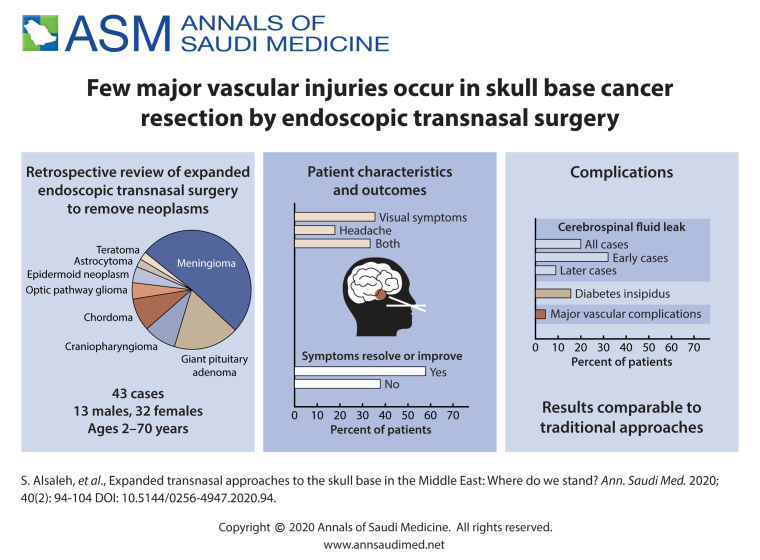
ABSTRACT
BACKGROUND:
Endoscopic transnasal surgery has gained rapid global acceptance over the last two decades. The growing literature and understanding of anterior skull base endoscopic anatomy, in addition to new dedicated endoscopic instruments and tools, have helped to expand the use of the transnasal route in skull base surgery.
OBJECTIVE:
Report our early experience in expanded endoscopic transnasal surgery (EETS) and approach to skull base neoplasms.
DESIGN:
Descriptive, retrospective case series.
SETTING:
Major tertiary care center.
PATIENTS AND METHODS:
A retrospective case review was conducted at King Saud University Medical City between December 2014 and August 2019. Cases with skull base neoplasms that underwent EETS were included. EETS was defined as endoscopic surgical exposure that extended beyond the sellar margins (prechiasmatic sulcus superiorly, clival recess inferiorly, cavernous carotid lines laterally). Routine transsphenoidal pituitary neoplasms, neoplasms of sinonasal origin and meningoencephaloceles were excluded.
MAIN OUTCOME MEASURES:
Preoperative clinical assessment, imaging results, surgical approach, and hospital course were all retrieved from the patient electronic charts. Clinical follow-up, perioperative complications, and gross residual tumor rates were documented and reviewed.
SAMPLE SIZE AND CHARACTERISTICS:
45 cases of EETS, 13 males and 32 females with mean age of 39.0 (17.7) years (range 2–70 years).
RESULTS:
The series comprised a wide range of pathologies, including giant pituitary adenoma (8 cases), meningioma (23 cases), craniopharyngioma (4 cases), chordoma (4 cases), optic pathway glioma (2 cases), epidermoid neoplasms (2 cases), astrocytoma (1 case), and teratoma (1 case). For the entire series, gross total resection was achieved in 25/45 operations (55.5%). Postoperative cerebrospinal fluid leak was the most common complication observed in 9 patients (20%) which were all managed endoscopically. Major vascular complications occurred in 2 patients (4.4%) and are described. Other complications are outlined as well. No mortality was observed.
CONCLUSIONS:
EETS to the skull base can be done with results comparable to traditional approaches. More work is needed to expand our experience, improve outcomes, and educate the public and medical community in our region about the usefulness of this approach.
LIMITATIONS:
Sample size and study design.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Skull base surgery is one of the most complex and technically challenging, since it involves deep locations related to important and critical structures and has a relatively high risk of morbidity and mortality. Various pathologies can involve the skull base, including neoplastic, inflammatory, infectious, traumatic, and congenital anomalies.1 Different techniques and approaches have been used in skull base surgery, including anterior, antero-lateral, and postero-lateral routes.2-5 Endoscopic transnasal surgery has gained rapid global acceptance over the last two decades. The growing literature and understanding of skull base endoscopic anatomy, in addition to the reduction of invasiveness when using these approaches to access the skull base, has helped to expand the use of the transnasal route in skull base surgery. The evolution of the standard endoscopic transsphenoidal approach has been witnessed in several centers to encompass many expanded endoscopic approaches to address various pathologies involving the anterior skull base.6-12 Endoscopic transsphenoidal surgery has become a preferred approach in pituitary surgery, mainly because of improved visualization, which leads to less perioperative complications in comparison with the microscopic approach. Other advantages include achieving a higher rate of gross total resection and shorter length of hospital stay.13 However, adopting expanded endonasal approaches to the skull base is still a challenge in many centers for various reasons. Lack of training, clinical collaborative efforts, equipment, and instrumentation are some of the obstacles that should be taken into consideration prior to embarking on such a journey. In the present study, we report our early experience in expanded endoscopic transnasal surgery (EETS) managing various skull base neoplasms at King Saud University Medical City in Riyadh, Saudi Arabia.
PATIENTS AND METHODS
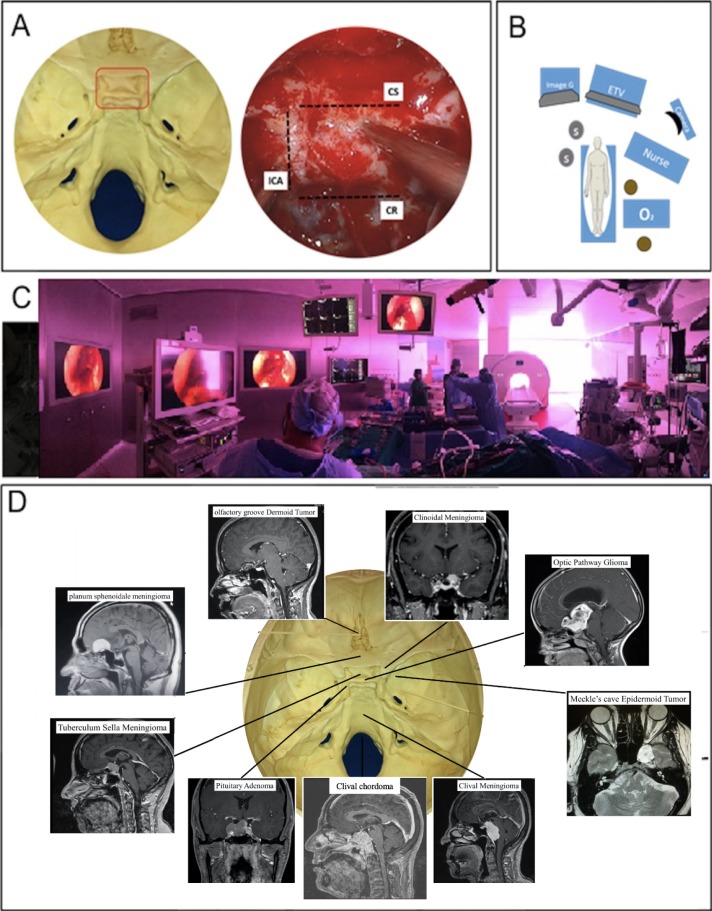
We reviewed cases from King Saud University Medical City between December 2014 and August 2019. The prospectively collected data was reviewed retrospectively. The surgical team consisted of a neurosurgeon (AA) and three rhinologists (SS, AR, SR) trained in endonasal skull base surgery. Cases with skull base neoplasms requiring EETS were only included. This was defined as endoscopic surgical exposure that extended beyond the sellar margins (prechiasmatic sulcus superiorly, clival recess inferiorly, cavernous carotid lines laterally) (Figure 1A). Routine transsphenoidal pituitary neoplasms, neoplasms of sinonasal origin and meningoencephaloceles were excluded from this analysis. The institutional review board of the college of medicine at King Saud University approved this study.
Figure 1.
Representation of the surgical access provided by expanded endoscopic transnasal surgery (EETS) (A) Endoscopic exposure extended beyond the sellar margins. EETS was considered pre-chiasmatic sulcus superiorly, clival recess inferiorly, and cavernous carotid lines laterally (ICA: internal carotid artery; CS: cavernous sinus; CR: clival recess). Operating room setup for expanded endoscopic endonasal approaches (B, C). One monitor was used to allow a clear view for both surgeons. Intraoperative neuronavigation was always available in any case when EETS was planned. Distribution of cases and representation of the surgical access provided by EETS (D).
Assessment of imaging results, surgical approach, and hospital course were all retrieved from the patient electronic charts. Clinical follow-up and perioperative complications were documented and reviewed as well. Gross residual tumor rates were assessed based on a contrast-enhanced magnetic resonance imaging (MRI) of the skull base done at 8 weeks postoperatively. Symptomatic outcomes following surgery were classified as resolved, improved, stable, or worsened for all of the symptom and function domains.
In all cases with skull base neoplasms, clinical assessment by both neurosurgery and rhinology services was conducted routinely, and imaging in the form of contrast-enhanced computed tomography (CT) and MRI of the skull base with neuronavigational protocols was obtained. Neuroendocrine and ophthalmology consultations were requested preoperatively as well. A multidisciplinary discussion about the preferred surgical approach and postoperative management was then conducted with the hospital tumor board. The operative suite was equipped with a neuronavigation system, an intraoperative MRI machine, and direct access to the neurointerventional unit.
Once the patient was admitted to the operative suite and a general anesthetic was administered, a lumbar drain was inserted, the head was rigidly fixated with pins, and the neuronavigation device was set up. A pedicled nasoseptal flap was raised in all cases and situated in either the nasopharynx (in most cases) or in the ipsilateral maxillary sinus (in cases of clival pathologies). One or both middle turbinates were partially excised and stored for possible later use. Sinonasal exposure, performed by the rhinologist, was decided preoperatively depending on the pathology, tumor size, and location along the anterior skull base.
Skull base osteotomies and intradural dissection was then performed using a three or four hands technique with the rhinolo-gist holding the endoscope and neurosurgeon dissecting the neoplasm (Figure 1). Skull base reconstruction was begun by filling the intradural defect with autologous fat. Then, a piece of autologous fascia lata that was larger than the defect was harvested and placed extracranially. This step was followed by MEDPOR (Stryker, Portage, MI, USA) plate placement using a “gasket seal” technique (Figure 2I). The raised nasoseptal flap was then placed covering the defect and fixed in place with fibrin glue, oxidized cellulose, and gelatin sponges. Multiple polyvinyl alcohol sponges were then placed on both sides abutting the absorbable packing. These were left in place along with the lumbar drain for a period ranging from 2 to 4 days postoperatively.
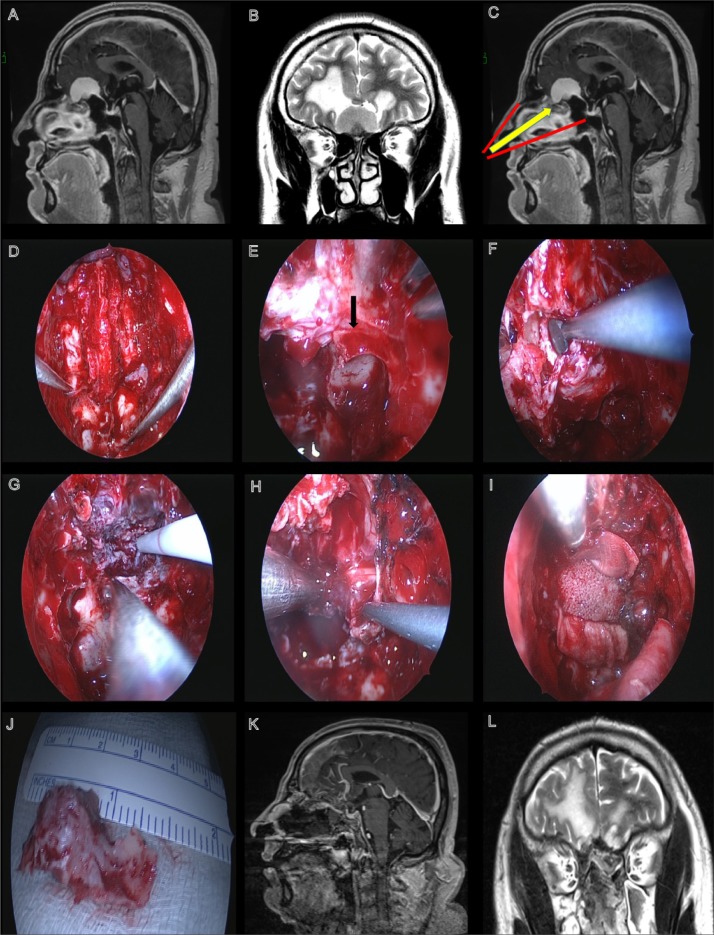
Figure 2.
Patient was a 50-year-old male presenting with seizures and anosmia. Preoperative T1-weighted image with gadolinium showing an olfactory groove meningioma that is homogeneously enhanced (A). Preoperative T1-weighted image showing an olfactory groove meningioma (B). The extent of the exposure for the transnasal approach in this case (C). View seen post complete bilateral sphenoethmoidectomy, septectomy, and an endoscopic modified Lothrop procedure (D). Posterior ethmoid artery seen on the left side (black arrow) (E). Dissection of mucosa along the skull base (F). Intraoperative views seen during (G) and after tumor resection (H). View after initial reconstruction using a MEDPOR porous plate and fascia lata (I). Resected portion of the crista galli (J). Postoperative images showing the complete removal of the lesion and the position of the nasoseptal flap (K, L).
Patients were routinely admitted to an intensive care unit directly postoperatively for neuromonitoring and then transferred to the neurosurgical ward on Day 2. Patients were usually discharged from the hospital between the postoperative Days 4 and 7, with scheduled appointments for both neurosurgical and rhinology outpatient clinics. A contrast-enhanced MRI of the skull base was routinely performed at 8 weeks to assess gross residual tumor rates.
RESULTS
The 45 cases of EETS included 13 males (28.9%) and 32 females (71.1%) with a mean (standard deviation) age at the time of the first surgical intervention of 39.0 (17.7) years (range 2–70 years). Patient characteristics, presentation, radiological result, complications, pathology, and outcome are presented in Table 1. A wide range of pathologies was identified in this cohort. The series included meningiomas (n=23), suprasellar giant pituitary adenoma (n=8), craniopharyngioma (n=4), chordoma (n=4), epidermoid tumor (n=2), optic pathway gliomas (n=2), suprasellar teratoma (n=1), and suprasellar pilocytic astrocytoma (n=1) (Table 2).
Table 1.
Characteristics in patients who underwent expanded endoscopic transnasal surgery.
| No | Age (years), sex | Clinicalpresentation | Post-op clinical status | Resectiontype | Post-op management | Pathology and location (MRI) | Complication(s) | Follow-up period (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 56, F | VS | Improved | STR | Observation, stable | Anterior clinoidal meningioma | Septal perforation | 12 |
| 2 | 8, M | HA | Improved | GTR | RT, progression | Clival chordoma | CSF leak | 24 |
| 3 | 24, M | HA, VS | Improved | GTR | RT, stable | Clival chordoma | None | 24 |
| 4 | 29, M | HA, seizures | Stable | STR | RT, regression of residual | Clival chordoma | Left 6th cranial nerve palsy, CSF leak, EVD-related ventriculitis | 12 |
| 5 | 15, F | Incidental finding on MRI | Worsened | STR | RT, regression of residual | Clival chordoma | Right ICA injury, intraventricular hemorrhage | 12 |
| 6 | 67, F | Incidental finding on MRI | Stable | STR | Loss of follow-up | Clival meningioma | None | Loss of follow-up |
| 7 | 20, F | VS | Improved | STR | RT, regression of residual | Craniopharyngioma | Permanent Dl, meningitis | 12 |
| 8 | 19, M | Hypopituitarism | Stable | GTR | Observation, stable | Craniopharyngioma | None | 48 |
| 9 | 23, M | HA, VS | Improved | STR | Loss of follow-up | Craniopharyngioma | Permanent Dl, CSF leak, hypopituitarism | Loss of follow-up |
| 10 | 55, F | VS | Improved | GTR | Observation, stable | Giant pituitary adenoma | Hypothyroidism | 24 |
| 11 | 60, M | Acromeqaly, VS, HA | Stable | STR | Loss of follow-up | Giant pituitary adenoma | None | Loss of follow-up |
| 12 | 52, M | HA, VS | Improved | GTR | Observation, stable | Giant pituitary adenoma | None | 12 |
| 13 | 50, F | VS | Stable | STR | Observation, stable | Giant pituitary adenoma | None | 12 |
| 14 | 38, M | Acromeqaly, VS, HA | Improved | STR | RT, regression of residual | Giant pituitary adenoma | None | 48 |
| 15 | 44, M | Acromeqaly, VS, HA | Improved | STR | RT, regression of residual | Giant pituitary adenoma | None | 24 |
| 16 | 58, M | Incidental finding on MRI | Stable | GTR | Observation, stable | Giant pituitary adenoma | SIADH | 12 |
| 17 | 56, F | VS | Improved | GTR | Observation, stable | Giant pituitary adenoma | Transient Dl | 12 |
| 18 | 48, F | VS | Improved | GTR | Observation, stable | Meckel's cave epidermoid tumor | CSF leak, meningitis Transient Dl, | 48 |
| 19 | 13, M | HA, VS | Stable | STR | Observation, stable Chemo, partial | Optic pathway glioma | panhypopituitarism, hydrocephalus | 36 |
| 20 | 6, F | VS | Stable | STR | response and open approach proposed for residual disease | Optic pathway glioma | Ventriculitis, CSF leak, meningitis, hydrocephalus | 48 |
| 21 | 47, F | HA | Improved | STR | Observation, stable | Petroclival meningioma | Abducens nerve palsy | 36 |
| 22 | 29, F | HA | Stable | GTR | Observation, stable | Planum sphenoidal epidermoid cyst | None | 48 |
| 23 | 65, F | VS | Improved | STR | Loss of follow-up | Planum sphenoidale meningioma | Adrenal insufficiency, hypothyroidism, hydrocephalus | Loss of follow-up |
| 24 | 48, F | HA | Improved | GTR | Observation, stable | Planum sphenoidale meningioma | None | 24 |
| 25 | 53, F | HA, VS | Improved | GTR | Observation, stable | Planum sphenoidale meningioma | None | 36 |
| 26 | 70, F | HA, VS | Improved | STR | Observation, stable | Planum sphenoidale meningioma | None | 36 |
| 27 | 46, F | Seizures, VS, HA | Improved | GTR | Observation, stable | Planum sphenoidale meningioma | None | 12 |
| 28 | 35, F | VS | Improved | GTR | Observation, stable | Planum sphenoidale meningioma | None | 12 |
| 29 | 38, M | VS | Improved | GTR | Observation, progression | Planum sphenoidale meningioma | None | 24 |
| 30 | 50, M | Seizures | Stable | GTR | Observation, stable | Planum sphenoidale meningioma | None | 24 |
| 31 | 36, F | HA | Improved | GTR | Observation, stable | Planum sphenoidale meningioma | None | 6 |
| 32 | 43, F | VS | Worsened | STR | Transferred to rehab center | Redo-olfactory groove meningioma | ACA infarction, Permanent Dl | Transferred to rehab center |
| 33 | 58, F | HA, psychiatric symptoms, anosmia | Improved | GTR | Observation, stable | Redo-olfactory groove meningioma | Right orbital hematoma, CSF leak, brain abscess | 24 |
| 34 | 56, F | HA, VS | Stable | GTR | Observation, stable | Supra-clinoidalmeningioma | CSF leak | 60 |
| 35 | 49, F | VS | Improved | GTR | Observation, stable | Supra-clinoidalmeningioma | None | 6 |
| 36 | 7, F | HA | Stable | STR | Observation, stable | Suprasellar and retroclival craniopharyngioma | Permanent Dl, Panhypopituitarism, Hydrocephalus | 48 |
| 37 | 18, F | Hormonal symptoms, VS | Stable | STR | Loss of follow-up | Suprasellar teratoma | Transient Dl, hypothyroidism | Loss of follow-up |
| 38 | 2, F | Unsteady gait | Stable | STR | Observation, stable | Suprasellar pilocytic astrocytoma | Panhypopituitarism | 10 |
| 39 | 36, F | HA, VS | Stable | GTR | Observation, stable | Tuberculum sella meningioma | None | 24 |
| 40 | 37, F | HA, VS | Stable | GTR | Loss of follow-up | Tuberculum sella meningioma | None | Loss of follow-up |
| 41 | 35, F | VS | Improved | GTR | Observation, stable | Tuberculum sella meningioma | None | 36 |
| 42 | 25, F | VS | Improved | GTR | Observation, stable | Tuberculum sella meningioma | CSF leak | 12 |
| 43 | 47, F | VS | Stable | STR | Observation, stable | Tuberculum sella meningioma | CSF leak | 36 |
| 44 | 31, F | HA, VS | Improved | GTR | Observation, stable | Tuberculum sella meningioma | None | 6 |
| 45 | 51, F | HA, VS | Improved | GTR | Observation, stable | Tuberculum sella meningioma | None | 4 |
M: male; F: female; GTR: gross total resection; STR: subtotal resection; CSF: cerebrospinal fluid; Dl: diabetes insipidus; HA: headache; VS: visual symptoms; RT: radiotherapy; EVD: external ventricular drainage; SIADH: syndrome of inappropriate antidiuretic hormone secretion; ACOM: anterior communicating artery; ACA: anterior cerebral artery; ICA: internal carotid artery.
Table 2.
Pathology and location of skull base neoplasms.
| Pathology and location (MRI) | |
|---|---|
| Planum sphenoidale meningioma | 9 (20.0) |
| Giant pituitary adenoma | 8 (17.8) |
| Tuberculum sella meningioma | 7 (15.6) |
| Clival chordoma | 4 (8.9) |
| Craniopharyngioma | 3 (6.6) |
| Supra-clinoidal meningioma | 2 (4.5) |
| Optic pathway glioma | 2 (4.5) |
| Redo-olfactory groove meningioma | 2 (4.5) |
| Anterior clinoidal meningioma | 1 (2.2) |
| Clival meningioma | 1 (2.2) |
| Meckel’s cave epidermoid tumor | 1 (2.2) |
| Petroclival meningioma | 1 (2.2) |
| Planum sphenoidal epidermoid cyst | 1 (2.2) |
| Suprasellar and retroclival craniopharyngioma | 1 (2.2) |
| Suprasellar pilocytic astrocytoma | 1 (2.2) |
| Suprasellar teratoma | 1 (2.2) |
Data are number (%).
The majority of patients (n=40) presented with either visual symptoms (n=16; 35.5%), headache (n=8; 17.8%), or both (n=15; 33.3%), followed by endocrinopathy, seizure, psychiatric changes, anosmia, or the neoplasm was an incidental MRI finding (Table 1). In terms of symptomatic outcomes, over half of the patients (57.8%) reported resolution or improvement in preoperative symptoms, and 37.8% had no changes in symptoms. Conversely, two patients (4.4%) had a worsening of their symptoms following surgery. One patient had a right internal carotid artery (ICA) injury, while the other had intraoperative anterior communicating artery (ACOM) bleeding and an anterior cerebral artery (ACA) infarction. (Table 1, Cases 5 and 32).
In regard to postoperative complications, cerebro-spinal fluid (CSF) leak was the most common complication in our series. Nine patients (20%) underwent endoscopic exploration and repair of postoperative CSF leaks. Three of these patients subsequently developed meningitis, and another one developed ventriculitis, which were successfully treated by medical therapy. Also, in one patient, during the repair, a brain abscess was noted intraoperatively, which necessitated drainage and medical therapy. Diabetes insipidus (DI) was another common complication that occurred in 15.6% of patients. In three patients, DI was transient; whereas four other patients had permanent DI. Other complications included deterioration in vision, abducens nerve palsy, hydrocephalus, hormonal disturbance, septal perforation, orbital hematoma, ventriculitis, and meningitis.
Intraoperative major vascular complications occurred in two patients (4.4%). The first case was a 43-year-old woman with a recurrent olfactory groove meningioma (Table 1, Case 32). During surgery, the anterior cerebral artery was injured, which led to massive bleeding intraoperatively that required embolization by interventional radiology as intraoperative methods of control were not sufficient to control it. Postoperatively, the patient was transferred to the surgical intensive care unit with a decreased level of consciousness, which improved over the next 3 weeks. Then the patient was moved to our surgical ward, where she gained full consciousness. During her hospital stay, the patient developed DI, meningitis, and right-sided hemiparesis. Endocrinology, infectious diseases, and physical therapy teams were involved in the patient care. Meningitis resolved with antibiotics, DI was controlled, and her hemiparesis showed significant improvement. She started to ambulate independently with extensive rehabilitation. After 8 months in the hospital, the patient was transferred to a rehabilitation center.
The other case (Table 1, Case 5) resulted from an injury to the right internal carotid artery during removal of a clival chordoma. Bleeding was controlled promptly by applying TachoSil (Baxter Inc., Deerfield, Illinois, USA) fibrin sealant patch and pressure on the site. An intra-operative MRI showed adequate patency of the internal carotid artery, allowing for the tumor resection to continue. Angiography was done on the first postoperative day to coil a pseudoaneurysm. The patient was extubated the second day postoperatively with left hemiparesis, which improved, and she returned to baseline during her 2-week hospital stay.
No mortality was observed in our cohort. The complications of this series are summarized in Table 3. For the entire series, gross total resection was achieved in 25 of 45 cases (55.5%), and the remaining 20 patients underwent subtotal resection (44.4%).
Table 3.
Complications following expanded endoscopic transnasal surgery.
| Complicationa | |
|---|---|
| CSF leak | 9 (20.0%) |
| Diabetes insipidusb | 7 (15.6%) |
| Hydrocephalus | 4 (8.9%) |
| Meningitis | 3 (6.6%) |
| Hypopituitarism | 3 (6.6%) |
| Hypothyroidism | 3 (6.6%) |
| Abducens nerve palsy | 2 (4.5%) |
| Ventriculitis | 1 (2.2%) |
| Brain abscess | 1 (2.2%) |
| Major vascular injury | 2 (4.4%) |
Data are number (%). CSF: cerebrospinal fluid;
Some patients experienced more than one complication;
Diabetes insipidus was permanent in four patients and transient in three
DISCUSSION
Minimally invasive skull base endoscopic approaches have been designed to improve the quality of visualization, minimize hospital stay, and reduce morbidity and mortality. In the past, these approaches were limited only to the pituitary fossa.14 Thanks to advances in instrumentation, a better understanding of skull base endoscopic anatomy, and experience, EETS has been recently introduced.15,16 With EETS, it is possible to gain access to skull base lesions located between the anterior cranial fossa down to the level of C2 in the sagittal plane. It is also possible to reach the medial orbit, orbital apex, Meckel’s cave, the petrous apex, and the temporal and infratemporal fossae in the coronal plane.10,17-19
To master the endonasal approaches to the skull base, it is essential that the surgeon have endoscopic skills and manual dexterity as well as an understanding of the anatomy and dissection principles. To improve teamwork collaboration between the neurosurgeon and rhinologist, the “two nostrils, four hands” technique is used.20 This collaborative technique expands the surgical field to the second nasal fossa, which significantly increases the visual field and improves outcomes.20 The multidisciplinary team approach between the otolaryngologist and the neurosurgeon improves the postoperative quality of care in their respective inpatient services and outpatient clinics.21
Advanced knowledge and the development of skull base surgery have resulted in better surgical access with minimal brain retraction and minimized vascular injury. Unlike with the transcranial route for the management of anterior skull base lesions, the risk of postoperative seizure is an exceedingly rare complication in EETS.22 Also, other surgical complications, such as subdural hygroma and postoperative edema, are also rare in EETS.22 Despite overcoming some limitations of the transcranial route, some complications occur in higher rates. Kassam et al23 reported the outcomes of 800 patients who underwent endoscopic endonasal surgery. The most common complication in their cohort was CSF leak (15.9%). Transient (2.5%) and permanent (1.8%) neurological deficits were also reported. Intracranial infection occurred in 1.6% and systemic complications in 2.1%. Mortality was observed in 0.9% of cases.23
Bleeding is a challenging and critical entity to consider while performing EETS. The source of bleeding can be from the nasal cavity, the cavernous sinus, or large vessel injuries, which can lead to catastrophic bleeding.23 As reported by Kassam et al,23 intraoperative vascular injuries occurred in 0.9% of patients; they have also been reported by other groups with rates ranging between 2-9%.22,24-26 Our major vascular injury rate is comparable at 4.4% with no mortality observed. The risk of brain abscess was reported in 6% of patients in a large series of olfactory groove meningioma managed with transnasal surgery.22 As with our case, abscess formation in that study was mainly seen with tumors >40 mm and multiple surgeries.22
Simple transsphenoidal surgery for pituitary tumors has a relatively low risk of CSF leak since the arachnoid is not usually breached. However, EETS possesses a higher risk of CSF leak due to large dural defects and direct ventricular communication, in some cases resulting in high flow leaks. In the literature, the rate of CSF leaks after transsphenoidal surgery ranges from 2% to 13%.27-29 The risk of CSF leaks during the early days of EETS was reported to be as high as 65%, with a range of 5–50% reported in earlier series.16,17,30-34 Nine cases in our series (20%) underwent CSF leak repair.
Several reconstruction techniques have been suggested, including the use of multiple layers as well as a combination of fat, fascia lata, septal cartilage, vascularized pedicle flaps, and artificial dura. However, no single type of repair has proved to be superior to the others, though it is difficult to compare them because most studies have evaluated the techniques in a small number of cases with defects at different sites.35 Nix et al36 reported the failure of primary repair and the need for secondary repair using endoscopic techniques in 10 of 51 cases. Defects were located in the planum sphenoidale in 7 of 20 cases (35%), the clivus in 2 of 5 cases (40%), and the pituitary fossa in 1 of 23 cases (4%).36 Proper surgical techniques based on anatomical understanding can reduce the risk of these complications.
We compared CSF leak rates in the first half of cases in our series versus the second half. We observed that in the first half, 7 of 22 cases (31.8%) developed CSF leaks compared to 2 of 23 cases (8.7%) in the second half. In our series, although a multilayered reconstruction technique was used in all cases using a “gasket seal” rigid reconstruction technique (as proposed by Schwartz)37 and vascularized flaps, the postoperative leak rate remained significant earlier in the series. Plausible causes of this could be classified into the following main categories: skull base reconstructive technique, postoperative site infection, and lack of reconstructive autologous material or adequate postoperative sedation in pediatric cases. Retrospectively reviewing cases of CSF leak post EETS, we noticed that use of fibrin glue between layers and application of excessive amounts could be one of the factors hindering healing of the reconstructed site. Thus, we restricted its application to limited amounts on the edges of the nasoseptal flap. Earlier in the series, fat was not used to fill intradural defects; instead, fascia lata was used in a layered fashion. We gradually replaced intradural fascia with fat, which may have also contributed to the decrease in CSF leak rates later on. Antibiotic prophylaxis, face disinfection, and a sterile technique were routine practice in all EETS cases in our center; thus, we are prospectively collecting data on these cases to identify possible causes of operative site infection. Pediatric cases present another challenge. A protocol which includes consideration of preoperative external ventricular drain (EVD) insertion, use of allograft or synthetic materials due to a lack of autologous grafts, and temporary postoperative sedation, is in place to improve surgical outcomes in that population.
Of note, the complexity of the cases described above should be considered when interpreting the complication rates and postoperative outcomes (Figure 3). As we have gradually moved from the third to fourth level in the Pittsburgh endoscopic skull base surgery training model,38 complication rates were expected to rise. However, a team-based approach during pre-operative planning along with cumulative experience have resulted in minimizing morbidity and improving outcomes. In an effort to continually improve, a fellow-ship in both rhinology and skull base neurosurgery was also developed in our center during the study period. This will positively impact our field at both national and international levels in the near future.
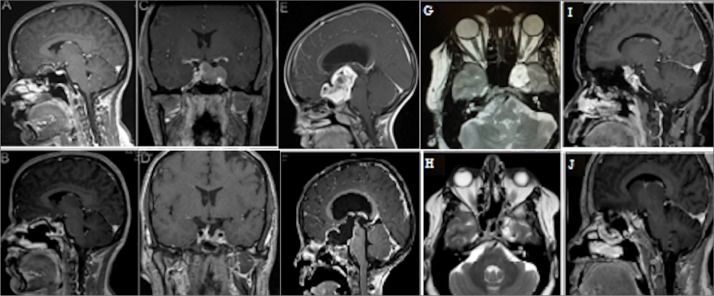
Figure 3.
Representative preoperative and postoperative magnetic resonance imaging scans of olfactory groove dermoid tumor (A, B), giant pituitary adenoma (C, D), optic pathway glioma (E, F), Meckel’s cave epidermoid tumor (G, H), and supra-clinoidal meningioma (I, J).
In conclusion, EETS is a promising minimally invasive procedure for selected neoplasms of the anterior skull base. Results can be comparable to those from traditional open approaches. More work needs to be done to expand our experience, improve the outcomes, and educate the public and medical community in our region about the feasibility and the usefulness of this technique. Currently, our results are comparable to those of larger published case series, and we strive for better outcomes in the near future.
Funding Statement
None.
REFERENCES
- 1.Policeni BA, Smoker WR.. Imaging of the skull base: anatomy and pathology. Radiol Clin North Am. 2015;53(1):1-14. doi: 10.1016/j.rcl.2014.09.005. PubMed PMID: 25476171. [DOI] [PubMed] [Google Scholar]
- 2.Samii M, Ammirati M.. The combined supra-infratentorial pre-sigmoid sinus avenue to the petro-clival region. Surgical technique and clinical applications. Acta Neurochir (Wien). 1988;95(1/2):6-12. PubMed PMID: 3218555. [DOI] [PubMed] [Google Scholar]
- 3.Hakuba A, Tanaka K, Suzuki T, Nishimura S.. A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989;71(5 Pt 1):699-704. doi: 10.3171/jns.1989.71.5.0699. PubMed PMID: 2809723. [DOI] [PubMed] [Google Scholar]
- 4.Cho CW, Al-Mefty O.. Combined petrosal approach to petroclival meningiomas. Neurosurgery. 2002;51(3):708-16; discussion 716-8. PubMed PMID: 12188949. [PubMed] [Google Scholar]
- 5.Fahlbusch R, Schott W.. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmo-logical and endocrinological outcomes. J Neurosurg. 2002;96(2):235-43. doi: 10.3171/jns.2002.96.2.0235. PubMed PMID: 11838796. [DOI] [PubMed] [Google Scholar]
- 6.Frank G, Pasquini E, Mazzatenta D.. Extended transsphenoidal approach. J Neurosurg. 2001;95(5):917-8. PubMed PMID: 11702890. [DOI] [PubMed] [Google Scholar]
- 7.de Divitiis E, Cappabianca P, Cavallo LM.. Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery. 2002;51(3):699-705; discussion 705-7. PubMed PMID: 12188948. [DOI] [PubMed] [Google Scholar]
- 8.Frank G, Pasquini E.. Endoscopic endonasal approaches to the cavernous sinus: surgical approaches. Neurosurgery. 2002;50(3):675. PubMed PMID: 11890157. [DOI] [PubMed] [Google Scholar]
- 9.Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, et al. . The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102(5):832-41. doi: 10.3171/jns.2005.102.5.0832. PubMed PMID: 15926706. [DOI] [PubMed] [Google Scholar]
- 10.Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R.. Expanded endonasal approach: fully endoscopic, completely trans-nasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(1):E6. PubMed PMID: 16078820. [PubMed] [Google Scholar]
- 11.de Divitiis E, Cavallo LM, Cappabianca P, Esposito F.. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: part 2. Neurosurgery. 2007;60(1):46-58; discussion 58-9. doi: 10.1227/01.NEU.0000249211.89096.25. PubMed PMID: 17228252. [DOI] [PubMed] [Google Scholar]
- 12.Solari D, Villa A, De Angelis M, Esposito F, Cavallo LM, Cappabianca P.. Anatomy and surgery of the endoscopic endonasal approach to the skull base. Transl Med UniSa. 2012;2:36-46. PubMed PMID: 23905043; PubMed Central PMCID: PMCPMC3728777. [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C, et al. . Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: a meta-analysis. World J Surg Oncol. 2014;12:94. doi: 10.1186/1477-7819-12-94. PubMed PMID: 24721812; PubMed Central PMCID: PMCPMC3991865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosegay H. Cushing’s legacy to transsphenoidal surgery. J Neurosurg. 1981;54(4):448-54. doi: 10.3171/jns.1981.54.4.0448. PubMed PMID: 7009800. [DOI] [PubMed] [Google Scholar]
- 15.Zada G, Kelly DF, Cohan P, Wang C, Swerdloff R.. Endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions. J Neurosurg. 2003;98(2):350-8. doi: 10.3171/jns.2003.98.2.0350. PubMed PMID: WOS:000180873300014. [DOI] [PubMed] [Google Scholar]
- 16.Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T.. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55(3):539-47. doi: 10.1227/01.Neu.0000134287.19377.A2. PubMed PMID: WOS:000224309000017. [DOI] [PubMed] [Google Scholar]
- 17.Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL.. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3. PubMed PMID: 16078817. [PubMed] [Google Scholar]
- 18.Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL.. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(1):E4. PubMed PMID: 16078818. [PubMed] [Google Scholar]
- 19.Kassam AB, Snyderman C, Gardner P, Carrau R, Spiro R.. The expanded endonasal approach: a fully endoscopic transnasal approach and resection of the odontoid process: technical case report. Neurosurgery. 2005;57(1 Suppl):E213; discussion E. PubMed PMID: 15987596. [DOI] [PubMed] [Google Scholar]
- 20.Castelnuovo P, Pistochini A, Locatelli D.. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique.” Rhinology. 2006;44(1):2-7. PubMed PMID: 16550942. [PubMed] [Google Scholar]
- 21.McLaughlin N, Carrau RL, Kelly DF, Prevedello DM, Kassam AB.. Teamwork in skull base surgery: an avenue for improvement in patient care. Surg Neurol Int. 2013;4:36. doi: 10.4103/2152-7806.109527. PubMed PMID: 23607058; PubMed Central PMCID: PMCPMC3622378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koutourousiou M, Fernandez-Miranda JC, Wang EW, Snyderman CH, Gardner PA.. Endoscopic endonasal surgery for olfac-tory groove meningiomas: outcomes and limitations in 50 patients. Neurosurg Focus. 2014;37(4):E8. PubMed PMID: 25391163. [DOI] [PubMed] [Google Scholar]
- 23.Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, et al. . Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients. J Neurosurg. 2011;114(6):1544-68. doi: 10.3171/2010.10.JNS09406. PubMed PMID: 21166570. [DOI] [PubMed] [Google Scholar]
- 24.Iacoangeli M, Di Rienzo A, Re M, Alva-ro L, Nocchi N, Gladi M, et al. . Endoscopic endonasal approach for the treatment of a large clival giant cell tumor complicated by an intraoperative internal carotid artery rupture. Cancer Manag Res. 2013;5:21-4. doi: 10.2147/CMAR.S38768. PubMed PMID: 23403482; PubMed Central PMCID: PMCPMC3565560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E.. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery. 2006;59(1 Suppl 1):ONS50-7; discussion ONS57. doi: 10.1227/01.NEU.0000219914.17221.55. PubMed PMID: 16888551. [DOI] [PubMed] [Google Scholar]
- 26.Cavallo LM, Solari D, Esposito F, Cappabianca P.. The endoscopic endonasal approach for the management of craniopharyngiomas involving the third ventricle. Neurosurg Rev. 2013;36(1):27-37; discussion 38. doi: 10.1007/s10143-012-0403-4. PubMed PMID: 22791074. [DOI] [PubMed] [Google Scholar]
- 27.Semple PL, Laws ER Jr.. Complications in a contemporary series of patients who underwent transsphenoidal surgery for Cushing’s disease. J Neurosurg. 1999;91(2):175-9. doi: 10.3171/jns.1999.91.2.0175. PubMed PMID: 10433303. [DOI] [PubMed] [Google Scholar]
- 28.Sudhakar N, Ray A, Vafidis JA.. Complications after trans-sphenoidal surgery: our experience and a review of the literature. Brit J Neurosurg. 2004;18(5):507-12. doi: 10.1080/02688690400012459. PubMed PMID: WOS:000225863000011. [DOI] [PubMed] [Google Scholar]
- 29.Fatemi N, Dusick JR, Neto MAD, Kelly DF.. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience. Neurosurgery. 2008;63(4):244-56. doi: 10.1227/01.Neu.0000327025.03975.Ba. PubMed PMID: WOS:000260578700009. [DOI] [PubMed] [Google Scholar]
- 30.Alobid I, Ensenat J, Marino-Sanchez F, Rioja E, de Notaris M, Mullol J, et al. . Expanded endonasal approach using vascularized septal flap reconstruction for skull base tumors has a negative impact on sinonasal symptoms and quality of life. Am J Rhinol Allergy. 2013;27(5):426-31. doi: 10.2500/ajra.2013.27.3932. PubMed PMID: WOS:000326759700022. [DOI] [PubMed] [Google Scholar]
- 31.Dehdashti AR, Ganna A, Witterick I, Gentili F.. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery. 2009;64(4):677-87. doi: 10.1227/01.Neu.0000339121.20101.85. PubMed PMID: WOS:000264783500026. [DOI] [PubMed] [Google Scholar]
- 32.Laws ER, Kanter AS, Jane JA Jr., Dumont AS.. Extended transsphenoidal approach. J Neurosurg. 2005;102(5):825-7; discussion 827-8. doi: 10.3171/jns.2005.102.5.0825. PubMed PMID: 15926704. [DOI] [PubMed] [Google Scholar]
- 33.Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A.. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19(1):E8. PubMed PMID: 16078822. [PubMed] [Google Scholar]
- 34.Kitano M, Taneda M.. Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery. 2004;54(3):653-60; discussion 660-1. PubMed PMID: 15028140. [DOI] [PubMed] [Google Scholar]
- 35.Zweig JL, Carrau RL, Celin SE, Schaitkin BM, Pollice PA, Snyderman CH, et al. . Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg. 2000;123(3):195-201. doi: 10.1067/mhn.2000.107452. PubMed PMID: 10964290. [DOI] [PubMed] [Google Scholar]
- 36.Nix P, Tyagi A, Phillips N.. Retrospective analysis of anterior skull base CSF leaks and endoscopic repairs at Leeds. Br J Neurosurg. 2016:1-5. doi: 10.3109/02688697.2016.1161176. PubMed PMID: 27008345. [DOI] [PubMed] [Google Scholar]
- 37.Leng LZ, Brown S, Anand VK, Schwartz TH.. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62(5 Suppl 2):ONSE342-3; discussion ONSE343. doi: 10.1227/01.neu.0000326017.84315.1f. PubMed PMID: 18596534. [DOI] [PubMed] [Google Scholar]
- 38.Lavigne P, Faden D, Gardner PA, Fernandez-Miranda JC, Wang EW, Snyderman CH.. Validation of training levels in endoscopic endonasal surgery of the skull base. Laryngoscope. 2019. doi: 10.1002/lary.27895. PubMed PMID: 30843604. [DOI] [PubMed] [Google Scholar]