Abstract
Background
Bronchoscopy is the gold standard for evaluating tracheomalacia; however, reliance on an invasive procedure limits understanding of normal airway dynamics. Self-gated ultrashort echo-time MRI (UTE MRI) can assess tracheal dynamics but has not been rigorously evaluated.
Methods
This study was a validation of UTE MRI diagnosis of tracheomalacia in neonates using bronchoscopy as the gold standard. Bronchoscopies were reviewed for the severity and location of tracheomalacia based on standardized criteria. The percent change in cross-sectional area (CSA) of the trachea between end-inspiration and end-expiration was determined by UTE MRI, and receiver-operating curves were used to determine the optimal cutoff values to predict tracheomalacia and determine positive and negative predictive values.
Results
Airway segments with tracheomalacia based on bronchoscopy had a more than threefold change in CSA measured from UTE MRI (54.4 ± 56.1% vs 14.8 ± 19.5%; P < .0001). UTE MRI correlated moderately with bronchoscopy for tracheomalacia severity (ρ = 0.39; P = .0001). Receiver-operating curves, however, showed very good ability of UTE MRI to identify tracheomalacia (area under the curve, 0.78). A “loose” definition (> 20% change in CSA) of tracheomalacia had good sensitivity (80%) but low specificity (64%) for identifying tracheomalacia based on UTE MRI, whereas a “strict” definition (> 40% change in CSA) was poorly sensitive (48%) but highly specific (93%).
Conclusions
Self-gated UTE MRI can noninvasively assess tracheomalacia in neonates without sedation, ionizing radiation, or increased risk. This technique overcomes major limitations of other diagnostic modalities and may be suitable for longitudinal population studies of tracheal dynamics.
Key Words: bronchoscopy, pediatric pulmonology, tracheomalacia
Abbreviations: CSA, cross-sectional area; CCHMC, Cincinnati Children’s Hospital Medical Center; TM, tracheomalacia; UTE, ultrashort echo-time
FOR EDITORIAL COMMENT, SEE PAGE 489
Tracheomalacia (TM) is characterized by weakening of the airway wall and results in dynamic collapse of the airway lumen during respiration. TM is estimated to occur in approximately 1:2,000 otherwise healthy children and can afflict more than one-half of high-risk populations such as neonates with bronchopulmonary dysplasia or tracheoesophageal fistulas.1, 2, 3 Despite the high prevalence of TM, there are few population studies that assess the implications of dynamic tracheal collapse on symptoms and outcomes or the response of TM to medical or surgical interventions.
The “gold standard” for the diagnosis of TM is bronchoscopy during spontaneous breathing. There are no standardized criteria for evaluating TM, nor is there consensus of normal airway collapse in children.4, 5, 6 Although there is high interrater reliability for the endoscopic diagnosis of TM in adults, this approach has not been assessed in pediatric patients.7 Furthermore, bronchoscopy requires sedation and poses increased risk, albeit small, to the patient. Consequently, bronchoscopy is not suitable for assessment of normal airway dynamics, limiting our understanding of normal tracheal collapse and changes over time.
Various modalities have been used to diagnose TM in young children. Pulmonary function testing is neither sensitive nor specific for diagnosing TM and requires sedation in infants and toddlers.2 Plain-film radiography and airway fluoroscopy are specific but poorly sensitive.5,8 Chest CT imaging is sensitive and specific for diagnosing TM in patients with severe TM.4 Although progress has been made to reduce radiation exposure from CT scans, neonates are particularly sensitive to the effects of ionizing radiation.9,10 Because all traditional methods to assess TM in neonates require sedation, ionizing radiation, or both, none is suitable for population or longitudinal studies of TM. Cine MRI has shown potential for the evaluation of airway dynamics in adults and cooperative older children but requires sedation in younger children and infants.11 In addition, the smaller anatomy and higher respiratory rates in young neonates necessitate an increase in both spatial and temporal resolution of traditional cine MRI, which is technically difficult to achieve, and thus cine MRI of the airway with high temporal and three-dimensional spatial resolution has not been implemented successfully in neonates.
Ultrashort echo-time MRI (UTE MRI) has been used to quantitatively evaluate airway dynamics.12 This UTE MRI technique implements a radial k-space acquisition (distinct from most conventional MRI sequences) and thus is more robust to motion artifacts.13,14 In addition, radial UTE MRI acquisitions allow for a retrospective motion-tracking approach that discards MRI data affected by bulk motion prior to image reconstruction, meaning sedation is not required for imaging. This retrospective motion-tracking method allows for imaging of respiratory dynamics during spontaneous tidal-breathing in nonsedated neonates. Importantly, UTE MRI does not require ionizing radiation.
UTE MRI has the potential to overcome the limitations of existing diagnostic techniques, but the ability of this method to identify TM has not been rigorously assessed. The purpose of the current study was to assess the ability of UTE MRI to diagnose TM compared with bronchoscopy as the gold standard.
Subjects and Methods
Subjects
Subjects were recruited from the neonatal ICU at Cincinnati Children’s Hospital Medical Center (CCHMC). Subjects who had undergone a research UTE MRI and a clinical bronchoscopy within 90 days of imaging were eligible for enrollment. Subjects were excluded if they had a tracheostomy. This study was approved by the institutional review board at CCHMC (#2018-0958), and informed consent was obtained for each patient for both the clinical bronchoscopy and UTE MRI.
Bronchoscopic Assessment of TM
Clinical bronchoscopies were performed under general anesthesia while maintaining spontaneous respiration. Twenty-five patients had both a flexible and rigid bronchoscopy. If both procedures were performed, a 2.8-mm flexible bronchoscope (Olympus BF-XP160F) was inserted through the vocal cords that were anesthetized with 0.5 mL of 1% topical lidocaine, and the airway was evaluated to the segmental bronchi. Following the flexible bronchoscopy, a Hopkins rod was inserted through the vocal cords. The trachea was evaluated to the level of the main bronchi. Eleven patients underwent evaluation by rigid bronchoscopy only.
All bronchoscopies performed at CCHMC are stored on a secure online server. Endoscopic videos were edited to allow evaluation of the airway from the vocal cords to the main carina and scored for the location and severity of dynamic trachea collapse by three independent reviewers (E. B. H., C. K. H., and D. B.) with expertise in clinical bronchoscopy. The location of collapse was defined as upper, middle, or lower trachea, and the severity of collapse was defined as none (0%-25% collapse), mild/moderate (26%-75% collapse), and severe (> 75% collapse). A visual aid with endoscopic photos representative of all severities of airway collapse was provided to each reviewer. Prior to scoring the endoscopies, a training session was held to review the scoring criteria and evaluate test cases until interrater agreement was > 90%.
The presence of TM by bronchoscopy was defined based on majority agreement of the three reviewers. The severity of dynamic trachea collapse was defined as the average severity score of all three reviewers. Ten bronchoscopies were chosen at random without knowledge of the reviewer for assessment of intrarater reliability.
UTE MRI Assessment of TM
Research UTE MRI scans were performed on a neonatal-sized 1.5T MRI without administering sedation for the purposes of imaging.15, 16, 17 Imaging was performed by using the “feed and swaddle” method with standard hearing protection and continuous monitoring for respiratory or hemodynamic distress during the procedure.18,19 MR images were acquired with a three-dimensional radial UTE sequence during spontaneous tidal breathing, yielding CT-like image resolution of 0.7 × 0.7 × 0.7 mm.14 As previously described,13,14,20 the following UTE parameters were used: repetition time ≈ 5 ms, echo time ≈ 200 μs, flip angle = 5°, field of view = 18 cm, matrix = 256 × 256 × 256, number of radial projections ∼200,000, and scan time ≈ 16 min. Periods of bulk motion were discarded by using the motion-modulated center of k-space; the remaining quiescent data were assigned to specified views (typically eight, one each for end-expiration and end-inspiration, and three each for inhalation and exhalation) throughout the respiratory cycle with a sliding-window binning algorithm in MATLAB (The MathWorks, Inc.). The respiratory-binned data were then used to reconstruct gated images showing the airway anatomy at various stages of respiration. These imaging and retrospective respiratory-gating methods have previously been described in detail.13,14,20
Virtual models of the airway surface at end-inspiration and end-expiration were created from image segmentations performed in ITK-SNAP (3.6.0; Penn Image Computing and Science Laboratory; www.itksnap.org).20 Airway center lines through these surfaces were generated, and a series of disks bounded by the tracheal surface were defined at an angle of 90° to this center line and spaced every 1 mm via in-house MATLAB code (The MathWorks, Inc.).21, 22, 23, 24 These disks were used to measure the luminal cross-sectional area (CSA). Because the disks follow the airway center line, they avoid errors associated with misalignment of the airway and image axes,25 as can occur when using axial image measurements. The CSA of each disk was calculated for end-expiration (CSAexp) and end-inspiration (CSAinsp) and plotted against the position along the trachea as previously described (Fig 1).12 The MRI-based assessment of TM was defined as the percent change in CSA from end-inspiration and end-expiration: [(CSAinsp –CSAexp)/CSAinsp]. The upper, middle, and lower regions of the trachea were defined by dividing the tracheal length into thirds from the cricoid to the carina. The maximum value of percent change in CSA from measurements in all disks in each third of the trachea was used to determine collapse in that segment for patients with and without TM based on bronchoscopy findings (Fig 2).
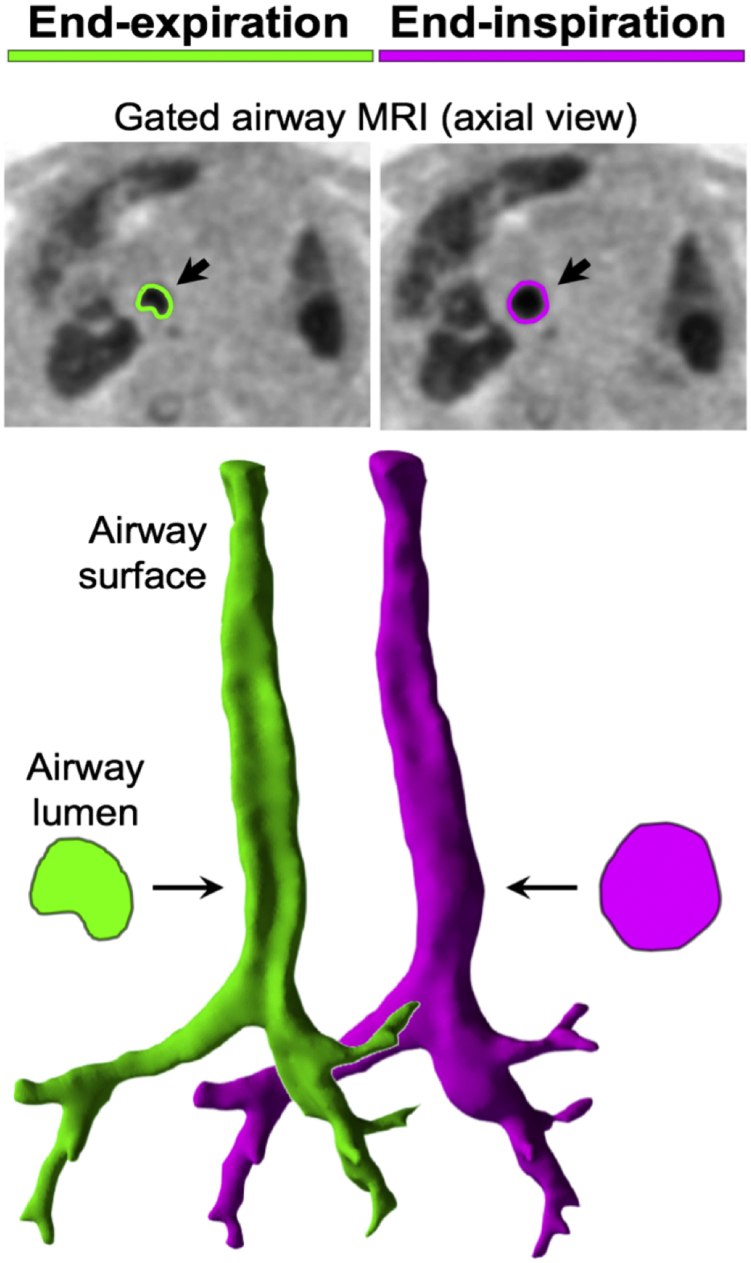
Figure 1.
Upper: Axial ultrashort echo-time MRI retrospectively gated to end-expiration (left) and end-inspiration (right) via the motion-modulated center of the MRI k-space. The edge of the tracheal lumen is highlighted in green and magenta, respectively. Lower: Airway surfaces generated from segmentations of the airway from the ultrashort echo-time MRI shown from the posterior aspect. The trachealis is concave in the end-expiration image. Trachea cross-sections are shown for each of the end-expiration (green) and end-inspiration (magenta) images. These cross-sections are used to calculate the cross-sectional area at the two points in the respiratory cycle.
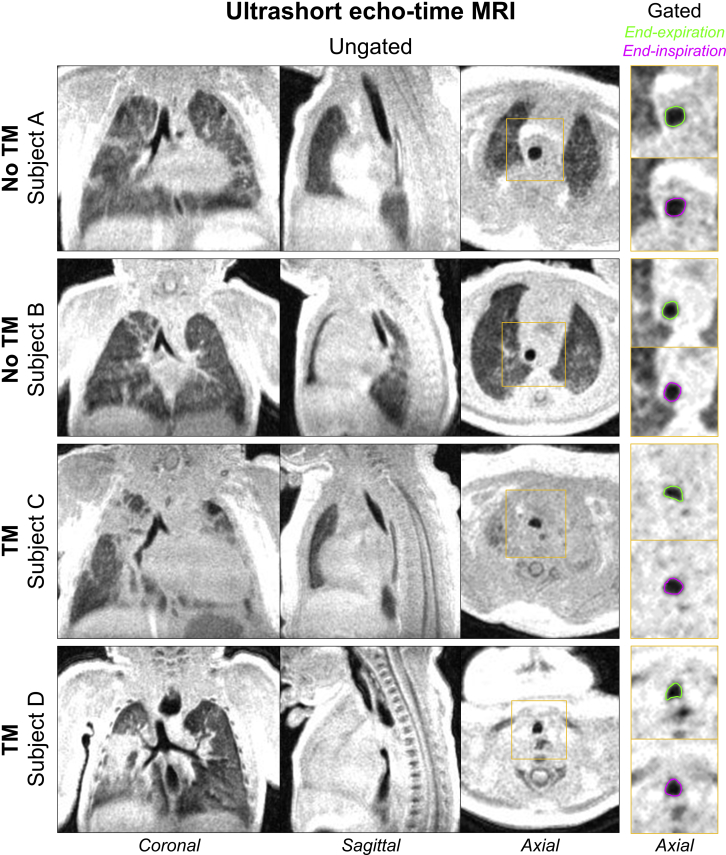
Figure 2.
Three-dimensional ultrashort echo-time MRI with chest and airway coverage in four neonatal patients (resolution, 0.7 × 0.7 × 0.7 mm). Coronal, sagittal, and axial planes of ungated images acquired during tidal breathing are shown in the three left columns. Insets (yellow): respiratory-gated images reconstructed from the same imaging data show axial views of tracheal dynamics at end-expiration (green) and end-inspiration (magenta). Endoscopic results in these four patients indicated absence of TM in Subjects A and B and presence of TM in Subjects C and D, which qualitatively agrees with the degree of dynamic tracheal collapse observed on the respiratory-gated images at right. TM = tracheomalacia.
Statistical Analysis
Interrater and intrarater reliability of bronchoscopic assessment of TM was calculated by using Cohen’s κ statistic. The correlation of TM severity based on bronchoscopy and UTE MRI was assessed by using the Pearson correlation coefficient. A receiver-operating curve was generated by using a bootstrap with 1,000 replications to assess the sensitivity and specificity of UTE MRI to identify TM compared with bronchoscopy as the gold standard. Segments of the trachea were clustered according to individual because segments within the same patient may not be independent.
The percent change in CSA that maximized accuracy based on the receiver-operating curve was chosen as the cutoff value. Positive and negative prediction values were determined by using the cutoff value. A P value < .05 was considered statistically significant. All data analysis was performed by using Stata version 14.0 (StataCorp).
Results
There were 36 patients recruited for this study. The average age at the time of MRI was 94.8 ± 88.3 days, and the average age at bronchoscopy was 102.5 ± 74.8 days. Subjects had a variety of respiratory disorders, including bronchopulmonary dysplasia (55.6%), tracheoesophageal fistula with esophageal atresia (25.0%), congenital diaphragmatic hernia (13.9%), and upper airway obstruction (5.6%) (Table 1).
Table 1.
Demographic Characteristics (N = 36)
| Age at UTE MRI, d | 94.8 ± 88.3 |
| Age at bronchoscopy, d | 102.5 ± 74.8 |
| Birth weight, g | 1,803 ± 1,260 |
| Weight at UTE MRI, g | 3341 ± 892 |
| Height at UTE MRI, cm | 48.4 ± 5.6 |
| Male sex | 19 (52.8%) |
| Respiratory comorbidities | |
| Bronchopulmonary dysplasia | 20 (55.6%) |
| Tracheoesophageal fistula/esophageal atresia | 9 (25.0%) |
| Congenital diaphragmatic hernia | 5 (13.9%) |
| OSA | 2 (5.6%) |
| Bronchoscopy-defined tracheomalacia | |
| Upper trachea | 2 (5.6%) |
| Middle trachea | 23 (63.8%) |
| Lower trachea | 25 (69.4%) |
Data are presented as mean ± SD unless otherwise indicated. UTE = ultrashort echo-time.
Based on rigid bronchoscopy, two neonates (5.6%) had TM in the upper trachea, 23 (63.8%) in the middle trachea, and 25 (69.4%) in the lower trachea. There was excellent intrarater agreement for all bronchoscopists, ranging from 93% to 100% (κ = 0.86-1.0; P < .0001). The interrater agreement for the diagnosis of TM across all segments was 87% (κ = 0.72; P < .0001).
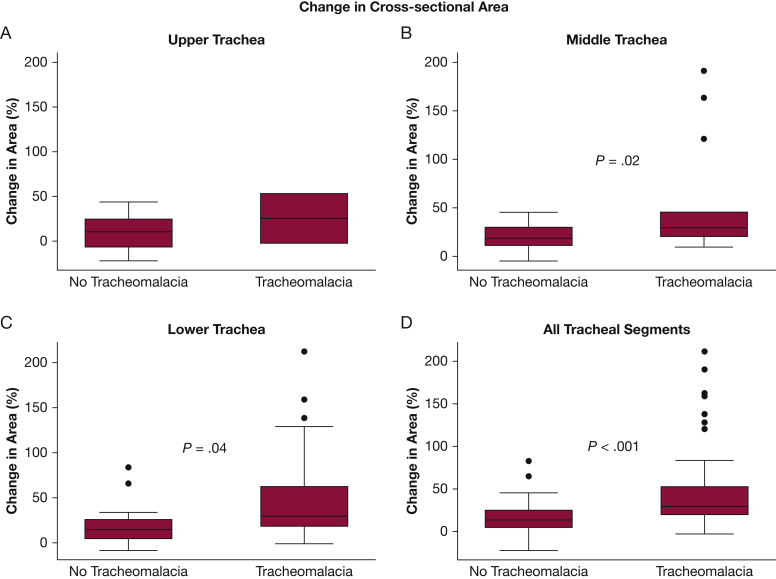
A total of 108 segments of the trachea were compared by using endoscopy and MRI. Neonates with TM based on rigid bronchoscopy had more than a threefold increased change in CSA measured from UTE MRI (54.4 ± 56.1% vs 14.8 ± 19.5%; P < .0001) (Fig 3). There was moderate correlation with severity of TM based on rigid bronchoscopy and change in CSA (ρ = 0.39; P = .0001).
Figure 3.
Percent change in tracheal cross-sectional area based on ultrashort echo-time MRI for the upper (A), middle (B), lower (C), and all (D) tracheal segments stratified according to endoscopically determined tracheomalacia. Box-and-whisker plot: box includes the 1st and 3rd quartiles, whiskers include the 95th percentile, circles represent outliers.
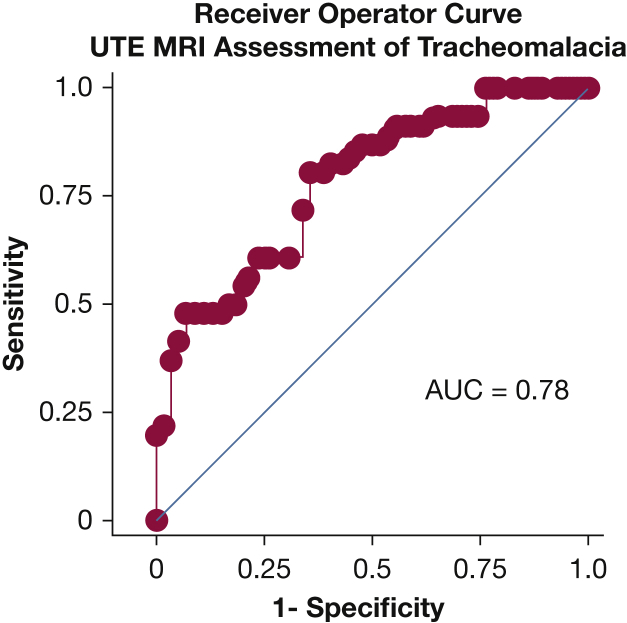
Receiver-operating curves showed good ability of UTE MRI to identify TM (area under the curve, 0.78) with a bias coefficient of –0.003 (Fig 4). A change in CSA of > 20% provided a sensitivity of 80% and a specificity of 64%. We defined this as a loose measure of TM. When this measure was applied, the positive predictive value was 62%, and the negative predictive value was 76% (Table 2).
Figure 4.
Receiver-operating curve showing the sensitivity and specificity of percent change in tracheal cross-sectional area from ultrashort echo-time MRI for assessing tracheomalacia. Endoscopically determined tracheomalacia was used as the gold standard. AUC = area under the curve.
Table 2.
Diagnostic Performance of UTE MRI for Assessment of Tracheomalacia
| Change in CSA | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 20% | 80 (61,100) | 64 (49,91) | 62 (48,75) | 76 (62,87) |
| 40% | 48 (13,89) | 93 (73,100) | 84 (64,96) | 69 (57,79) |
Data are presented as the point estimate and 95% CI. CSA = cross-sectional area; NPV = negative predictive value; PPV = positive predictive value. See Table 1 legend for expansion of other abbreviation.
We also developed a strict definition of TM as a change in CSA of > 40%, which provided a sensitivity of 48% and a specificity of 94%. When the strict definition was used, the positive predictive value was 84%, and the negative predictive value was 69% (Table 2).
A subset of 25 patients had a flexible bronchoscopy with 73 tracheal segments available for evaluation. As with rigid bronchoscopy, UTE MRI showed good ability to identify TM compared with flexible bronchoscopy (area under the curve, 0.77) and had moderate correlation with severity of TM (ρ = 0.42; P < .0001). Our previous loose definition again provided good sensitivity (82%) but was less specific (67%) for the identification of TM, and our strict definition was less sensitive (50%) but highly specific (86%).
Discussion
In this study, we developed a novel method for evaluating TM in neonates using gated UTE MRI. This is the only current method that permits a purely quantitative assessment of tracheal dynamics in neonates along the entire length of the trachea during spontaneous respiration without sedation and/or radiation. We also describe values of airway collapse on imaging to define TM and compare imaging with both flexible and rigid bronchoscopy. Our loose definition of TM based on UTE MRI provides good sensitivity, and our strict definition provides excellent specificity.
Bronchoscopy remains the gold standard for the assessment of TM; however, bronchoscopy is limited by the lack of standardized criteria for defining TM, lack of standardized endoscopic technique, and the ability to define normality. Previous studies have used rigid and flexible bronchoscopy as the gold standard for defining TM in children.4,26 There are no data defining which technique correlates best with clinical presentation and minimal data comparing the endoscopic findings of flexible and rigid bronchoscopy. Choi et al27 recently compared rigid and flexible bronchoscopy for evaluation of TM using the color histogram technique.28 Although the authors concluded that there is no difference in airway collapse as judged by the two techniques, 33% of patients were defined as having TM on rigid bronchoscopy that were not identified on flexible bronchoscopy.27 Thus, there are important differences between the gold standards for evaluation of TM. These differences likely contribute to some of the discordance with UTE MRI and bronchoscopic assessment of TM.
In addition to lack of standardization of the bronchoscopic technique, there are no standardized bronchoscopic criteria for diagnosing TM. Currently, bronchoscopy relies on a semi-quantitative assessment of airway collapse during spontaneous breathing; unfortunately, bronchoscopy does not take the depth of sedation into account nor the transmural airway pressure, which may alter airway dynamics. The presence of a bronchoscope itself can also alter respiratory mechanics, and it is not clear what effect this may have on tracheal dynamics.29,30 Furthermore, the lack of a standardized definition also allows for interrater variability when interpreting TM based on bronchoscopy, although we achieved very good interrater agreement with agreed upon definitions and extensive training cases. Quantitative methods have been described to determine collapse of the trachea based on bronchoscopy; however, this is not routinely implemented in clinical practice.26,28,31 Perhaps the most challenging aspect of using bronchoscopy for assessing TM is that the use of an invasive procedure prevents evaluation of the trachea in healthy children, severely limiting the understanding of normal airway dynamics.
UTE MRI provides several advantages for the assessment of TM in neonates compared with other imaging modalities that require ionizing radiation. Due to the concerns of the carcinogenic effects of ionizing radiation, the use of CT scanning in pediatrics has declined in the last decade.9,32 Although recent advances in multi-detector CT scanning have reduced the exposure to ionizing radiation, there is still the potential for adverse events that are entirely avoided with UTE MRI.10
The use of MRI has traditionally required sedation in pediatric patients. Although anesthesia is generally well tolerated in healthy children, young infants and those with complex medical disease are at higher risk for major adverse events.33 In addition to the increased risk related to sedation, anesthesia can significantly alter airway morphology,34 and it is not currently clear what effect anesthesia has on diagnosing TM. We have developed a self-gated UTE image reconstruction technique that yields views at several time points during the respiratory cycle without sedation.12 This technique is also able to remove data corrupted by patient bulk motion.12,13 Consequently, UTE MRI can be used to assess the dynamics of the trachea during spontaneous respiration without significant additional risk35 or distortion of airway morphology related to sedation.
TM has been linked to increased respiratory morbidity in the neonatal period and throughout childhood.1,26,36,37 Because all current methods for assessing TM require sedation, ionizing radiation, or both, it has previously not been feasible to study the impact of TM on large populations of children. Limitations of existing modalities have also made it difficult to study the natural history of TM in pediatric patients. UTE MRI provides a unique opportunity to fill this gap in knowledge. Similarly, UTE MRI provides an objective and quantitative evaluation of TM in neonates, which could be useful for objectively evaluating the response of the trachea and tracheal dynamics to treatments such as aortopexy, posterior tracheopexy, and bethanechol.
Although UTE MRI provides many advantages for assessing TM, there was not complete concordance with bronchoscopy. Using our “loose” definition, UTE MRI was 73% accurate for identifying TM and 74% accurate with our strict definition. Agreement between bronchoscopists was 87%; thus, we cannot expect agreement with bronchoscopy and UTE MRI to exceed this value. There are important limitations of UTE MRI when assessing airway dynamics. The use of retrospective self-gating evaluates the average collapse of the trachea during spontaneous breathing and discards bulk motion. Some children may only develop significant collapse during respiratory maneuvers such as forced expiration that would not be captured by using this technique. This factor likely accounts for the disagreements between imaging and bronchoscopy.
This study relied on a population of neonates undergoing bronchoscopy, which is inherently a biased patient population, and our results may not be generalizable to other patient populations. Currently, bronchoscopy is the gold standard for evaluation of TM, so this is similarly a challenge for any validation study of a novel technique for diagnosing TM in children. Our previous study has shown that a normal neonatal airway collapses by slightly more than 20%,12 which is very consistent with our loose definition of TM. We have also included patients with a very wide range of airway collapse; thus, the risk of selection bias has been limited as much as possible.
Conclusions
Respiratory-gated UTE MRI was able to identify TM in neonates during spontaneous respiration without sedation or ionizing radiation. Because there is no additional risk to the patient, UTE MRI provides the first opportunity to evaluate TM in entire populations and to assess the natural progression of central airway collapse and the impact on respiratory outcomes in neonates.
Acknowledgments
Author contributions: E. B. H. takes responsibility for the content of THE manuscript.
E. B. H. and A. J. B. conceived and designed the study, acquired data, analyzed and interpreted data, and drafted the initial manuscript. N. S. H., D. B., C. K. H., R. J. F., G. B., and A.D. A. designed the study, acquired data, and revised the manuscript. P. S. K. and J. C. W. interpreted the data and revised the manuscript. All authors approved the final version of the manuscript and have agreed to be accountable for all aspects of the work.
Financial/nonfinancial disclosures: None declared.
Footnotes
Drs Hysinger and Bates contributed equally to this manuscript.
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [Grant R01 HD093363].
References
- 1.Hysinger E.B., Friedman N.L., Padula M.A., et al. Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia. Ann Am Thorac Soc. 2017;14(9) doi: 10.1513/AnnalsATS.201702-178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boogaard R., Huijsmans S.H., Pijnenburg M.W., Tiddens H.A., de Jongste J.C., Merkus P.J. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest. 2005;128(5):3391–3397. doi: 10.1378/chest.128.5.3391. [DOI] [PubMed] [Google Scholar]
- 3.Hysinger E., Friedman N., Jensen E., Zhang H., Piccione J. Bronchoscopy in neonates with severe bronchopulmonary dysplasia in the NICU. J Perinatol. 2019;39(2):263–268. doi: 10.1038/s41372-018-0280-y. [DOI] [PubMed] [Google Scholar]
- 4.Ngerncham M., Lee E.Y., Zurakowski D., Tracy D.A., Jennings R. Tracheobronchomalacia in pediatric patients with esophageal atresia: comparison of diagnostic laryngoscopy/bronchoscopy and dynamic airway multidetector computed tomography. J Pediatr Surg. 2015;50(3):402–407. doi: 10.1016/j.jpedsurg.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez M.O., Greer M.C., Masters I.B., Chang A.B. A comparison of fluoroscopic airway screening with flexible bronchoscopy for diagnosing tracheomalacia. Pediatric Pulmonol. 2012;47(1):63–67. doi: 10.1002/ppul.21517. [DOI] [PubMed] [Google Scholar]
- 6.Mair E.A., Parsons D.S. Pediatric tracheobronchomalacia and major airway collapse. Ann Otol Rhinol Laryngol. 1992;101(4):300–309. doi: 10.1177/000348949210100403. [DOI] [PubMed] [Google Scholar]
- 7.Majid A., Gaurav K., Sanchez J.M., et al. Evaluation of tracheobronchomalacia by dynamic flexible bronchoscopy. A pilot study. Ann Am Thorac Soc. 2014;11(6):951–955. doi: 10.1513/AnnalsATS.201312-435BC. [DOI] [PubMed] [Google Scholar]
- 8.Walner D.L., Ouanounou S., Donnelly L.F., Cotton R.T. Utility of radiographs in the evaluation of pediatric upper airway obstruction. Ann Otol Rhinol Laryngol. 1999;108(4):378–383. doi: 10.1177/000348949910800411. [DOI] [PubMed] [Google Scholar]
- 9.Miglioretti D.L., Johnson E., Williams A., et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E.Y., Strauss K.J., Tracy D.A., Bastos M., Zurakowski D., Boiselle P.M. Comparison of standard-dose and reduced-dose expiratory MDCT techniques for assessment of tracheomalacia in children. Acad Radiol. 2010;17(4):504–510. doi: 10.1016/j.acra.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Faust R.A., Remley K.B., Rimell F.L. Real-time, cine magnetic resonance imaging for evaluation of the pediatric airway. Laryngoscope. 2001;111(12):2187–2190. doi: 10.1097/00005537-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Bates A.J., Higano N.S., Hysinger E.B., et al. Quantitative assessment of regional dynamic airway collapse in neonates via retrospectively respiratory-gated 1H ultrashort echo time MRI. J Magn Reson Imaging. 2019;49(3):659–667. doi: 10.1002/jmri.26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higano N.S., Hahn A.D., Tkach J.A., et al. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med. 2017;77(3):1284–1295. doi: 10.1002/mrm.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn A.D., Higano N.S., Walkup L.L., et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. 2017;45(2):463–471. doi: 10.1002/jmri.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tkach J.A., Hillman N.H., Jobe A.H., et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol. 2012;42(11):1347–1356. doi: 10.1007/s00247-012-2444-9. [DOI] [PubMed] [Google Scholar]
- 16.Merhar S.L., Tkach J.A., Woods J.C., et al. Neonatal imaging using an on-site small footprint MR scanner. Pediatr Radiol. 2017;47(8):1001–1011. doi: 10.1007/s00247-017-3855-4. [DOI] [PubMed] [Google Scholar]
- 17.Tkach J.A., Merhar S.L., Kline-Fath B.M., et al. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. AJR Am J Roentgenol. 2014;202(1):W95–W105. doi: 10.2214/AJR.13.10613. [DOI] [PubMed] [Google Scholar]
- 18.Mathur A.M., Neil J.J., McKinstry R.C., Inder T.E. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38(3):260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 19.Windram J., Grosse-Wortmann L., Shariat M., Greer M.L., Crawford M.W., Yoo S.J. Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol. 2012;42(2):183–187. doi: 10.1007/s00247-011-2219-8. [DOI] [PubMed] [Google Scholar]
- 20.Yushkevich P.A., Piven J., Hazlett H.C., et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Bates A.J., Comerford A., Cetto R., Schroter R.C., Tolley N.S., Doorly D.J. Power loss mechanisms in pathological tracheas. J Biomech. 2016;49(11):2187–2192. doi: 10.1016/j.jbiomech.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Bates A.J., Cetto R., Doorly D.J., Schroter R.C., Tolley N.S., Comerford A. The effects of curvature and constriction on airflow and energy loss in pathological tracheas. Respir Physiol Neurobiol. 2016;234:69–78. doi: 10.1016/j.resp.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton N.J., Kanani M., Roebuck D.J., et al. Tissue-engineered tracheal replacement in a child: a 4-year follow-up study. Am J Transplant. 2015;15(10):2750–2757. doi: 10.1111/ajt.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccinelli M., Veneziani A., Steinman D.A., Remuzzi A., Antiga L. A framework for geometric analysis of vascular structures: application to cerebral aneurysms. IEEE Trans Med Imaging. 2009;28(8):1141–1155. doi: 10.1109/TMI.2009.2021652. [DOI] [PubMed] [Google Scholar]
- 25.Bates A.J., Schuh A., McConnell K., et al. A novel method to generate dynamic boundary conditions for airway CFD by mapping upper airway movement with non-rigid registration of dynamic and static MRI. Int J Numer Method Biomed Eng. 2018;34(12):e3144. doi: 10.1002/cnm.3144. [DOI] [PubMed] [Google Scholar]
- 26.Masters I.B., Zimmerman P.V., Pandeya N., Petsky H.L., Wilson S.B., Chang A.B. Quantified tracheobronchomalacia disorders and their clinical profiles in children. Chest. 2008;133(2):461–467. doi: 10.1378/chest.07-2283. [DOI] [PubMed] [Google Scholar]
- 27.Choi J., Dharmarajan H., Yu J., et al. Diagnostic flexible versus rigid bronchoscopy for the assessment of tracheomalacia in children. J Laryngol Otol. 2018:1–5. doi: 10.1017/S0022215118002050. [DOI] [PubMed] [Google Scholar]
- 28.Masters I.B., Eastburn M.M., Wootton R., et al. A new method for objective identification and measurement of airway lumen in paediatric flexible videobronchoscopy. Thorax. 2005;60(8):652–658. doi: 10.1136/thx.2004.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson R.W., Peters J.I., Shelledy D.C. Effects of fiberoptic bronchoscopy during mechanical ventilation in a lung model. Chest. 2000;118(3):824–831. doi: 10.1378/chest.118.3.824. [DOI] [PubMed] [Google Scholar]
- 30.Hsia D., DiBlasi R.M., Richardson P., Crotwell D., Debley J., Carter E. The effects of flexible bronchoscopy on mechanical ventilation in a pediatric lung model. Chest. 2009;135(1):33–40. doi: 10.1378/chest.08-1000. [DOI] [PubMed] [Google Scholar]
- 31.Masters I.B., Ware R.S., Zimmerman P.V., et al. Airway sizes and proportions in children quantified by a video-bronchoscopic technique. BMC Pulm Med. 2006;6:5. doi: 10.1186/1471-2466-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce M.S., Salotti J.A., Little M.P., et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen M.M., Cameron C.B., Duncan P.G. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70(2):160–167. doi: 10.1213/00000539-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Litman R.S., Weissend E.E., Shrier D.A., Ward D.S. Morphologic changes in the upper airway of children during awakening from propofol administration. Anesthesiology. 2002;96(3):607–611. doi: 10.1097/00000542-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Tkach J.A., Li Y., Pratt R.G., et al. Characterization of acoustic noise in a neonatal intensive care unit MRI system. Pediatr Radiol. 2014;44(8):1011–1019. doi: 10.1007/s00247-014-2909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagatta J.M., Hysinger E.B., Zaniletti I., et al. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J Pediatrics. 2018;203:218–224.e213. doi: 10.1016/j.jpeds.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer A.J., Singh S.B., Adam R.J., et al. Tracheomalacia is associated with lower FEV1 and Pseudomonas acquisition in children with CF. Pediatr Pulmonol. 2014;49(10):960–970. doi: 10.1002/ppul.22922. [DOI] [PMC free article] [PubMed] [Google Scholar]