516–524. doi: 10.1016/j.carbpol.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Shanura Fernando IP, Asanka Sanjeewa KK, Samarakoon KW, Kim H-S, Gunasekara UKDSS, Park Y-J, Abeytunga DTU, Lee WW, Jeon Y-J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J Appl Phycol. 2018;30(6):3223–3232. doi: 10.1007/s10811-018-1415-4. [DOI] [Google Scholar]
- Souza BWS, Cerqueira MA, Bourbon AI, Pinheiro AC, Martins JT, Teixeira JA, Coimbra MA, Vicente AA. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocolloid. 2012;27(2):287–292. doi: 10.1016/j.foodhyd.2011.10.005. [DOI] [Google Scholar]
- Sudharsan S, Giji S, Seedevi P, Vairamani S, Shanmugam A. Isolation, characterization and bioactive potential of sulfated galactans from Spyridia hypnoides (Bory) Papenfuss. Int J Biol Macromol. 2018;109:589–597. doi: 10.1016/j.ijbiomac.2017.12.097. [DOI] [PubMed] [Google Scholar]
- Thomas NV, Kim SK. Beneficial effects of marine algal compounds in cosmeceuticals. Mar Drugs. 2013;11(12):146–164. doi: 10.3390/md11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhao X, Lv Y, Li M, Liu X, Li G, Yu G. Structural and compositional characteristics of hybrid carrageenans from red algal Chondracanthus chamissoi. Carbohydr Polym. 2012;89(3):914–919. doi: 10.1016/j.carbpol.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algal. Carbohydr Polym. 2011;84(1):14–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- Xu SY, Huang X, Cheong KL. Recent advances in marine algal polysaccharides: isolation, structure, and activities. Mar Drugs. 2017;15(12):388. doi: 10.3390/md15120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Kan J, Hu Z, Liu Y, Du H, Pang GC, Cheong KL. Quantification of neoagaro-oligosaccharide production through enzymatic hydrolysis and its anti-oxidant activities. Molecules. 2018;23(6):1354. doi: 10.3390/molecules23061354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Liu JP, Huang X, Du LP, Shi FL, Dong R, Huang XT, Zheng K, Liu Y, Cheong KL. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT. 2018;90:577–582. doi: 10.1016/j.lwt.2018.01.007. [DOI] [Google Scholar]
- Xu SY, Aweya JJ, Li N, Deng R-Y, Chen W-Y, Tang J, Cheong K-L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019;289:177–186. doi: 10.1016/j.foodchem.2019.03.050. [DOI] [PubMed] [Google Scholar]
- Yaphe W, Arsenault GP. Improved resorcinol reagent for the determination of fructose, and of 3,6-anhydrogalactose in polysaccharides. Anal Biochem. 1965;13(1):143–148. doi: 10.1016/0003-2697(65)90128-4. [DOI] [Google Scholar]
- Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Hu Y, Yang B, Zhao X, Wang P, Ji G, Wu J, Guan H. Extraction, isolation and structural characterization of polysaccharides from a red alga Gloiopeltis furcata. J Ocean Univ. 2010;9(2):193–197. doi: 10.1007/s11802-010-0193-7. [DOI] [Google Scholar]
- Yun EJ, Choi IG, Kim KH. Red macroalgal as a sustainable resource for bio-based products. Trends Biotechnol. 2015;33(5):247–249. doi: 10.1016/j.tibtech.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang X, Aweya JJ, Huang ZX, Kang ZY, Bai ZH, Li KH, He XT, Liu Y, Chen XQ, Cheong KL. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr Polym. 2020;234:115894. doi: 10.1016/j.carbpol.2020.115894. [DOI] [PubMed] [Google Scholar]
- Zheng LX, Chen XQ, Cheong KL. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int J Biol Macromol. 2020;151:344–354. doi: 10.1016/j.ijbiomac.2020.02.168. [DOI] [PubMed] [Google Scholar]