Abstract
Background
Reduced BMI is an absolute contraindication for lung transplantation (LTx) at most centers in the United States. The objective of this study was to quantify post-LTx survival of moderate to severely underweight patients with cystic fibrosis (CF) (BMI < 17 kg/m2) in the United States relative to normal-weight recipients with CF and other frequently transplanted patient cohorts.
Methods
Using United Network for Organ Sharing Registry data (undergoing transplant from June 2005-November 2015), Kaplan-Meier estimates of median posttransplant survival were calculated for all patients with CF, COPD, and idiopathic pulmonary fibrosis (IPF), as well as low and normal weight CF subgroups. Cox regression modeling stratified according to transplant center assessed risk of posttransplant mortality in recipients with CF and a BMI < 17 kg/m2 compared with recipients with COPD (reference).
Results
Median posttransplant survival (95% CI) for CF, COPD, and IPF was 7.9 (7.2-8.6), 5.9 (5.6-6.2), and 5.5 (5.2-5.8) years, respectively. Although an absolute decrease was noted in posttransplant survival for recipients with CF and a BMI < 17 kg/m2, compared with those with CF and a BMI ≥ 17 kg/m2 (7.0 years [4.5-7.9] vs 8.2 years [7.3-9.0]), Cox modeling found no increased mortality risk (adjusted hazard ratio, 1.09; 95% CI, 0.90-1.32; P = .38). There was no difference in posttransplant mortality between patients with CF and a BMI < 17 kg/m2 and recipients with COPD and all BMIs (adjusted hazard ratio, 1.04; 95% CI, 0.86-1.25; P = .71).
Conclusions
Transplant recipients with CF and a BMI < 17 kg/m2 had posttransplant survival rates comparable to those of other groups frequently undergoing transplantation. BMI < 17 kg/m2 as a single risk factor in the CF population should not be treated as an absolute contraindication to LTx.
Key Words: BMI, cystic fibrosis, lung transplant, survival, underweight
Abbreviations: CF, cystic fibrosis; IPF, idiopathic pulmonary fibrosis; LTx, lung transplantation; UNOS, United Network for Organ Sharing
FOR EDITORIAL COMMENT, SEE PAGE 757
Lung transplantation (LTx) can improve quality of life and survival in a variety of end-stage pulmonary diseases, but it remains a high-risk treatment with outcomes inferior to other solid organ transplants.1,2 Candidate selection is crucial for distinguishing patients who will have reasonable benefit following LTx from those destined for poor outcomes. Low preoperative BMI has been associated with increased hospital length of stay, overall mortality, and death from infection following transplantation in analyses involving comparisons for individuals with the same diagnosis.3, 4, 5, 6, 7, 8 Singer et al9 found a 35% increase in 1-year mortality associated with a pretransplant BMI < 18.5 kg/m2, and Fernandez et al10 reported incrementally greater risks at lower BMIs, with BMI ≤ 20 kg/m2 associated with increased 90-day mortality. Thus, International Society for Heart and Lung Transplantation candidate selection recommendations consider severe malnutrition a relative contraindication to LTx,11 and many centers use BMI < 17 kg/m2 or < 18 kg/m2 as an absolute exclusion criterion12 based on worsened expected posttransplant survival.
Cystic fibrosis (CF) is a disease strongly associated with malnutrition, especially among individuals with advanced lung disease. Similar to the general LTx population, among individuals with CF, low pretransplant BMI has been associated with worse posttransplant outcomes3, 4, 5 and often serves as a contraindication to offering transplant, particularly when patients are losing or unable to gain weight prior to the procedure.
Candidate selection involves assessing patients’ underlying lung disease prognosis and their expected chance of garnering an acceptable posttransplant outcome.11 The threshold for acceptability is by nature subjective, but conventional practices factor in reported survival estimates among all LTx recipients, an expectation that a recipient should have a reasonable likelihood of 5-year survival, and ethical principles in organ allocation. Although candidate selection criteria vary across centers, it can be argued that in principle this acceptable threshold is assessed in the context of an individual’s expected survival outcome relative to the overall population in need of LTx, rather than their survival compared only with others with the same underlying lung disease. Conversely, in practice, a selection committee may decline to list an individual with CF whose BMI is < 17 kg/m2 based on assumptions that worsened outcomes related to malnutrition would not meet an acceptable expected posttransplant outcome. However, it is unknown how the posttransplant survival of patients with CF and a BMI below usual exclusion thresholds compares with that of the general LTx candidate population.
The objective of the current study was to quantify posttransplant survival of CF LTx recipients with moderate/severe malnutrition (BMI < 17 kg/m2) at the time of LTx and compare their survival vs that of other common transplant cohorts. We hypothesized that although a lower BMI would be associated with worse outcomes compared with higher BMI within the CF cohort, the median survival of patients with CF and a BMI < 17 kg/m2 would be comparable to that of patients with other commonly transplanted diagnoses. Especially relevant for patients near common BMI exclusion thresholds, we also examined whether upward change in pretransplant BMI among CF transplant recipients is associated with posttransplant survival.
Patients and Methods
Population
Using United Network for Organ Sharing (UNOS) Registry data provided by the Organ Procurement and Transplantation Network as of December 13, 2016, patients with a first LTx at a US center between June 1, 2005, and November 30, 2015, were included. Subjects were included if they were aged ≥ 12 years at first LTx with a diagnosis of CF, COPD (without notation of alpha-1-antitrypsin disease), or idiopathic pulmonary fibrosis (IPF). Exclusion criteria included re-transplantation (first LTx before June 1, 2005), multiorgan transplantation, and living donor LTx. An exploratory analysis of waitlist deaths included all individuals with CF who were listed for LTx between June 1, 2005, and November 30, 2015. The University of Washington Human Subjects Division determined that this study does not involve human subjects and further review by the institutional review board was not required (Study #02131).
Data
Pretransplant variables were recorded either at the time of LTx or nearest to LTx. BMI was recorded at time of LTx. For CF, BMI was stratified according to ≥ 17 kg/m2 or < 17 kg/m2 (e-Appendix 1, e-Table 1). Transplant year was categorized as 2005 to 2009 or 2010 to 2015 to account for effects of calendar time.
Statistical Analysis
Among LTx centers with at least one CF LTx in a given year of the study, descriptive statistics were used to describe the percentage of centers with zero CF LTx recipients with BMI ≤ 16.9 kg/m2 and the percentage of centers with zero CF LTx recipients with BMI ≤ 17.9 kg/m2 as a proxy for identifying center-specific BMI thresholds of < 17 kg/m2 and < 18 kg/m2, respectively.
The primary outcome was posttransplant mortality risk. As in previous studies, patients who were lost to follow-up or underwent re-transplantation (eg, second lung transplant) were censored at re-transplant date or date of last follow-up when status was recorded as lost to follow-up.1,13,14 We calculated Kaplan-Meier estimates15 of median posttransplant survival time together with 1-year and 5-year posttransplant survival percentages for CF, COPD, and IPF cohorts, and for BMI strata of the CF cohort. To estimate 1-year conditional survival, median survival time following transplantation (restricted to the subgroup of patients who survived at least 1 year after LTx) was calculated. For the primary analysis, Cox regression modeling was used to estimate posttransplant mortality risk of patients with CF and a BMI < 17 kg/m2 relative to COPD (reference group).16 Patients with IPF were also compared with the COPD group in this Cox regression model. All Cox models included transplant year category as a covariate and were stratified according to LTx center, allowing each center to have its own baseline hazard to account for between-center variability. Patient-level covariates were not included in the Cox models because of the nonoverlapping characteristics of the patients in the different diagnosis cohorts. The proportional hazard assumption was formally tested by using Schoenfeld residuals and by evaluating the significance of interactions between the predictor of interest and time (e-Appendix 1).
Sensitivity Analyses
Waitlist Analyses
An exploratory analysis was performed among all individuals with CF listed for LTx, stratified according to BMI at listing (< 17 kg/m2 compared with ≥ 17 kg/m2) to determine the proportion who died while on the waitlist. In addition, the median BMI [interquartile range] was calculated for individuals with CF who died while on the waitlist.
Posttransplant Survival Analyses
Sensitivity analyses were performed to assess the stability of the primary analyses, repeating survival estimates and Cox regression modeling of posttransplant death in: (1) subjects aged ≥ 18 years; and (2) the cohort of COPD and IPF LTx recipients that underwent bilateral LTx. To confirm results from previous studies in the current study cohort, Cox modeling was used to compare posttransplant mortality risk of patients with CF and a BMI < 17 kg/m2 vs patients with CF and a BMI ≥ 17 kg/m2 (reference group). A separate Cox regression model compared posttransplant mortality risk of the entire CF cohort (all BMIs) vs COPD (reference group).
BMI Change Analyses
A continuous measure of BMI change was calculated from BMI at listing minus BMI at LTx, without adjustment for time spent on the waitlist. In underweight subjects with CF, three separate Cox regression models were used to investigate the association between pretransplant BMI change and posttransplant survival among recipients with low BMI at the time of transplant or the time of listing. Model 1 was developed to assess association of BMI change while on the waitlist for LTx recipients with CF and a BMI < 17 kg/m2 at the time of transplant compared with individuals with BMI ≥ 17 kg/m2 at transplant. Model 2 assessed the association of BMI change for LTx recipients with CF and a BMI < 17 kg/m2 at listing (compared with individuals with CF listed with BMI ≥ 17 kg/m2) with posttransplant survival; model 3 assessed the association of BMI change for recipients with a BMI < 18.5 kg/m2 at listing (compared with individuals with CF listed with a BMI ≥ 18.5 kg/m2). The three models were repeated using a categorical approach to change in BMI from listing to transplant: increased (≥ 1 kg/m2 increase) or decreased (≥ 1 kg/m2 decrease), with unchanged (< 1 kg/m2 change) as the reference group.
All testing was two-sided and conducted at the 0.05 level of significance without correction for multiple comparisons. Statistical analyses were performed by using Stata version 15 (StataCorp).
Results
Transplant Recipient Characteristics
A total of 13,510 transplant recipients were analyzed: 2,195 with CF, 4,858 with COPD, and 6,457 with IPF (e-Fig 1). Median age was 28, 61, and 62 years in the CF, COPD, and IPF groups, respectively (Table 1). Bilateral LTxs were performed in 99.9% of the CF group compared with 63% in the COPD group and 53% in the IPF group. Median lung allocation score at transplantation was 41 for CF, 34 for COPD, and 46 for IPF. Eleven percent of patients with CF were supported with mechanical ventilation prior to LTx, compared with 3% in the COPD group and 6% in the IPF group. In CF, 352 (16%) had a BMI < 17 kg/m2, 1,831 (83%) had a BMI ≥ 17 kg/m2, and 12 were missing BMI data at LTx. Subjects with CF and a BMI < 17 kg/m2 at LTx were younger but were otherwise comparable to subjects with CF and a BMI > 17 kg/m2 (e-Table 2). Moderate to severe malnutrition was present almost exclusively in patients with CF (e-Table 3).
Table 1.
Characteristics of the Lung Transplant Recipients
| Characteristic | CF (n = 2,195) | COPD (n = 4,858) | IPF (n = 6,457) |
|---|---|---|---|
| Recipient age, median (IQR), y | 28 (23-37) | 61 (56-65) | 62 (57-66) |
| Age ≥ 18 y | 1,989 (91) | 4,857 (100) | 6,446 (100) |
| Male sex | 1,129 (51) | 2,569 (53) | 4,676 (72) |
| Race/ethnicity | |||
| White | 2,076 (95) | 4,459 (92) | 5,285 (82) |
| Black | 29 (1) | 305 (6) | 419 (6) |
| Hispanic | 83 (4) | 54 (1) | 558 (9) |
| Asian | 0 | 12 (0) | 144 (2) |
| American Indian/Alaska Native | 4 (0) | 8 (0) | 29 (0) |
| Native Hawaiian/other Pacific Islander | 0 | 1 (0) | 8 (0) |
| Multiracial | 3 (0) | 19 (0) | 14 (0) |
| Transplant year 2010-2015 (reference, 2005-2009) | 1,288 (59) | 2,722 (56) | 4,155 (64) |
| Private insurance | 1,288 (59) | 2,155 (44) | 3,750 (58) |
| Height at transplant, median (IQR), cm | 165 (159-173) | 170 (163-177) | 173 (165-178) |
| Weight at transplant, median (IQR), kg | 53 (47-60) | 70 (59-81) | 82 (71-91) |
| BMI at transplant, median (IQR), kg/m2 | 19 (18-21) | 24 (21-28) | 28 (25-30) |
| BMI < 17 kg/m2 | 352 (16) | 76 (2) | 31 (0) |
| Diabetes present | 1,034 (47) | 420 (9) | 1,196 (19) |
| Oxygen requirement at rest at transplant, median (IQR), L/min | 4 (2-6) | 3 (2-4) | 4 (3-8) |
| FEV1 % at transplant, median (IQR) | 22 (18-27) | 20 (16-26) | 50 (40-62) |
| FVC % at transplant, median (IQR) | 37 (30-46) | 52 (41-64) | 45 (36-57) |
| Lung allocation score at transplant, median (IQR) | 41 (37-50) | 34 (32-35) | 46 (40-61) |
| On ventilator at transplant | 250 (11) | 158 (3) | 402 (6) |
| ECMO at transplanta | 101 (5) | 17 (0) | 169 (3) |
| Bilateral transplant | 2,193 (100) | 3,082 (63) | 3,430 (53) |
| Repeat transplant | 157 (7) | 102 (2) | 195 (3) |
Data are presented as No. (%) unless otherwise indicated. CF = cystic fibrosis; ECMO = extracorporeal membrane oxygenation; IPF = idiopathic pulmonary fibrosis; IQR = interquartile range.
ECMO is not consistently captured in the United Network for Organ Sharing Registry for a majority of the study period.
Transplant Center BMI Thresholds
Depending on the year within the study, 54% to 69% of centers had zero LTx recipients with CF and a BMI ≤ 16.9 kg/m2, and 30% to 49% had zero recipients with CF and a BMI ≤ 17.9 kg/m2 (e-Table 1). These results are consistent with BMI < 17 kg/m2 as a threshold for contraindication to LTx at a majority of US LTx centers, whereas BMI < 18 kg/m2 may be the threshold at fewer centers.
Waitlist Outcomes
Among individuals with CF and a BMI < 17 kg/m2 at listing, 14% died while on the waitlist, compared with 13% of individuals with CF and a BMI ≥ 17 kg/m2. The median BMI [interquartile range] for individuals with CF who died while on the waitlist was 18.7 kg/m2 [17.6-20.4] compared with a median BMI of 19.1 kg/m2 [17.6-21.0] among transplant recipients with CF.
Posttransplant Survival
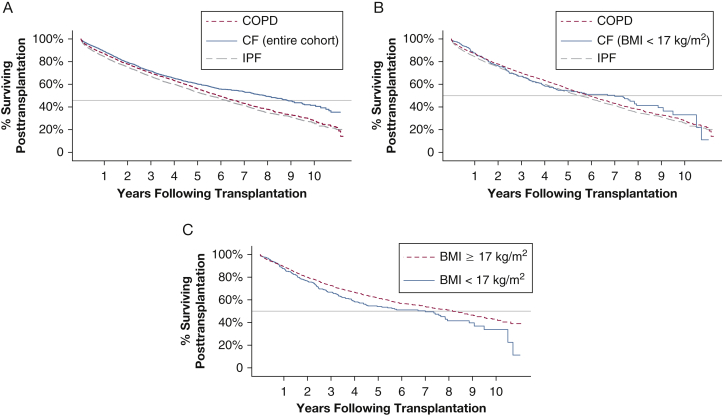
Median posttransplant survival in the CF cohort was 7.9 years (95% CI, 7.2-8.6) (Table 2). This finding compared favorably to median survival for subjects with COPD (5.9 years; 95% CI, 5.6-6.2) and subjects with IPF (5.5 years; 95% CI, 5.2-5.8) (Fig 1). Loss to follow-up was documented for 1% of patients in all diagnosis groups. Within the CF cohort, as expected, subjects with BMI < 17 kg/m2 at LTx had shorter posttransplant survival than subjects with BMI ≥ 17 kg/m2 (7.0 vs 8.2 years), but Cox modeling found no increased mortality risk (adjusted hazard ratio, 1.09; 95% CI, 0.90-1.32; P = .38). In Cox modeling, in which the COPD cohort was the reference group, patients with CF and a BMI < 17 kg/m2 had no survival disadvantage (adjusted hazard ratio, 1.04; 95% CI, 0.64-1.25; P = .71). Median survival conditional on survival for 1-year posttransplant was 9.1 years (95% CI, 8.4-10.3) in the CF cohort; LTx recipients with CF and a BMI ≥ 17 kg/m2 had 1-year conditional survival of 9.5 years vs 7.8 years among those with CF and a BMI < 17 kg/m2 (Table 3). IPF LTx recipients had lower 1-year survival (84%) compared with CF and COPD LTx recipients (87%-89%) (Table 4). Five-year survival estimates were similar for patients with CF and a BMI < 17 kg/m2 (54%), all patients with IPF (53%), and all patients with COPD (56%). In sensitivity analyses that included only patients with bilateral LTx or patients aged ≥ 18 years, results were similar (e-Tables 4-7).
Table 2.
Kaplan-Meier Estimates of Median Posttransplant Survival Time
| Variable | No. | Median Posttransplant Survival Time (y) | 95% CI |
|---|---|---|---|
| CF, entire cohorta | 2,195 | 7.9 | 7.2-8.6 |
| CF, BMI ≥ 17 kg/m2 | 1,831 | 8.2 | 7.3-9.0 |
| CF, BMI < 17 kg/m2 | 352 | 7.0 | 4.5-7.9 |
| COPD | 4,858 | 5.9 | 5.6-6.2 |
| IPF | 6,457 | 5.5 | 5.2-5.8 |
See Table 1 legend for expansion of abbreviations.
Twelve subjects with CF had missing BMI data.
Figure 1.
A-C, Kaplan-Meier survival curves for COPD, CF, and IPF. Analyses are censored at loss to follow-up or re-transplantation. A, Subjects with COPD, IPF, and CF (all BMIs). B, Subjects with COPD, IPF, and CF with BMI < 17 kg/m2. C, Subjects with CF, stratified according to BMI ≥ 17 kg/m2 or < 17 kg/m2. CF = cystic fibrosis; IPF = idiopathic pulmonary fibrosis.
Table 3.
Kaplan-Meier Estimates of Median Posttransplant Survival Time, Conditional on Surviving at Least 1 Year Posttransplant
| Variable | No. | Median Posttransplant Survival Time (y) | 95% CI |
|---|---|---|---|
| CF, entire cohort a | 1,827 | 9.1 | 8.4-10.3 |
| CF, for BMI subgroups | |||
| BMI ≥ 17 kg/m2 | 1,527 | 9.5 | 8.6-a |
| BMI < 17 kg/m2 | 292 | 7.8 | 7.0-9.5 |
| COPD, entire cohort | 3,998 | 6.9 | 6.7-7.2 |
| IPF, entire cohort | 5,044 | 6.8 | 6.5-7.1 |
See Table 1 legend for expansion of abbreviations.
No upper confidence limit for the CF BMI ≥ 17 kg/m2 subgroup, as the upper bound of the 95% CI for the survival function does not cross the 50% survival mark.
Table 4.
Kaplan-Meier Survival Estimates (% Surviving) at 1 and 5 Years’ Posttransplant
| Variable | 1 Year (95% CI) | 5 Year (95% CI) |
|---|---|---|
| CF, entire cohorta | 89% (88-90) | 60% (58-63) |
| CF, BMI ≥ 17 kg/m2 | 89% (88-91) | 61% (59-64) |
| CF, BMI < 17 kg/m2 | 88% (84-91) | 54% (48-60) |
| COPD | 87% (86-88) | 56% (54-58) |
| IPF | 84% (84-85) | 53% (51-54) |
See Table 1 legend for expansion of abbreviations.
Twelve subjects with CF had missing BMI data.
Change in BMI
Among 352 underweight recipients with CF, only 39 (11%) had a decline in BMI between listing and transplantation. The mean ± SD decline in BMI for these subjects was 0.4 ± 1.4 kg/m2. Subjects with a decline in BMI during waitlist time were similar in characteristics to those with stable BMI. Cox modeling found no association between BMI decline prior to transplantation and posttransplant mortality among individuals with BMI < 17 kg/m2 at LTx (hazard ratio, 0.98; 95% CI, 0.86-1.11; P = 0.74) or low BMI at listing (e-Table 8).
Discussion
The complexity of patients with end-stage pulmonary disease and the difficulties with survival prediction make candidate selection for LTx a challenging process that balances transplant urgency (likelihood of death without LTx) with transplant benefit (likelihood of survival following LTx).11 In this large study using the UNOS dataset, we found a median posttransplant survival in CF of 7.9 years; much like the International Society for Heart and Lung Transplantation Registry data,1 this time frame was the longest survival among all transplanted diagnoses. Most importantly, the median survival in patients with CF and BMI < 17 kg/m2 was 7.0 years, which was comparable to subjects with the other most commonly transplanted diagnoses. Underweight patients with CF have been shown to have a higher risk of death without LTx,17,18 underscoring transplant urgency. At the same time, low BMI is frequently an absolute contraindication to LTx.12 Importantly, transplant programs do not tend to make candidacy decisions based on transplant urgency or the chance of waitlist mortality. Rather, candidate selection is influenced by what the transplant program determines to be appropriate or acceptable listing criteria, expected outcomes (eg, survival, recovery, quality of life), and contraindications. In the setting of a potential contraindication, selection committees attempt to estimate the individual’s expected posttransplant outcomes (assuming they make it to transplant). The current study highlights reasonable posttransplant survival for a cohort of underweight individuals with CF who, based on previous considerations, may be unjustifiably excluded from LTx at most centers in the United States.
Several intrinsic components of CF worsen in advanced lung disease and directly affect nutrition, including dyspnea, anorexia, and increased basal energy expenditure due to chronic respiratory infection.12,19, 20, 21, 22, 23, 24, 25 Thus, impaired nutrition can be thought of as an inherent component of disease progression in CF26 but one that clinical experience suggests is highly reversible following LTx. In fact, underweight status has been shown to be common in advanced CF lung disease, with one study reporting a pretransplant BMI < 18.5 kg/m2 in 42.4%,3 whereas the current study found a pretransplant BMI < 18.5 kg/m2 in 40.0% (< 17 kg/m2 in 16%) of subjects with CF undergoing transplant. These data strengthen the case that this relatively large percentage of the CF population should not be reflexively excluded from LTx.
An argument can be made that comparing the survival outcomes of underweight transplant recipients with CF vs recipients with COPD yielded an expected result. Certainly, much of the relative success in LTx for CF likely resides in patients being younger and lacking medical comorbidities, perhaps along with familiarity with chronic illness management. However, because the allocation process does not limit the number of organs allocated to one particular disease, selection committees must analyze risk factors in a given patient in the context of the underlying diagnosis, age, and other clinical characteristics. Inherently, this implies comparisons of expected outcomes across different diagnoses and age groups, for which the results of this study are pragmatic. When assessing relative or absolute contraindications to LTx for individuals with CF, it is important to consider whether an individual meets a more general acceptable threshold for posttransplant outcomes rather than assessing whether their outcome is likely to be as good as others with CF.
This study did have limitations. First, it is likely that underweight subjects with CF who were selected for and able to survive to LTx during the study period otherwise carried factors that positively influenced their candidacy and posttransplant outcomes. Studies that evaluate any pretransplant risk factor and the associated posttransplant outcomes are always subject to this bias: those who made it to transplant will likely be less severely ill than those who died while on the waitlist. Our waitlist analyses did not show a clinically meaningful difference in waitlist deaths among individuals with CF listed with a BMI < 17 kg/m2 compared with those listed with higher weight. Stratified regression modeling allowing for center-specific baseline hazards should have accounted for between-center variability, but the center-specific decision to list individuals with CF and low BMI was likely affected by history of medical adherence, social support, body protein stores, frailty, transplant program risk aversion, steroid exposure, and/or other factors that could not be accounted for in this analysis. Although the results remain an accurate representation of outcomes in this presumably carefully selected cohort, this necessary limitation and related selection bias underscore the importance of a thorough pretransplant risk assessment in underweight patients with CF. Furthermore, as lung disease progresses in this population, we believe every effort should be made to optimize nutrition prior to LTx.27
Second, the covariates that are usually considered as confounders for survival models are nonoverlapping for the diagnoses currently studied (CF, IPF, and COPD). For instance, age, FEV1, and presence of diabetes differ in value or prevalence and have different physiologic context across diagnoses. We therefore did not include patient-level covariates in the multivariable model, and our analyses are subject to residual confounding. Third, we were unable to completely evaluate for the complex effect of changes in BMI over time. Our BMI change analyses revealed no association between BMI change and posttransplant survival among individuals with low BMI at listing or time of transplant, but these analyses were not adjusted for other markers of disease severity and only included transplant recipients. Patients with CF and the largest decreases in BMI may have died without LTx. Outcomes in underweight patients with CF may in fact be influenced by nutritional changes over time (either pretransplant or posttransplant), but nutritional status may be more accurately described with measurement of degree of lean body mass, protein stores, or relative sarcopenia rather than BMI.28 There is surgical literature showing the protective effect of a positive nitrogen balance on postoperative outcomes,29, 30, 31 but these data are not captured in the UNOS registry. Further study is needed to evaluate the effects of these parameters on posttransplant outcomes to enable more refined risk stratification in the pretransplant period. Further study will also be useful for associations with other posttransplant complications, including primary graft dysfunction, wound healing, acute cellular rejection, chronic lung allograft dysfunction, and other outcomes that may be affected by malnutrition in the pretransplant and/or posttransplant CF population.
Conclusions
BMI < 17 kg/m2 often serves as an absolute contraindication for LTx for individuals with CF in the United States. In the current study, patients with CF and a pretransplant BMI < 17 kg/m2 had posttransplant survival that was similar to reported survival across diagnoses in data from the Registry of International Society for Heart and Lung Transplantation.1 Thus, we propose that BMI < 17 kg/m2 as a single risk factor in the CF population should not be treated as an absolute contraindication to LTx.
Acknowledgments
Author contributions: K. J. R. is the guarantor of the content of the manuscript, including the data and analyses. K. J. R., S. G. K., and M. C. B. had full access to the data in the study and take responsibility for the accuracy of the analyses and interpretation. K. J. R., S. G. K., M. C. B., and C. H. G. contributed substantially to the data analysis and interpretation, and writing the first draft of the manuscript. All authors contributed substantially to study design, interpretation, and revisions of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: K. J. R. has received funding from the National Institutes of Health (NIH) [1K23HL138154-01], the Cystic Fibrosis Foundation (CFF) [RAMOS17A0], the CHEST Foundation Cystic Fibrosis Grant in partnership with Vertex Pharmaceuticals Incorporated, and CFF Lung Transplant Consortium [LEASE16A3]. M. C. B. has received funding from Children’s Core for Biomedical Statistics, partly supported by the University of Washington Institute of Translational Health Sciences [under award UL1 TR002319] from the National Center for Advancing Translational Sciences of the NIH. R. S. has received funding from the Canadian Institutes for Health Research [364568], CF Canada [593925, 3185], and CFF [541972]. E. M. has received funding from the NIH [K23 HL144916], the CFF Research Development Program [SINGH19R0], and Parker B. Francis Foundation. J. M. P. has received funding from the CFF Research Development Program and Lung Transplant Consortium, NIH [P30 DK72506]. E. D. L. has received funding from the CFF Lung Transplant Consortium [LEASE16A3]. M. L. A. has received funding from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases [P30 DK089507], CFF (Therapeutics Development Network) [CC030-17AD, AITKEN09XX0, RDP, 14Q10, CCRX065-16, CMHC065-16, ARC], and clinical trial research (Vertex Incorp, Novoteris, LLC, and Savara, Inc.). C. H. G. receives funding from the CFF, the NIH [R01HL113382, R01AI101307, U M1HL119073, P30DK089507, and UL1TR000423], and the US Food and Drug Administration [R01FD003704]. He has also received financial support from Gilead Sciences for grant reviews and from Boehringer Ingelheim for clinical trial design consulting; has received honoraria to serve as the Chair of a Data Safety Monitoring Board for a clinical trial jointly supported by the European Commission and Novartis; and was part of a research group that received a clinical research grant from Vertex Pharmaceuticals. None declared (S. G. K., M. S. M., C. J. G.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The content of this article is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported in part by the Health Resources and Services Administration [Contract 234-2005-370011C]. K. J. R. is supported by National Institutes of Health (NIH) [1K23HL138154-01], the Cystic Fibrosis Foundation (CFF) [RAMOS17A0], and CFF Lung Transplant Consortium [LEASE16A3]; M. C. B. is supported by the Children’s Core for Biomedical Statistics, partly supported by the University of Washington Institute of Translational Health Sciences [under award UL1 TR002319] from the National Center for Advancing Translational Sciences of the NIH; R. S. is supported by Canadian Institutes for Health Research [364568], CF Canada [593925, 3185], and CFF [541972]; J. M. P. is supported by CFF Research Development Program and Lung Transplant Consortium, NIH [P30 DK72506]; E. D. L. is supported by CFF Lung Transplant Consortium [LEASE16A3]; M. L. A. is supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases [P30 DK089507]; C. H. G. is supported by NIH P30DK089507; and E. D. M. is supported by NIH K23 HL144916, and Parker B. Francis Foundation.
Supplementary Data
References
- 1.Chambers D.C., Yusen R.D., Cherikh W.S. The Registry of the International Society for Heart and Lung Transplantation: thirty-fourth Adult Lung and Heart-Lung Transplantation Report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36(10):1047–1059. doi: 10.1016/j.healun.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Valapour M., Lehr C.J., Skeans M.A. OPTN/SRTR 2016 annual data report: lung. Am J Transplant. 2018;18(suppl 1):363–433. doi: 10.1111/ajt.14562. [DOI] [PubMed] [Google Scholar]
- 3.Lederer D.J., Wilt J.S., D’Ovidio F. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med. 2009;180(9):887–895. doi: 10.1164/rccm.200903-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen J.G., Arnaoutakis G.J., Weiss E.S., Merlo C.A., Conte J.V., Shah A.S. The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant. 2010;29(9):1026–1033. doi: 10.1016/j.healun.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Madill J., Gutierrez C., Grossman J. Nutritional assessment of the lung transplant patient: body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant. 2001;20(3):288–296. doi: 10.1016/s1053-2498(00)00315-6. [DOI] [PubMed] [Google Scholar]
- 6.Upala S., Panichsillapakit T., Wijarnpreecha K., Jaruvongvanich V., Sanguankeo A. Underweight and obesity increase the risk of mortality after lung transplantation: a systematic review and meta-analysis. Transplant Int. 2016;29(3):285–296. doi: 10.1111/tri.12721. [DOI] [PubMed] [Google Scholar]
- 7.Plochl W., Pezawas L., Artemiou O., Grimm M., Klepetko W., Hiesmayr M. Nutritional status, ICU duration and ICU mortality in lung transplant recipients. Intensive Care Med. 1996;22(11):1179–1185. doi: 10.1007/BF01709333. [DOI] [PubMed] [Google Scholar]
- 8.Schwebel C., Pin I., Barnoud D. Prevalence and consequences of nutritional depletion in lung transplant candidates. Eur Respir J. 2000;16(6):1050–1055. doi: 10.1034/j.1399-3003.2000.16f05.x. [DOI] [PubMed] [Google Scholar]
- 9.Singer J.P., Peterson E.R., Snyder M.E. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med. 2014;190(9):1012–1021. doi: 10.1164/rccm.201405-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez R., Safaeinili N., Kurihara C. Association of body mass index with lung transplantation survival in the United States following implementation of the lung allocation score. J Thorac Cardiovasc Surg. 2018;155(4):1871–1879.e1873. doi: 10.1016/j.jtcvs.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weill D., Benden C., Corris P.A. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Morrell M.R., Pilewski J.M. Lung transplantation for cystic fibrosis. Clin Chest Med. 2016;37(1):127–138. doi: 10.1016/j.ccm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Hayes D., Jr., Tobias J.D., Tumin D. Center volume and extracorporeal membrane oxygenation support at lung transplantation in the lung allocation score era. Am J Respir Crit Care Med. 2016;194(3):317–326. doi: 10.1164/rccm.201511-2222OC. [DOI] [PubMed] [Google Scholar]
- 14.Thabut G., Christie J.D., Kremers W.K., Fournier M., Halpern S.D. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304(1):53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]
- 15.Altman D.G., Bland J.M. Time to event (survival) data. BMJ. 1998;317(7156):468–469. doi: 10.1136/bmj.317.7156.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 17.Snell G.I., Bennetts K., Bartolo J. Body mass index as a predictor of survival in adults with cystic fibrosis referred for lung transplantation. J Heart Lung Transplant. 1998;17(11):1097–1103. [PubMed] [Google Scholar]
- 18.Ramos K.J., Quon B.S., Heltshe S.L. Heterogeneity in survival in adult patients with cystic fibrosis with FEV1 < 30% of predicted in the United States. Chest. 2017;151(6):1320–1328. doi: 10.1016/j.chest.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barni G.C., Forte G.C., Forgiarini L.F., Abrahao C.L.O., Dalcin P.T.R. Factors associated with malnutrition in adolescent and adult patients with cystic fibrosis. J Bras Pneumol. 2017;43(5):337–343. doi: 10.1590/S1806-37562016000000319. [DOI] [PubMed] [Google Scholar]
- 20.Culhane S., George C., Pearo B., Spoede E. Malnutrition in cystic fibrosis: a review. Nutr Clin Pract. 2013;28(6):676–683. doi: 10.1177/0884533613507086. [DOI] [PubMed] [Google Scholar]
- 21.Matel J.L., Milla C.E. Nutrition in cystic fibrosis. Semin Respir Crit Care Med. 2009;30(5):579–586. doi: 10.1055/s-0029-1238916. [DOI] [PubMed] [Google Scholar]
- 22.Panagopoulou P., Fotoulaki M., Nikolaou A., Nousia-Arvanitakis S. Prevalence of malnutrition and obesity among cystic fibrosis patients. Pediatr Int. 2014;56(1):89–94. doi: 10.1111/ped.12214. [DOI] [PubMed] [Google Scholar]
- 23.Pencharz P.B., Durie P.R. Pathogenesis of malnutrition in cystic fibrosis, and its treatment. Clinical Nutr. 2000;19(6):387–394. doi: 10.1054/clnu.1999.0079. [DOI] [PubMed] [Google Scholar]
- 24.Hadjiliadis D. Special considerations for patients with cystic fibrosis undergoing lung transplantation. Chest. 2007;131(4):1224–1231. doi: 10.1378/chest.06-1163. [DOI] [PubMed] [Google Scholar]
- 25.Singer L.G., Brazelton T.R., Doyle R.L., Morris R.E., Theodore J. Weight gain after lung transplantation. J Heart Lung Transplant. 2003;22(8):894–902. doi: 10.1016/s1053-2498(02)00807-0. [DOI] [PubMed] [Google Scholar]
- 26.Cystic Fibrosis Foundation Patient Registry . Cystic Fibrosis Foundation; Bethesda, MD: 2019. 2018 Annual Data Report. [Google Scholar]
- 27.Schindler T., Michel S., Wilson A.W. Nutrition management of cystic fibrosis in the 21st century. Nutr Clin Pract. 2015;30(4):488–500. doi: 10.1177/0884533615591604. [DOI] [PubMed] [Google Scholar]
- 28.Yusen R.D. Body composition, lung transplant candidacy, and patient outcomes. Am J Respir Crit Care Med. 2014;190(9):971–973. doi: 10.1164/rccm.201410-1767ED. [DOI] [PubMed] [Google Scholar]
- 29.Weimann A., Braga M., Harsanyi L. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clinical Nutr. 2006;25(2):224–244. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Braga M., Ljungqvist O., Soeters P., Fearon K., Weimann A., Bozzetti F. ESPEN guidelines on parenteral nutrition: surgery. Clinical Nutr. 2009;28(4):378–386. doi: 10.1016/j.clnu.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Rombeau J.L., Barot L.R., Williamson C.E., Mullen J.L. Preoperative total parenteral nutrition and surgical outcome in patients with inflammatory bowel disease. Am J Surg. 1982;143(1):139–143. doi: 10.1016/0002-9610(82)90144-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.