Abstract
Background
The relationship between 25-hydroxyvitamin D (25-OH-vitamin D) and COPD outcomes remains unclear. Using the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), we determined associations among baseline 25-OH-vitamin D and cross-sectional and longitudinal lung function and COPD exacerbations.
Methods
Serum 25-OH-vitamin D level was measured in stored samples from 1,609 SPIROMICS participants with COPD. 25-OH-vitamin D levels were modeled continuously and dichotomized as deficient (< 20 ng/mL) vs not deficient (≥ 20 ng/mL). Outcomes of interest included % predicted FEV1 (current and 1-year longitudinal decline) and COPD exacerbations (separately any and severe, occurring in prior year and first year of follow-up).
Results
Vitamin D deficiency was present in 21% of the cohort and was more prevalent in the younger, active smokers, and blacks. Vitamin D deficiency was independently associated with lower % predicted FEV1 (by 4.11%) at enrollment (95% CI, –6.90% to –1.34% predicted FEV1; P = .004), 1.27% predicted greater rate of FEV1 decline after 1 year (95% CI, –2.32% to –0.22% predicted/y; P = .02), and higher odds of any COPD exacerbation in the prior year (OR, 1.32; 95% CI, 1.00-1.74; P = .049). Each 10-ng/mL decrease in 25-OH-vitamin D was associated with lower baseline lung function (–1.04% predicted; 95% CI, –1.96% to –0.12% predicted; P = .03) and increased odds of any exacerbation in the year before enrollment (OR, 1.11; 95% CI, 1.01-1.22; P = .04).
Conclusions
Vitamin D deficiency is associated with worse cross-sectional and longitudinal lung function and increased odds of prior COPD exacerbations. These findings identify 25-OH-vitamin D levels as a potentially useful marker of adverse COPD-related outcomes.
Key Words: COPD, COPD epidemiology, COPD exacerbations, lung function, vitamin D
Abbreviations: 25-OH-vitamin D, 25-hydroxyvitamin D; AECOPD, acute exacerbation of COPD; FSAD, functional small-airway disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; Pi10, standardized airway wall thickness at an internal perimeter of 10 mm; SPIROMICS, Subpopulations and Intermediate Outcome Measures in COPD Study; VDD, vitamin D deficiency
FOR EDITORIAL COMMENT, SEE PAGE 755
Nutritional and vitamin deficiencies are prevalent in COPD.1, 2, 3 Relationships between 25-hydroxyvitamin D (25-OH-vitamin D) and COPD outcomes are of interest because of the effects of 25-OH-vitamin D on the immunologic and musculoskeletal systems, which may contribute to respiratory outcomes.4 Vitamin D deficiency (VDD) (25-OH-vitamin D level < 20 ng/mL5) has been implicated as a risk factor for the development and severity of airflow limitation6, 7, 8 and physical function in those with COPD.9, 10, 11, 12, 13, 14 Analyses of the general US population in the National Health and Nutrition Examination Study have reported lower lung function and increased chronic bronchitis in vitamin D-deficient participants.8,15 However, longitudinal declines in FEV1 among individuals with COPD are heterogeneous.16 Prior studies limited to those without COPD17,18 have not described an association between vitamin D levels and lung function decline. To date, the impact of 25-OH-vitamin D levels on individuals with established COPD is not established.
Randomized controlled trials have failed to show lung function improvement,19 decreased risk of exacerbation,19,20 or improved COPD symptoms with vitamin D supplementation.21 These trial findings are limited by including participants at or near vitamin D sufficiency.19,20 Despite these trial findings, participants with lower vitamin D levels may benefit from supplementation.4,22,23 As equipoise remains regarding the role VDD may play in prospective COPD outcomes, an analysis of large, well-characterized COPD-specific cohorts with detailed and standardized clinical phenotyping and longitudinal follow-up may be of particular interest to clinicians who treat patients with COPD and VDD.
We use the detailed demographic, clinical, and spirometric data from the multicenter, prospective Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS)24 cohort to characterize cross-sectional and longitudinal independent associations among 25-OH-vitamin D, lung function, and acute exacerbations of COPD (AECOPD). We hypothesize that VDD in the SPIROMICS cohort will be associated with poorer baseline and longitudinal lung function and greater risk of AECOPD at enrollment and over the first year of the SPIROMICS study.
Methods
Study Participants
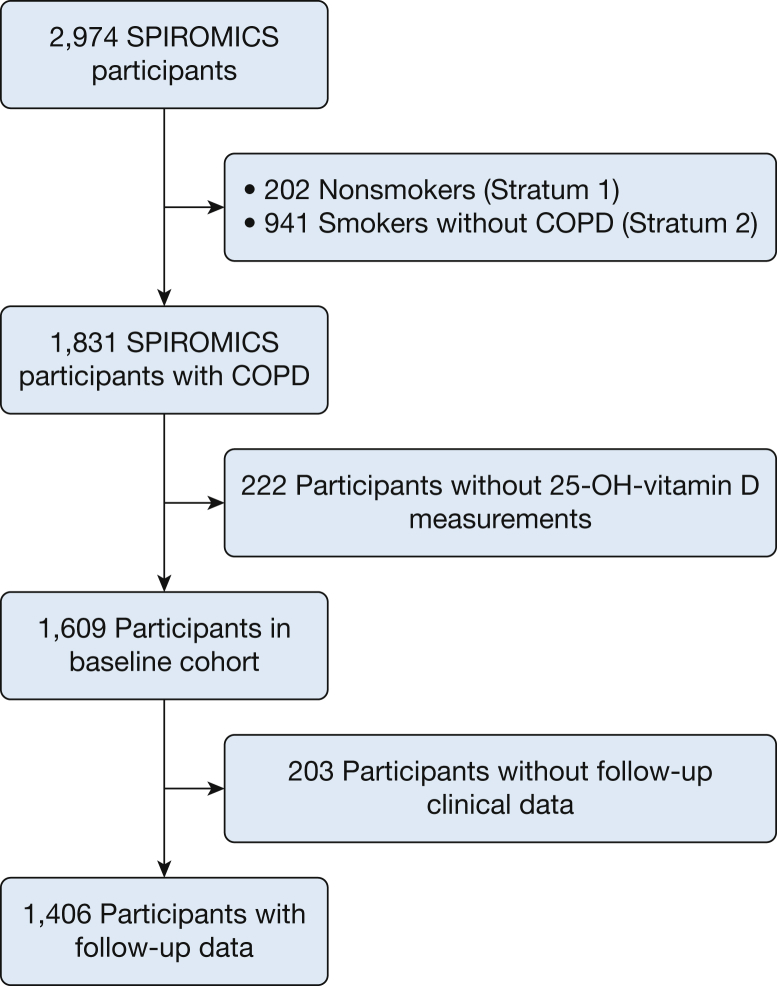
SPIROMICS is a multicenter, observational, prospective, cohort study that includes current or former smokers (≥ 20 pack-years), with or without chronic airflow obstruction, between the ages of 40 and 80 years, and nonsmoking control subjects recruited from 12 clinical centers (n = 2,974).24,25 Participants included in this analysis had spirometry-confirmed COPD (FEV1/FVC < 0.70) and available baseline clinical data (n = 1,609) (Fig 1). Institutional review boards at each center approved SPIROMICS and participants provided informed, written consent (e-Table 1).
Figure 1.
CONSORT diagram showing selection of analytical cohort. 25-OH-vitamin D = 25-hydroxyvitamin D; CONSORT = Consolidated Standards of Reporting Trials; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Data Collection
The SPIROMICS cohort provided participant-reported demographic data, medical history, smoking history, and serum samples at enrollment. Postbronchodilator FEV1 and FVC were determined at enrollment and at the 1-year follow-up visit. Use of vitamin D supplementation was determined via patient report without specific ascertainment of formulation. An AECOPD was defined as the report of any worsening of COPD symptoms requiring antibiotics or steroids. Severe AECOPD was defined as the report of worsening COPD symptoms requiring an ED visit or hospitalization.26 Baseline AECOPD data were obtained through participant self-report at the time of enrollment. Longitudinal AECOPD data were collected by self-report during quarterly telephone calls and at a yearly follow-up visit. CT scan metrics (percent emphysema, functional small-airway disease [FSAD], airway wall thickness at an internal perimeter of 10 mm [Pi10] for airways ≤ 20 mm) were assessed as previously defined.27, 28, 29 Serum 25-OH-vitamin D levels were measured post hoc from stored baseline serum samples, using radioimmunoassay (enzyme immunoassay [IDS]; intraassay coefficient of variance, 8.14%; limit of detection, 3.7 ng/mL).
Statistical Methods
Two-sample Student t-test or Kruskal-Wallis testing and χ2 testing were performed to identify relationships between VDD and continuous or categorical variables, respectively. 25-OH-vitamin D levels were modeled continuously and as VDD (< 20 ng/mL) vs not deficient (≥ 20 ng/mL).5 Multilevel linear regression modeling was used to determine relationships between 25-OH-vitamin D levels and % predicted FEV1 at baseline as well as the rate of change over 1 year. Exacerbations were modeled in binary fashion (0 vs ≥ 1 episode). Logistic regression analysis was used to determine the association between 25-OH vitamin D and the odds of experiencing an AECOPD in the year before and after enrollment. Severe AECOPD in the year before and after enrollment was also separately explored as outcomes. Covariates in the multivariable model were selected on the basis of clinical relevance.30 Covariates in linear regression include pack-years of smoking (per 10 pack-years), smoking status, and season of 25-OH-vitamin D blood draw (spring, summer, and fall with winter as referent). As age, sex, race, and height are incorporated into the % predicted FEV1 calculation, these covariates were not separately included in % predicted FEV1 models. Covariates in logistic regression models include age (per 10 years), race, sex, pack-years of smoking (per 10 pack-years), current smoking status, and season of blood draw. The association between 25-OH vitamin D and CT scan metrics was assessed in bivariate and multivariable linear regression models. Adjusted CT models included age, race, sex, BMI, current smoking, pack-years of smoking, and site of study. Total lung capacity was also included for the models incorporating Pi10. Sensitivity analyses were performed by incorporating reported yearly income (< $50,000/y vs ≥ $50,000/y) into statistically significant models. Baseline postbronchodilator % predicted FEV1 was added to statistically significant AECOPD models (with removal of age, race, and sex to avoid collinearity). Main modeling approaches were performed restricting to participants not reporting vitamin D supplementation. For all comparisons, P < .05 was considered significant; CT scan associations were corrected for multiple comparisons. Statistical analysis was performed with SAS version 9.4 (SAS Institute).
Results
Cohort Characteristics
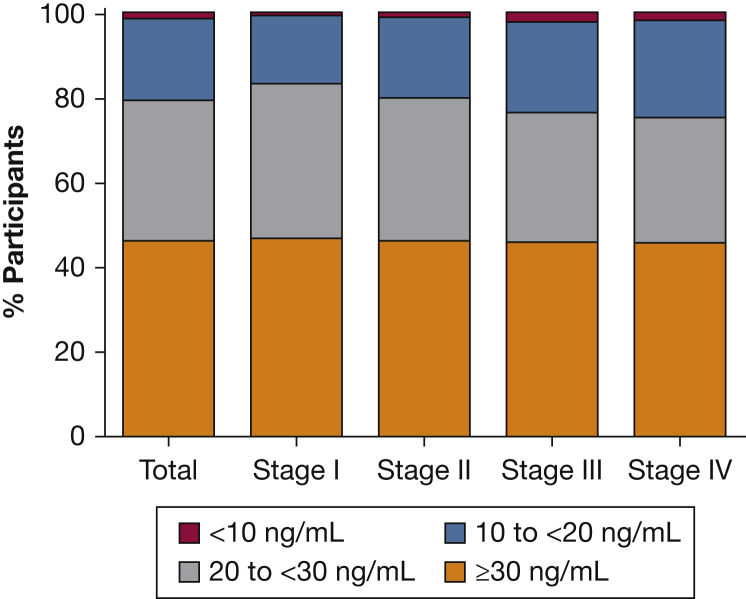
There were 1,609 participants in the analytical cohort (Table 1). Participants had a mean age of 65 years, 42% were female, and 14% were black. Severe COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage III-IV) was present in 34% of participants, and 31% reported one or more AECOPD in the year before enrollment. All participants had 25-OH-vitamin D levels above the limit of detection (> 3.7 ng/mL). The median vitamin D level in the cohort was 28.9 ng/mL (quartile 1-quartile 3, 21.3-36.5 ng/mL), with 338 (21%) having VDD. By vitamin D category, 1% had vitamin D < 10 ng/mL, 20% between ≥ 10 and < 20 ng/mL, 33% between ≥ 20 and < 30 ng/mL, and 46% ≥ 30 ng/mL. Figure 2 shows the distribution of vitamin D levels stratified by GOLD FEV1 severity. A total of 24% of the cohort reported some form of oral vitamin D supplementation (6% in the VDD group and 29% in the vitamin D-nondeficient group). VDD was associated with younger age (62 vs 66 years; P < .0001), black race (29% vs 10%;P < .0001), and current smoking (48% vs 30%; P < .0001). The VDD group had lower postbronchodilator % predicted FEV1 at baseline (57.5% vs 62.0% predicted; P = .001) and at 1 year (56.7% vs 63.4% predicted; P < .001). There was a greater proportion of baseline visits occurring during winter among VDD participants compared with non-VDD participants (27% vs 18%; P = .003). A greater proportion of participants with VDD experienced one or more AECOPD in the year before enrollment (39% vs 30%; P = .005), as well as one or more severe AECOPD in the year before enrollment (22% vs 14%; P < .001).
Table 1.
Cohort Characteristics
| Total | Vitamin D < 20 | Vitamin D ≥ 20 | P Value | |
|---|---|---|---|---|
| Patients | 1,609 | 338 | 1,271 | … |
| Age, y | 65.3 (7.9) | 62.0 (7.9) | 66.1 (7.7) | < .001 |
| Sex, female | 673 (42) | 154 (46) | 519 (41) | .12 |
| Black race | 226 (14) | 97 (29) | 139 (10) | < .001 |
| BMI, kg/m2 | 27.3 (5.3) | 27.4 (5.7) | 27.3 (5.1) | .71 |
| Current smoker | 529 (33) | 161 (48) | 368 (30) | < .001 |
| Pack-years smoking, median (Q1-Q3) | 46 (35-62) | 45 (35-60) | 48 (36-63) | .20 |
| History of asthma | 363 (24) | 88 (28) | 275 (23) | .055 |
| Yearly income < $50,000 | 819 (51.0) | 211 (62.6) | 608 (47.8) | < .001 |
| FEV1/FVC ratio, baseline | 0.51 (0.13) | 0.50 (0.13) | 0.51 (0.13) | .13 |
| FEV1 % predicted, post-BD, baseline | 61.1 (23.1) | 57.5 (22.7) | 62.0 (23.1) | .001 |
| FVC % predicted, post-BD, baseline | 88.8 (19.9) | 86.1 (19.4) | 89.5 (20) | .005 |
| GOLD FEV1 severity | ||||
| Stage I | 346 (22) | 59 (17) | 287 (23) | .115 |
| Stage II | 712 (44) | 145 (43) | 567 (45) | |
| Stage III | 383 (24) | 92 (27) | 291 (23) | |
| Stage IV | 159 (10) | 40 (12) | 119 (9) | |
| Vitamin D supplementation | 383 (24) | 20 (6) | 363 (29) | < .001 |
| Vitamin D category | ||||
| < 10 ng/mL | 24 (1.05) | 24 (7) | 0 (0) | N/A |
| 10 to < 20 ng/mL | 314 (19.5) | 314 (93) | 0 (0) | |
| 20 to < 30 ng/mL | 535 (33.2) | 0 (0) | 535 (42) | |
| ≥ 30 ng/mL | 736 (45.7) | 0 (0) | 736 (58) | |
| Season of baseline visit | ||||
| Spring | 441 (27) | 94 (28) | 347 (27) | .003 |
| Summer | 423 (26) | 72 (21) | 351 (28) | |
| Fall | 414 (26) | 80 (24) | 334 (26) | |
| Winter | 330 (21) | 92 (27) | 238 (18) | |
| FEV1 % predicted, post-BD, 1 y | 61.1 (23.1) | 56.7 (22.4) | 63.4 (22.6) | < .001 |
| AECOPD in previous year, any | ||||
| 0 | 1,092 (69) | 207 (61) | 885 (70) | .005 |
| 1 | 273 (17) | 75 (23) | 198 (16) | |
| 2+ | 224 (14) | 53 (16) | 171 (14) | |
| AECOPD in previous year, severe | ||||
| 0 | 1,336 (84) | 258 (78) | 1,078 (86) | < .001 |
| 1 | 173 (11) | 50 (15) | 123 (10) | |
| 2+ | 76 (5) | 24 (7) | 52 (4) | |
| AECOPD in first year, any | ||||
| 0 | 1,125 (74) | 226 (72) | 899 (74) | .35 |
| 1 | 226 (15) | 48 (15) | 178 (15) | |
| 2+ | 172 (11) | 40 (13) | 132 (11) | |
| AECOPD in first year, severe | ||||
| 0 | 1,324 (87) | 273 (86) | 1,051 (88) | .59 |
| 1 | 123 (8) | 24 (8) | 99 (8) | |
| 2+ | 71 (5) | 22 (6) | 50 (4) |
All values represent No. (%) or mean (SD) unless otherwise indicated. P value, comparing vitamin D strata. AECOPD = acute exacerbation of COPD; BD = bronchodilator; GOLD = Global Initiative for Chronic Obstructive Lung Disease; Q1, Q3 = quartile 1, quartile 3.
Figure 2.
Distribution of vitamin D levels in the SPIROMICS cohort, stratified by GOLD FEV1 stage. GOLD = Global Initiative for Chronic Obstructive Lung Disease. See Figure 1 caption for expansion of other abbreviation.
25-OH-Vitamin D and FEV1 Associations
In bivariate linear regression, a 10-ng/mL decrease in 25-OH-vitamin D was associated with lower % predicted FEV1 (–1.14% predicted; 95% CI, –2.26% to –0.28% predicted; P = .01) at enrollment. Similarly, VDD was associated with lower % predicted FEV1 (–4.98% predicted; 95% CI, –7.66% to –2.30% predicted; P = .003) at enrollment. Multivariable multilevel linear regression modeling, adjusting for covariates described in Methods, was performed (Table 2). In this model, a 10-ng/mL lower vitamin D level at baseline was associated with lower % predicted FEV1 (–1.04% predicted; 95% CI, –1.96% to –0.12% predicted; P = .03) at enrollment. Continuous 25-OH-vitamin D was not associated with a more rapid rate of lung function decline in the first year of follow-up. In multivariate modeling, VDD was associated with lower % predicted FEV1 (–4.11% predicted; 95% CI, –6.90% to –1.34% predicted; P = .004) at baseline. VDD was associated with a significantly greater rate of % predicted FEV1 decline over the first year of follow-up (–1.27% predicted; 95% CI, –2.32% to –0.22% predicted; P = .02).
Table 2.
Multilevel Linear Regression Modelinga of Associations Between 25-Hydroxyvitamin D and FEV1 % Predicted at Baseline and First Year of Follow-Up
| FEV1 % Predicted (Continuous Vitamin D) |
FEV1 % Predicted (Vitamin D Deficiency Status) |
|||||
|---|---|---|---|---|---|---|
| FEV1 (% Predicted) | 95% CI | P Value | FEV1 (% Predicted) | 95% CI | P Value | |
| 25-OH-vitamin D (per 10-ng/mL decrease) | –1.04 | –1.96 to –0.12 | .03 | … | … | … |
| Annual FEV1 rate of change, % predicted (per 10-ng/mL decrease) | –0.19 | –0.53 to 0.15 | .28 | … | … | … |
| 25-OH-vitamin D (< 20 vs ≥ 20 ng/mL) | … | … | … | –4.11 | –6.90 to –1.34 | .004 |
| Annual FEV1 rate of change, % predicted (< 20 vs ≥ 20 ng/mL) | … | … | … | –1.27 | –2.32 to –0.22 | .02 |
| Visit 1 vs baseline | –1.19 | –0.15 to 0.53 | .04 | –0.36 | –0.83 to 0.12 | .14 |
| Current smoking | –0.37 | –1.69 to 0.96 | .58 | –0.27 | –1.59 to 1.06 | .69 |
| Pack-years smoking (per 10 pack-years) | –0.16 | –0.56 to 0.25 | .45 | –0.16 | –0.57 to 0.24 | .43 |
| Season of blood draw | ||||||
| Spring | 3.04 | –0.23 to 6.31 | .07 | 3.07 | –0.20 to 6.33 | .07 |
| Summer | 5.56 | 2.26 to 8.85 | .001 | 5.38 | 2.09 to 8.67 | .001 |
| Fall | –1.88 | –5.19 to 1.42 | .26 | –2.02 | –5.32 to 1.28 | .23 |
| Winter | (Ref) | … | … | (Ref) | … | … |
25-OH-vitamin D = 25-hydroxyvitamin D; Ref = referent.
Adjusted for all covariates in table.
25-OH-Vitamin D and AECOPD Associations
In bivariate analysis, a 10-ng/mL decrease in 25-OH-vitamin D was associated with 14% higher odds of an AECOPD in the year before enrollment (95% CI, 1.04-1.24; P = .005). Similarly, VDD was associated with 48% increased odds of an AECOPD in the year before enrollment (OR, 1.48; 95% CI, 1.15-1.91; P = .002). In addition, a 10-ng/mL decrease in vitamin D was associated with a 17% increase in the odds of a severe AECOPD in the year before enrollment (OR, 1.17; 95% CI, 1.04-1.32; P = .01). VDD was associated with a 77% increase in the odds of a severe AECOPD in the year before enrollment (OR, 1.77; 95% CI, 1.30-2.39; P < .0001) in bivariate analysis.
In multivariable analysis adjusted for covariates described in Methods, every 10-ng/mL decrease in 25-OH-vitamin D was associated with an 11% increase in the odds of an AECOPD in the year before enrollment (OR, 1.11; 95% CI, 1.01-1.22; P = .04) (Table 3). Similarly, VDD was associated with 32% higher odds of an AECOPD in the year before enrollment (OR, 1.32; 95% CI, 1.00-1.74; P = .049). Neither continuous nor dichotomized vitamin D levels were associated with prior severe AECOPD in adjusted analyses (e-Table 2).
Table 3.
Logistic Regression Modelinga of Associations Between Vitamin D and Odds of COPD Exacerbation in the Year Before Enrollment
| Odds of COPD Exacerbation in Prior Year (Continuous Vitamin D) |
Odds of COPD Exacerbation in Prior Year (Vitamin D Deficiency Status) |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| 25-OH-vitamin D (per 10-ng/mL decrease) | 1.11 | 1.01-1.22 | .04 | … | … | … |
| 25-OH-vitamin D (< 20 vs ≥ 20 ng/mL) | … | … | … | 1.32 | 1.00-1.74 | .049 |
| Age (per 10 y) | 0.60 | 0.52-0.71 | < .001 | 0.60 | 0.54-0.74 | < .001 |
| Sex, female | 1.73 | 1.38-2.16 | < .001 | 1.67 | 1.34-2.10 | < .001 |
| Race, black | 1.28 | 0.93-1.77 | .09 | 1.28 | 0.93-1.77 | .09 |
| Current smoking | 0.49 | 0.37-0.63 | < .001 | 0.48 | 0.37-0.63 | < .001 |
| Pack-years smoked (per 10 pack-years) | 0.97 | 0.93-1.02 | .19 | 0.97 | 0.93-1.02 | .19 |
| Season of blood draw | ||||||
| Spring | 0.89 | 0.65-1.23 | .96 | 0.88 | 0.64-1.21 | .63 |
| Summer | 0.91 | 0.66-1.26 | .99 | 0.91 | 0.66-1.26 | .91 |
| Fall | 0.90 | 0.65-1.25 | .97 | 0.90 | 0.65-1.25 | .81 |
| Winter | (ref) | … | … | (ref) | … | … |
Adjusted for all covariates in table. See Table 2 legend for expansion of abbreviation.
There were no associations between continuous 25-OH-vitamin D or VDD and the odds of an AECOPD at 1 year in bivariate or multivariable analysis (e-Table 3). Moreover, there was no association between vitamin D levels (bivariate or multivariable) and a severe AECOPD at 1 year (e-Table 4).
CT Analysis
In comparing VDD with nondeficient participants, there were no differences in percent emphysema (11.6% vs 11.3%; adjusted P > .99), FSAD (26.5% vs 27.4%; adjusted P > .99), and Pi10 (3.73 vs 3.72 mm; adjusted P = .22). Modeling 25-OH-vitamin D continuously (per 10-ng/mL increase), there was no association with percent emphysema (0.065%; adjusted P > .99) or FSAD (0.64%; P = .18). For every 10-ng/mL increase in 25-OH-vitamin D, Pi10 increased 0.004 mm (95% CI, 0.007-0.002; P = .03). However, this association was attenuated in multivariable modeling (P = .11).
Sensitivity Analyses
Yearly self-reported income category (reported by 82% of the analytical cohort) was added to the statistically significant FEV1 and AECOPD models. Inclusion of income in FEV1 models did not attenuate the relationship between VDD and baseline or longitudinal % predicted FEV1. Inclusion of income did attenuate the relationship between continuous vitamin D level and baseline % predicted FEV1 (per 10-ng/mL decrease in 25-OH-vitamin D: –0.43% predicted FEV1; 95% CI, –0.57% to 0.37% predicted; P = .43). Income also attenuated the relationship between VDD and prior AECOPD (OR, 1.20; 95% CI, 0.87-1.63; P = .27) and the relationship between continuous vitamin D and prior AECOPD (OR, 0.95; 95% CI, 0.85-1.07; P = .41). Inclusion of baseline lung function into AECOPD models did not attenuate the association between continuous 25-OH-vitamin D or VDD with prior AECOPD.
Sensitivity analysis restricting the analytical cohort to the 76% of participants not reporting vitamin D supplementation (n = 1,226) was performed (e-Table 5). The associations between continuous vitamin D and baseline lung function and any prior exacerbation were not attenuated. Similarly, the associations between VDD and baseline lung function and prior COPD exacerbations were not attenuated. However, restricting the analysis to participants not reporting supplementation attenuated the association between VDD and rate of lung function decline (–1.05% predicted; 95% CI, –2.21 to 0.12% predicted; P = .09).
Discussion
This study presents the relationship between 25-OH-vitamin D levels and COPD outcomes among 1,609 participants in the SPIROMICS cohort. VDD was seen in 21% of the study cohort and was associated with black race, younger age, and current smoking. VDD was independently associated with lower FEV1 at baseline, a greater rate of lung function decrement after 1 year, and experiencing AECOPD in the year before study enrollment. These findings demonstrate the association between VDD and COPD outcomes, and indicate the need for a future randomized study analyzing whether attaining vitamin D sufficiency prevents adverse COPD outcomes.
Patients with COPD are more likely to have VDD, including when compared with age- and sex-matched control subjects.12,31 This relationship may be due to heavier smoking history, current smoking, more advanced pulmonary disease, or a lower BMI.12 Our study reports lower baseline lung function, increased rate of lung function decline, and higher odds of reported prior AECOPD independent of these factors. A randomized study has shown no effect on lung function decline with supplementation in individuals with COPD and baseline 25-OH-vitamin D near 20 ng/mL.19 However, understanding relationships between VDD and clinical outcomes may inform the importance of remedying VDD in people with COPD.
Our analysis demonstrates that VDD is independently associated with lower % predicted FEV1 (by 4.11%) at study enrollment. These findings are consistent with previous observational studies associating VDD with lower FEV1 and higher incident COPD.8,12,13,32 Our study extends these observations by showing that VDD was associated with a 1.27/y greater decrease in FEV1 % predicted over 1 year in our study. This differs from other cohort studies of those with and without COPD, where VDD has not been shown to be associated with greater lung function decline.17,18 Our cohort, exclusively those with spirometry-confirmed COPD, may contain participants with differential inflammatory profiles. Although the mechanism cannot be determined from this analysis, altered inflammatory responses to noxious stimulants and decreased innate immune system effectiveness may be a potential explaination.33, 34, 35 Vitamin D sufficiency has been associated with a protective effect against lung function decline in smokers,18 suggesting an antiinflammatory role for vitamin D in the airways.36 Smoking status or history did not independently contribute to FEV1 decline in our models. Future investigation in smokers with preserved lung function may further inform the interaction of smoking, vitamin D status, and lung function.
Beyond the immunologic role of vitamin D, VDD is associated with demographic factors, poorer health status, and poor diet37 and may be an indicator of poor general health in those with lower lung function or rapidly progressing COPD. These potential explanations are supported by the attenuation of some of our results by inclusion of income as a surrogate for socioeconomic status into models. These findings illustrate the potential for reverse causality in the relationship between 25-OH-vitamin D levels and COPD outcomes.
This study demonstrates an increase in the odds of experiencing an AECOPD in the year before enrollment in those with VDD. An association between VDD and any or severe AECOPD in the year after enrollment was not observed. However, the numerically higher odds of experiencing an AECOPD in the year after study enrollment in those with low 25-OH-vitamin D is similar to another study,23 and the potential to observe statistically significant associations may be hindered by the low number of exacerbations and severely vitamin D-deficient participants in the SPIROMICS cohort.26 Studies regarding AECOPD and VDD have differential results, with meta-analyses suggesting that VDD is not significantly associated with an increased risk of AECOPD.4,38 In our models, the association between VDD and prior AECOPD is not attenuated by the addition of % predicted FEV1, suggesting VDD was not mediating exacerbation risk through an association with lung function. Implementing vitamin D supplementation as an intervention to prevent AECOPD has been investigated,20,39,40 with those most likely to benefit being patients with 25-OH-vitamin D levels < 10 ng/mL.19,20,23 Our analysis highlights the need for further studies of COPD populations to identify who may benefit from 25-OH-vitamin D supplementation.
This study has several limitations. The observational nature of this study does not allow us to conclude causality in the relationship between 25-OH-vitamin D levels and COPD outcomes. Lung function data were collected only at baseline and 1 year. SPIROMICS captures patient-reported outcomes to assess AECOPD rather than chart-adjudicated events. These events were collected with greater frequency in the year after enrollment, potentially introducing differential recall bias contributing to the differential associations of vitamin D on prior vs 1-year AECOPD assessments. Moreover, reliable outdoor time, frailty, and diet metrics were not assessed. As with any cohort study, those too unwell to present for study visits would not be represented, reducing the inclusion of those with decreased outdoor time and higher risk for VDD. Vitamin D supplementation was collected by participant report without ascertainment of dosage. Longitudinal lung function is attenuated in restricted analysis of those not receiving vitamin D supplementation. As vitamin D supplementation data were collected by patient report in SPIROMICS and the dosage was not adjudicated, this effect may be due to reducing the analytical cohort size. Radioimmunoassay analysis used in this study is not the “gold standard” for assessing 25-OH-vitamin D levels. Although the kit manufacturer does participate in the Centers for Disease Control and Prevention Vitamin D Standardization Certification Program, the performing laboratory did not participate in vitamin D external quality assessment. However, all testing included standard curves, kit controls, and internal laboratory controls. This method is used to assess 25-OH-vitamin D levels in the clinical setting, making our results relevant to clinical practice. We do not have longitudinal 25-OH-vitamin D levels to assess the impact of varying vitamin D levels on lung function decline estimates over multiple years. Effect size of our findings cannot be reliably reported. The predominance of white participants may limit generalizability to other populations with COPD and greater racial diversity.
Conclusion
We have observed VDD in approximately one of five participants with COPD in a multicenter cohort. Active smokers, blacks, and younger participants were more likely to have VDD. Lower 25-OH-vitamin D levels are independently associated with lower baseline lung function as well as greater odds of an AECOPD in the year before enrollment. VDD was associated with a greater rate of lung function decline over 1 year. These findings describe potential adverse effects of VDD on lung function decline in those with COPD.
Acknowledgments
Author contributions: R. M. B. had access to the data and is the guarantor for the content of the manuscript including data and analysis. R. M. B. and M. B. D. had full access to the data and take responsibility for the integrity of the data and accuracy of analysis presented herein. M. B. D. and R. M. B. contributed substantially to the design of the study, data interpretation, and drafting of the manuscript. A. S. C., M. B. D., and R. M. B. contributed to data analysis. D. C., E. A. H., A. P. C., R. G. B., J. A. K., C. C., W. W. L., V. E. O., J. M. W., G. J. C., P. G. W., R. P. B., C. S. P., N. N. H., and M. B. D. contributed to data collection in the SPIROMICS cohort. C. M. D., D. C., E. A. H., A. P. C., R. G. B., J. A. K., C. C., W. W. L., V. E. O., J. M. W., G. J. C., P. G. W., R. P. B., C. S. P., N. N. H., R. A. W., and T. T. B. critically appraised drafts of the manuscript for critically important intellectual content and contributed substantially to revisions of the manuscript. All authors agreed on the manuscript in its final, submitted form.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. M. B. has received a grant from the NIH-NHLBI related to this work. C. M. D. has received grants from the NIH and the Department of Defense unrelated to this work. D. C. reports funding from the NIH and the COPD Foundation unrelated to this work. E. A. H. is a founder of and shareholder in VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa; activities that are unrelated to this study. A. P. C. reports funds from the NIH-NHLBI, compensated and noncompensated work for VIDA, and personal fees from GlaxoSmithKline (GSK), all unrelated to this work. J. A. K. reports grant funding from the NIH-NHLBI unrelated to this work. C. C. reports working part-time on external scientific engagement and internal medical education for the GSK Global Respiratory Franchise, all unrelated to this work. J. M. W. reports grants from the NIH-NHLBI, NIH-NCATS (National Center for Advancing Translational Sciences), Beyer, Boehringer-Ingelheim, Mereo BioPharma, and financial relationships with Boehringer-Ingelheim, Mereo BioPharma, and PRA, all unrelated to this work. G. J. C. receives grants from Boehringer-Ingelheim, Novartis, Astra Zeneca, Respironics, MedImmune, Actelion, Forest, Pearl, Ikaria, Aeris, PneumRx, Pulmonx, and other fees from HGE Health Care Solutions Inc, Almirall, Boehringer-Ingelheim, and Nuvaira, all unrelated to this work. P. G. W. reports personal fees from Theravance, GSK, NGM Pharmaceuticals, Amgen, Glenmark Pharmaceuticals, Regeneron, Sanofi, Clarus Ventures, 23andMe, Astra Zeneca, all unrelated to this work. R. P. B. served on the advisory boards (GlaxoSmithKline, Boehringer Ingelheim, and Mylan Pharmaceuticals) and received research grants from GSK and Boehringer-Ingelheim, all activities unrelated to this work. N. N. H. reports grants and personal fees from AstraZeneca; grants from Boehringer-Ingelheim, the NIH, and the COPD Foundation; and personal fees from Mylan; all unrelated to this work. R. A. W. reports grants and personal fees from AstraZeneca/Medimmune, Boehringer-Ingelheim, and GSK outside the submitted work. He reports grants from Pearl Therapeutics and Sanofi-Aventis outside the submitted work. He reports personal fees from ContraFect, Pulmonx, Roche, Spiration, Sunovion, Merck, Circassia, Pneuma, Verona, Mylan/Theravance, Propeller Health, AbbVie, and GSK, all unrelated to this work. T. T. B. reports personal fees from Gilead, ViiV, Merck, EMS-Serono, and Theratechnologies, all unrelated to this work. M. B. D. reports grants from the NIH-NHLBI during the conduct of the study related to this work as well as personal fees from Boehringer-Ingelheim, GSK, AstraZeneca, Mylan-Theravance, Novavax, Parion, Midmark, and Philips and grants from the Department of Defense and Boehringer-Ingelheim, unrelated to this work. None declared: A. S. C., R. G. B., W. W. L., V. E. O., and C. S. P.
Role of sponsors: Industry sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
*SPIROMICS collaborators: The authors acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, MD; Wayne H. Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R. Graham Barr, MD, DrPH; Lori A. Bateman, MSc; Surya P. Bhatt, MD; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Gerard J. Criner, MD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Brad Drummond, MD; Christine M. Freeman, PhD; Craig Galban, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Yvonne Huang, MD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Jerry A. Krishnan, MD, PhD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; Wendy C. Moore, MD; John D. Newell Jr, MD; Robert Paine III, MD; Laura Paulin, MD, MHS; Stephen P. Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Victor E. Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P. Tashkin, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN.
Other contributions: The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data may be found at www.spiromics.org. SPIROMICS was supported by contracts from the NIH/NHLBI [HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C], by a grant from the NIH/NHLBI [U01 HL137880], and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: R. M. B. has received support from the National Institutes of Health, National Heart, Lung, and Blood Institute (NIH-NHLBI) [Grant F32HL143867-01] related to this work. M. B. D. has received support from the NIH-NHLBI [Grant R01HL125432-01A1] related to this work. For SPIROMICS funding, see Acknowledgments.
Contributor Information
Robert M. Burkes, Email: robert.burkes@unchealth.unc.edu.
SPIROMICS Investigators:
Neil E. Alexis, Wayne H. Anderson, Mehrdad Arjomandi, Igor Barjaktarevic, R. Graham Barr, Lori A. Bateman, Surya P. Bhatt, Eugene R. Bleecker, Richard C. Boucher, Russell P. Bowler, Stephanie A. Christenson, Alejandro P. Comellas, Christopher B. Cooper, David J. Couper, Gerard J. Criner, Ronald G. Crystal, Jeffrey L. Curtis, Claire M. Doerschuk, Mark T. Dransfield, Brad Drummond, Christine M. Freeman, Craig Galban, MeiLan K. Han, Nadia N. Hansel, Annette T. Hastie, Eric A. Hoffman, Yvonne Huang, Robert J. Kaner, Richard E. Kanner, Eric C. Kleerup, Jerry A. Krishnan, Lisa M. LaVange, Stephen C. Lazarus, Fernando J. Martinez, Deborah A. Meyers, Wendy C. Moore, John D. Newell, Jr., Robert Paine, III, Laura Paulin, Stephen P. Peters, Cheryl Pirozzi, Nirupama Putcha, Elizabeth C. Oelsner, Wanda K. O’Neal, Victor E. Ortega, Sanjeev Raman, Stephen I. Rennard, Donald P. Tashkin, J. Michael Wells, Robert A. Wise, Prescott G. Woodruff, Lisa Postow, and Lisa Viviano
Supplementary Data
References
- 1.Horadagoda C., Dinihan T., Roberts M., Kairaitis K. Body composition and micronutrient deficiencies in patients with an acute exacerbation of chronic obstructive pulmonary disease. Intern Med J. 2017;47(9):1057–1063. doi: 10.1111/imj.13453. [DOI] [PubMed] [Google Scholar]
- 2.Rawal G., Yadav S. Nutrition in chronic obstructive pulmonary disease: a review. J Transl Intern Med. 2015;3(4):151–154. doi: 10.1515/jtim-2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persson L.J., Aanerud M., Hiemstra P.S., Hardie J.A., Bakke P.S., Eagan T.M. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu M., Wang T., Wang C., Ji Y. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:2597–2607. doi: 10.2147/COPD.S101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 6.Herr C., Greulich T., Koczulla R.A. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zosky G.R., Berry L.J., Elliot J.G., James A.L., Gorman S., Hart P.H. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183(10):1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 8.Black P.N., Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 9.Tsiligianni I.G., van der Molen T. A systematic review of the role of vitamin insufficiencies and supplementation in COPD. Respir Res. 2010;11(1) doi: 10.1186/1465-9921-11-171. 171-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssens W., Lehouck A., Carremans C., Bouillon R., Mathieu C., Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179(8):630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 11.Skaaby T., Husemoen L.L., Thuesen B.H. Vitamin D status and chronic obstructive pulmonary disease: a prospective general population study. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanket S., Madireddi J., Stanley W., Sura P., Prabhu M. Relation between vitamin D deficiency and severity of chronic obstructive pulmonary disease: a case control study. J Clin Diagn Res. 2016;10(1):OC16–OC19. doi: 10.7860/JCDR/2016/15404.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendy A., Forno E., Niyonsenga T., Gasana J. Blood biomarkers as predictors of long-term mortality in COPD. Clin Respir J. 2018;12(5):1891–1899. doi: 10.1111/crj.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persson L.J., Aanerud M., Hiemstra P.S. Vitamin D, vitamin D binding protein, and longitudinal outcomes in COPD. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao G., Ford E.S., Tsai J., Li C., Croft J.B. Low concentrations of serum 25-hydroxyvitamin D associated with increased risk for chronic bronchitis among US adults. Br J Nutr. 2012;107(9):1386–1392. doi: 10.1017/S0007114511004417. [DOI] [PubMed] [Google Scholar]
- 16.Vestbo J., Edwards L.D., Scanlon P.D. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 17.Kunisaki K.M., Niewoehner D.E., Singh R.J., Connett J.E. Vitamin D status and longitudinal lung function decline in the Lung Health Study. Eur Respir J. 2011;37(2):238–243. doi: 10.1183/09031936.00146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange N.E., Sparrow D., Vokonas P., Litonjua A.A. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am J Respir Crit Care Med. 2012;186(7):616–621. doi: 10.1164/rccm.201110-1868OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehouck A., Mathieu C., Carremans C. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 20.Martineau A.R., James W.Y., Hooper R.L. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120–130. doi: 10.1016/S2213-2600(14)70255-3. [DOI] [PubMed] [Google Scholar]
- 21.Rafiq R., Prins H.J., Boersma W.G. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: a pilot trial. Int J Chron Obstruct Pulmon Dis. 2017;12:2583–2592. doi: 10.2147/COPD.S132117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malinovschi A., Masoero M., Bellocchia M. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res. 2014;15:131. doi: 10.1186/s12931-014-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolliffe D.A., Greenberg L., Hooper R.L. Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019;74(4):337–345. doi: 10.1136/thoraxjnl-2018-212092. [DOI] [PubMed] [Google Scholar]
- 24.Couper D., LaVange L.M., Han M. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodruff P.G., Barr R.G., Bleecker E. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han M.K., Quibrera P.M., Carretta E.E. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulin L.M., Smith B.M., Koch A. Occupational exposures and computed tomographic imaging characteristics in the SPIROMICS cohort. Ann Am Thorac Soc. 2018;15(12):1411–1419. doi: 10.1513/AnnalsATS.201802-150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieren J.P., Newell J.D., Jr., Barr R.G. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith B.M., Hoffman E.A., Rabinowitz D. Comparison of spatially matched airways reveals thinner airway walls in COPD: the Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(11):987–996. doi: 10.1136/thoraxjnl-2014-205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederer D.J., Bell S.C., Branson R.D. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 31.Janssens W., Bouillon R., Claes B. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 32.Afzal S., Lange P., Bojesen S.E., Freiberg J.J., Nordestgaard B.G. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax. 2014;69(1):24–31. doi: 10.1136/thoraxjnl-2013-203682. [DOI] [PubMed] [Google Scholar]
- 33.Gombart A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Leung D.Y., Richers B.N. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantorna M.T., Yu S., Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29(6):369–375. doi: 10.1016/j.mam.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szekely J.I., Pataki A. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: a short review. Expert Rev Respir Med. 2012;6(6):683–704. doi: 10.1586/ers.12.57. [DOI] [PubMed] [Google Scholar]
- 37.Jolliffe D.A., James W.Y., Hooper R.L. Prevalence, determinants and clinical correlates of vitamin D deficiency in patients with chronic obstructive pulmonary disease in London, UK. J Steroid Biochem Mol Biol. 2018;175:138–145. doi: 10.1016/j.jsbmb.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Holmgaard D.B., Mygind L.H., Titlestad I.L. Serum vitamin D in patients with chronic obstructive lung disease does not correlate with mortality: results from a 10-year prospective cohort study. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moosavi S.A.J., Shoushtari M.H. The effects of vitamin D supplementation on pulmonary function of chronic obstructive pulmonary disease patients, before and after clinical trial. Diseases. 2015;3(4):253–259. doi: 10.3390/diseases3040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ClinicalTrials.gov, U.S. National Library of Medicine Cathelicidin and Vitamin D: Impact on Populations At-Risk and With COPD. NCT02464059https://clinicaltrials.gov/ct2/show/NCT02464059
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.