Inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC), is an autoimmune digestive system disease (1). A recent cohort study using the U.K. Clinical Practice Research Datalink (CPRD) involving 7,231 dipeptidyl peptidase 4 inhibitor (DPP4i) users with 49 IBD cases indicated that new use of DPP4is over a median duration of 1.6 years was associated with IBD risk in patients with type 2 diabetes compared with other noninsulin antihyperglycemic drugs (hazard ratio 1.75, 95% CI 1.22, 2.49) (2). Current evidence regarding the effect of DPP4is on IBD risk is very limited. We thus performed a disproportionality analysis using the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) database, which contains all adverse events spontaneously reported to the Food and Drug Administration since 2004 (3).
We downloaded the FAERS data files from 2004 1st quarter to 2017 3rd quarter, used generic and trade names to identify DPP4is (sitagliptin, saxagliptin, linagliptin, alogliptin, and vildagliptin) and comparator drugs, and identified outcomes using Medical Dictionary for Regulatory Activities terms. The primary outcome was IBD (both CD and UC), and secondary outcomes were CD and UC, separately. The safety signal of DPP4is and IBD was assessed by reporting odds ratio (ROR) using two-by-two contingency tables (4). A signal was defined as an ROR of ≥2. The data were analyzed by SAS 9.4 software (SAS Institute, Cary, NC).
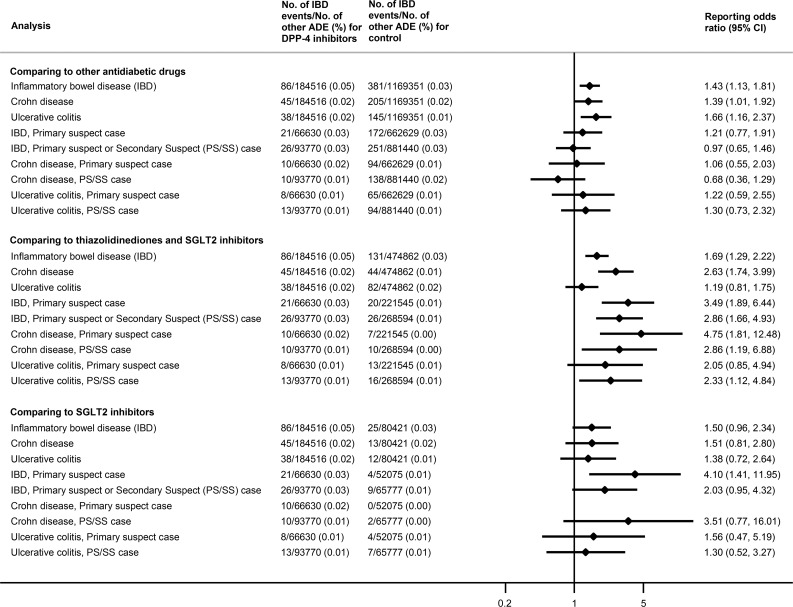
We conducted three comparisons (Fig. 1). First, we compared DPP4is to all other antidiabetic drugs, including metformin, sulfonylureas, thiazolidinediones, glucagon-like peptide 1 receptor agonists, sodium–glucose cotransporter 2 inhibitors (SGLT2is), insulin, α-glucosidase inhibitors, and glinides.
Figure 1.
Number of IBD events, other adverse drug events (ADE), and RORs in different drug comparisons.
Second, we compared DPP4is to two classes of therapeutic alternatives: thiazolidinediones (pioglitazone, rosiglitazone) and SGLT2is (canagliflozin, dapagliflozin, empagliflozin). These two classes were selected because they are used to treat similar stages of diabetes and have no known association with IBD risk.
Third, we compared DPP4is to SGLT2is only, as SGLT2is are a new class of antidiabetic drugs that may have more similar reporting rates to DPP4is.
For each comparison, we performed sensitivity analyses by restricting to events with drugs reported as 1) “primary suspect” (those drugs directly suspected of causing the adverse events when submitted in the case report) or as 2) “primary suspect” or “secondary suspect,” respectively.
A total of 86 DPP4i-associated IBD cases were extracted from FAERS through 30 September 2017, of which 45 and 38 were reported as CD and UC cases, respectively. The RORs (95% CIs) for IBD, CD, and UC were 1.43 (1.13, 1.81), 1.39 (1.01, 1.92), and 1.66 (1.16, 2.37), respectively, compared with all other antidiabetic drugs (Fig. 1). Sensitivity analyses restricted to primary suspect cases, or primary suspect and secondary suspect cases, moved RORs toward the null (95% CI crossed 1). When compared with thiazolidinediones and SGLT2is, the RORs for IBD, CD, and UC were 1.69 (1.29, 2.22), 2.63 (1.74, 3.99), and 1.19 (0.81, 1.75), respectively; the ROR for IBD was 3.49 (1.89, 6.44) when restricted to primary suspect cases. When compared with SGLT2is only, the ROR for IBD was 1.50 (0.96, 2.34). Overall, across the three comparisons, sensitivity analyses were consistent with the primary analysis but yielded lower RORs (compared with other antidiabetic drugs) or wider CIs (compared with therapeutic alternatives).
In this analysis of the FAERS database, we found a weak-to-moderate signal for IBD associated with DPP4i use when DPP4i was compared with therapeutic alternatives. Our FAERS analysis involving 184,516 DPP4i users with 86 IBD cases adds to existing evidence as we extend analyses with a comparison with therapeutic alternatives, which may help control for confounding bias. Notably, we observed a potential signal for IBD and CD, whereas Abrahami et al. (2) suggested an increased risk of IBD and UC. Preclinical studies on mouse models suggest that DPP4is may alleviate inflammatory disease and are protective against colitis (5). Some clinical studies indicate that the concentration of the DPP4 enzyme in serum may be inversely associated with IBD activity scores (5). More studies are needed to further explore the association and the underlying mechanism.
Our study has limitations. We were unable to fully control for confounding as the FAERS database is prone to reporting bias and channeling bias and cannot be used to calculate incidence. Additionally, cases were often missing data on comorbidities, previous treatment, or the duration of treatment. Finally, although our analysis suggests a weak-to-moderate signal of IBD risk for DPP4i use, the elevated RORs are not precise due to a limited number of events. Future large population-based studies are needed to assess the risk of this rare event.
Article Information
Funding and Duality of Interest. W.L. receives Student-Innovation funding (69003Y0052) from the School of Pharmaceutical Sciences, Peking University. J.B.B.’s contracted consulting fees are paid to the University of North Carolina by Adocia, AstraZeneca, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Senseonics, and vTv Therapeutics. J.B.B. receives grant support from Novo Nordisk, Sanofi, and vTv Therapeutics, is a consultant to Neurimmune AG, holds stock options in Mellitus Health, PhaseBio, and Stability Health, and is supported by a grant from the National Institutes of Health (UL1-TR-002489). T.S. receives investigator-initiated research funding and support from the National Institute on Aging as a principal investigator (R01-AG-056479) and from National Institutes of Health as co-investigator (R01-CA-174453, R01-HL-118255, and R21-HD-080214). He also receives salary support as co-Director of the Biostatistics, Epidemiology, and Research Design (BERD), North Carolina Translational and Clinical Sciences Institute (UL1-TR-002489) and from the Center for Pharmacoepidemiology (current members are GlaxoSmithKline, UCB BioSciences, Merck, and Shire). He also receives research support from pharmaceutical companies (Amgen, AstraZeneca, and Novo Nordisk) to the Department of Epidemiology, University of North Carolina. He does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.W. conceived the study and performed statistical analysis. T.W. and W.L. wrote the first draft of the paper. T.W., W.L., D.L., H.T., J.Y.Y., J.B.B., and T.S. approved the final version of the manuscript. T.W., H.T., J.Y.Y., J.B.B., and T.S. were involved in data review and interpretation. T.W., J.Y.Y., J.B.B., and T.S. contributed to critical revision of the manuscript for important intellectual content. T.W., J.B.B., and T.S. designed the study. W.L. and D.L. developed the protocol. T.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the International Society for Pharmacoepidemiology’s 11th Asian Conference on Pharmacoepidemiology, Xi’an, China, 27–29 October 2018.
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778 [DOI] [PubMed] [Google Scholar]
- 2.Abrahami D, Douros A, Yin H, et al. Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. BMJ 2018;360:k872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. Questions and answers on FDA's Adverse Event Reporting System (FAERS). Availablefrom https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed 10 April 2014
- 4.Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther 2007;82:157–166 [DOI] [PubMed] [Google Scholar]
- 5.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009;30:600–607 [DOI] [PubMed] [Google Scholar]