Abstract
OBJECTIVE
Impaired insulin sensitivity is associated with hyperfiltration (i.e., elevated glomerular filtration rate [GFR]) in adolescents with type 2 diabetes (T2D) and adults with prediabetes. Yet, these relationships are based on studies that relied on estimated GFR (eGFR), estimates of insulin sensitivity, or both. We aimed to verify the relationship between insulin sensitivity and renal hemodynamic function by gold standard methods in adults with T2D.
RESEARCH DESIGN AND METHODS
Insulin sensitivity was assessed by hyperinsulinemic-euglycemic clamp (M value) (glucose infusion rate in mg/kglean/min) and renal hemodynamic function by urinary inulin (GFR) and para-aminohippuric acid (effective renal plasma flow [ERPF]) clearances in participants with T2D without overt kidney disease. Filtration fraction (FF) (GFR/ERPF) was calculated. Relationships between insulin sensitivity and renal hemodynamic parameters were examined by multivariable linear regression. Renal hemodynamic parameters were examined across tertiles of M values.
RESULTS
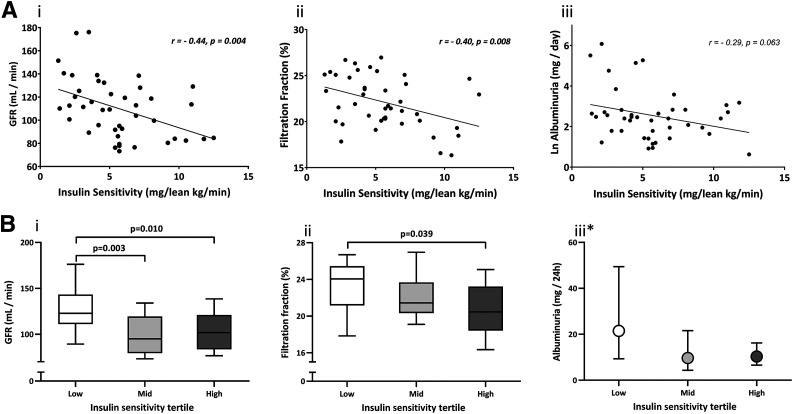
We tested 44 adults with T2D, of whom 77% were male, with mean ± SD age 63 ± 7 years, BMI 31.2 ± 4.0 kg/m2, and HbA1c 7.4 ± 0.6%. Average GFR was 110 ± 26 mL/min, with an FF of 22.1 ± 2.8% and median 24-h urinary albumin excretion of 11.3 mg (interquartile range 5.8–17.0). Average M value was 5.6 ± 2.9 mg/kglean/min. Insulin sensitivity inversely correlated with GFR (r = −0.44, P < 0.01) and FF (r = −0.40, P < 0.01), and these associations remained significant after multivariable adjustments for age, sex, renin-angiotensin system inhibitor use, and HbA1c. In addition, GFR, FF, and urinary albumin excretion were highest in the participants in the lowest M value tertile.
CONCLUSIONS
For the first time, we demonstrate that impaired insulin sensitivity is associated with intrarenal hemodynamic dysfunction by gold standard techniques in adults with T2D treated with metformin monotherapy.
Introduction
Approximately 40% of people with type 2 diabetes (T2D) will develop diabetic kidney disease (DKD), clinically manifested by impaired glomerular filtration rate (GFR) and/or increased urinary protein excretion (1). Glomerular hyperfiltration, a supraphysiological increase in GFR, is thought to contribute to progressive renal damage by increasing glomerular pressure and shear stress (2,3). It is typically defined as increased whole-kidney GFR, which is a function of the number of nephrons and single-nephron GFR, and can precede overt DKD or even diabetes onset (2,4). However, whole-kidney GFR may mask single-nephron hyperfiltration, as a person with low nephron numbers is likely to hyperfiltrate at the single-nephron level, while whole-kidney GFR is normal or low (2,5). This is particularly problematic in adults >30 years of age who experience age-related nephron mass decline. Increased filtration fraction (FF), a product of GFR divided by effective renal plasma flow (ERPF), has been proposed to be a more suitable marker to define hyperfiltration in this population (2,5).
T2D is a complex metabolic disorder that is characterized by hyperglycemia due to impaired insulin sensitivity and progressive β-cell failure, factors that have been causally implicated in the pathogenesis of DKD (6,7). Interestingly, a cross-sectional study in adolescents with youth-onset T2D indicated that measured insulin sensitivity is an important determinant of renal health—potentially even stronger than glycemic, blood pressure, and lipemic control (8). Impaired estimated insulin sensitivity has also been associated with elevated urinary albumin excretion and glomerular hyperfiltration in longitudinal studies in adolescents with youth-onset T2D (9) and adults with prediabetes (10).
This relationship between insulin sensitivity and renal hemodynamic function has not been directly studied in adults with T2D. In addition, previous studies in pediatric populations and adult populations without diabetes have relied on estimates of insulin sensitivity (10), estimated GFR (eGFR) (8), or both (9) to examine the relationship between insulin sensitivity and renal hemodynamic function. To our knowledge, there are no studies that have examined the relationship between directly measured insulin sensitivity and renal hemodynamic function, including FF. Such investigations are important, as 1) measured GFR is accurate at the normal-to-elevated range, while eGFR is not; 2) elevated FF is more likely to capture hyperfiltration in adults; and 3) estimating insulin sensitivity may be inaccurate in relatively small studies. Accordingly, the objective of this analysis was to define the relationships between insulin sensitivity and parameters of renal hemodynamic function by gold standard methods in adults with T2D on metformin monotherapy. We hypothesized that insulin resistance would relate to renal hemodynamic dysfunction including increased GFR and FF in adults with T2D without overt DKD.
Research Design and Methods
This cross-sectional study was performed at the Amsterdam University Medical Centers, location VUmc, and used the baseline data from the RED trial, a randomized clinical trial studying the renoprotective effects of dapagliflozin in people with T2D (11). The study protocol, protocol amendments, and any other protocol-specific documents were reviewed and approved by local authorities and the ethics review board of the Amsterdam University Medical Centers, location VUmc. The study complied with the Declaration of Helsinki and Good Clinical Practice guidelines and was registered at the ClinicalTrials.gov (NCT02682563).
Study Participants
Participants were recruited from our database and by advertisements in local newspapers. Eligible participants were men or postmenopausal women, aged 35–75 years, diagnosed with T2D with an HbA1c of 6.5–9.0% (48–75 mmol/mol) and a BMI >25 kg/m2. Participants were treated with metformin as the only glucose-lowering agent (stable dose for ≥3 months). Use of other antihyperglycemic medication was not allowed. Blood pressure was under control (i.e., <140/90 mmHg), and macroalbuminuria (i.e., albumin-to-creatinine ratio >300 mg/g) was not allowed; in case of previously diagnosed hypertension and/or albuminuria, treatment included at least a stable dose of a renin-angiotensin system (RAS) inhibitor for ≥3 months. Exclusion criteria included a history of unstable or rapidly progressing renal or malignant disease (excluding basal cell carcinoma), eGFR <60 mL/min/1.73 m2, urinary retention (bladder ultrasonography at screening visit was performed to objectively assess bladder emptying), (re)current urinary tract or genital infection, and the use of nonsteroidal anti-inflammatory drugs or diuretics that could not be stopped 3 months prior to the study day. Written informed consent was obtained from all participants before any trial-related activities.
Study Protocol, Measurements, and Calculations
The week before testing, the participants adhered to a controlled sodium (9–12 g/day) and protein (1.5–2.0 g/kg/day) diet in order to minimize variation in renal physiology due to salt and protein intake. After an overnight fast, blood and urine were obtained for fasting outcome variables. Then the renal tests commenced by a weight-calculated bolus infusion of 22.5 mg/kg inulin (Inutest; Fresenius Kabi Austria, Graz, Austria) and 3 mg/kg para-aminohippuric acid (PAH) (4-Aminohippuric Acid Solution 20%; Bachem Distribution Services, Weil am Rhein, Germany) in 10 min after which infusion continued at a lower rate (675 and 320 mg/h, respectively) for the remainder of the day. The participants remained fasted until the study day ended but were hydrated with tap water to secure urine collection. After 33 participants completed the trial, inulin was retracted from the market due to anaphylactic reactions in another center. Since iohexol and inulin have a similar pharmacokinetic profile, and clearances correlate almost perfectly (r = 0.986) (12), we subsequently used iohexol in our protocol to measure GFR in the remaining 11 participants (bolus 36 mg/kg in 10 min, followed by 906 mg/h). Separate analysis in participants tested with inulin and iohexol clearance yielded equivalent results (data not shown). We did not use different substances within a participant.
After 2 h of bed rest, the hyperinsulinemic-euglycemic clamp was initiated, with insulin (NovoRapid, Novo Nordisk, Denmark) infusion at 40 mU/min ⋅ m2 while plasma glucose was maintained at 5.0 mmol/L by variable glucose 20% infusion. After 90 min of equilibration, urine was collected by spontaneous voiding for two 45-min periods. Supplementary Fig. 1 provides an overview of the study protocol used for this study; the full protocol has previously been published (11). GFR and ERPF were determined from inulin/iohexol and PAH clearances, respectively, based on timed blood and urine sampling. Calculations for measured GFR, ERPF, filtration fraction (FF), effective renal blood flow (ERBF), and renal vascular resistance (RVR) have previously been described (13,14). FF was calculated by dividing measured GFR by ERPF, ERBF by dividing ERPF by (1 − hematocrit), and RVR by dividing mean arterial pressure (MAP) by ERBF.
Insulin sensitivity was measured by glucose infusion rate (corrected for urinary glucose excretion) during the last 30 min of the clamp and corrected for lean body mass (M value) (mg/kglean/min). We assessed lean body mass by single-frequency bioelectrical impedance analyzer (Maltron BF-906; Maltron International, Essex, U.K.). The ISI (M/I ratio) was calculated by dividing M value by the mean of insulin concentration during the same 30 min. The M/I ratio represents the amount of glucose metabolized per unit of plasma insulin.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), MAP, and heart rate were determined during all three phases at the brachial artery of the nondominant arm by an automated oscillometric device (DINAMAP; GE Healthcare, Little Chalfont, U.K.). Measurements were performed in triplicate at 1- to 2-min intervals using the mean of the last two measurements.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 24; IBM Statistics). The results are expressed as mean ± SD when variables were normally distributed or median with interquartile range (IQR) when they were not. Categorical variables are shown as n with %. Correlations between two variables were assessed using Pearson correlations (all normally distributed). Multivariable linear regression models were built to examine the relationship between insulin sensitivity and renal hemodynamics correcting for traditional risk factors; β and SE are shown. Participants were also stratified into tertiles based on insulin sensitivity (M value for lean body mass), and renal hemodynamics were examined across the tertiles. One-way ANOVA was used to assess whether there were differences between tertiles and, when significant, individual tertiles were compared with the Bonferroni post hoc test. Statistical significance was defined as a two-sided P value of <0.05.
Results
Patient Recruitment
Between July 2016 and March 2018, 75 participants were screened, of whom 50 were found eligible. Of these, five people withdrew consent before testing and one was excluded from the analyses due to insufficient bladder emptying on the test day (Fig. 2). One participant was excluded from analyses because of an M value >3 SDs from the mean, thus resulting in 43 included subjects in the current analyses.
Insulin Sensitivity and Renal Hemodynamic Function
The mean glucose infusion rate needed to maintain plasma glucose at 5 mmol/L (90 mg/dL) during the hyperinsulinemic-euglycemic clamp was 5.6 ± 2.9 mg/kglean/min (M value). Average insulin concentration during the last 30 min of the clamp was 542 ± 132 pmol/L. The mean ± SD M/I ratio was 0.011 ± 0.007 mg/kglean · min · (pmol/L)−1. Mean GFR and ERPF were 110 ± 26 mL/min and 500 ± 111 mL/min, respectively. This resulted in an FF of 22.1 ± 2.8%. Lastly, mean ERBF was 848 ± 206 mL/min and mean RVR was 0.13 ± 0.03 mmHg/L/min (Table 1).
Table 1.
Participant characteristics (n = 43)
| Clinical characteristics | |
| Age, years | 63 ± 7 |
| Male, n (%) | 33 (77) |
| Current smoker, n (%) | 4 (9) |
| BMI, kg/m2 | 31.2 ± 4.0 |
| Fasting plasma glucose, mg/dL | 162 ± 28 |
| Fasting plasma glucose, mmol/L | 9.0 ± 1.5 |
| HbA1c, % | 7.4 ± 0.6 |
| HbA1c, mmol/mol | 57 ± 7 |
| Diabetes duration, years | 10.4 ± 5.8 |
| Fasting systemic hemodynamic parameters | |
| SBP, mmHg | 135 ± 13 |
| DBP, mmHg | 83 ± 6 |
| MAP, mmHg | 101 ± 7 |
| Heart rate, bpm | 67 ± 11 |
| Renal hemodynamic parameters | |
| GFR, mL/min | 110 ± 26 |
| ERPF, mL/min | 500 ± 111 |
| FF, % | 22.1 ± 2.8 |
| ERBF, mL/min | 848 ± 206 |
| RVR, mmHg/L/min | 0.13 ± 0.03 |
| Albuminuria, mg/day | 11.3 (5.8–17.0) |
| Insulin sensitivity | |
| M value, mg/kglean · min | 5.6 ± 2.9 |
| M/I ratio, mg/kglean · min · (pmol/L)−1 | 0.011 ± 0.007 |
| Medication | |
| Metformin dose, mg | 1,500 (1,000–2,000) |
| Statin use, n (%) | 30 (70) |
| Anticoagulant medication use, n (%) | 6 (14) |
| RAS inhibitor use, n (%) | 32 (74) |
Values are expressed as mean ± SD, n (%), or median (IQR).
Insulin sensitivity was inversely associated with GFR (r = −0.44, P = 0.004) and FF (r = −0.40, P = 0.008). The negative relationship with urinary albumin excretion did not reach statistical significance (Table 2 and Fig. 1A). The relationship between insulin sensitivity and GFR (β = −3.5 ± 1.0, P = 0.001) and FF (β = 20.36 ± 0.14, P = 0.013) remained significant after adjustments for age, sex (male/female), RAS inhibitor use (yes/no), and HbA1c (Table 2).
Table 2.
Correlation between insulin sensitivity (lean M value) and renal hemodynamic function
| Univariable analyses | ||
|---|---|---|
| r | P | |
| GFR, mL/min | −0.44 | 0.004 |
| ERPF, mL/min | −0.22 | 0.162 |
| FF, % | −0.40 | 0.008 |
| ERBF, mL/min | −0.27 | 0.076 |
| RVR, mmHg/mL/min | 0.26 | 0.087 |
| ln albuminuria, mg/day | −0.29 | 0.063 |
| Multivariable analyses with GFR (mL/min) | ||
| Model 1 (adjustment for age, sex, RASi use, and HbA1c) | Model 2 (model 1 adjustments + M value) | |
| Age, years | β ± SE = −1.12 ± 0.50, P = 0.032 | β ± SE = −1.13 ± 0.44, P = 0.015 |
| Sex, male/female | β ± SE = −22.05 ± 8.25, P = 0.011 | β ± SE = −20.00 ± 7.26, P = 0.009 |
| RAS inhibitor, yes/no | β ± SE = 16.91 ± 7.95, P = 0.040 | β ± SE = 16.47 ± 6.97, P = 0.024 |
| HbA1c, mmol/mol | β ± SE = −0.15 ± 0.49, P = 0.765 | β ± SE = −0.26 ± 0.43, P = 0.551 |
| M value, mg/kglean · min | — | β ± SE = −3.50 ± 0.99, P = 0.001 |
| R2 = 0.38/P = 0.001 | R2 = 0.53/P < 0.001 | |
| Multivariable analyses with FF (mL/min) | ||
| Model 1 (adjustment for age, sex, RASi use, and HbA1c) | Model 2 (model 1 adjustments + M value) | |
| Age, years | β ± SE = 0.03 ± 0.07, P = 0.701 | β ± SE = 0.02 ± 0.6, P = 0.693 |
| Sex, male/female | β ± SE = −2.27 ± 1.07, P = 0.040 | β ± SE = −2.06 ± 1.00, P = 0.046 |
| RASi, yes/no | β ± SE = 0.15 ± 1.03, P = 0.885 | β ± SE = 0.11 ± 0.96, P = 0.913 |
| HbA1c, mmol/mol | β ± SE = 0.01 ± 0.06, P = 0.880 | β ± SE = −0.00 ± 0.06, P = 0.977 |
| M value, mg/kglean · min | — | β ± SE = −0.36 ± 0.14, P = 0.013 |
| R2 = 0.12/P = 0.300 | R2 = 0.26/P = 0.045 | |
Univariable analysis with Pearson correlation is shown. Also shown is multivariable approach to assess whether GFR and FF are still significantly associated with insulin sensitivity after correction for clinical characteristics. Model 1 included age, sex, RAS inhibitor (RASi) use, and HbA1c. In model 2, M value was added. Significant correlations are highlighted in boldface type. ERPF, effective renal plasma flow.
Figure 1.
A: Correlations between insulin sensitivity (lean M value) and renal measures. i: GFR. ii: FF. iii: log albuminuria. Pearson correlations are shown. B: Tertiles of insulin sensitivity (lean M value) compared with regard to renal function. i: GFR. ii: FF. iii: Albuminuria. Box and whisker plots are shown in i and ii, and geometric mean and 95% CI are shown in iii. The P values are calculated by Bonferroni post hoc test after significant one-way ANOVA. *ANOVA nonsignificant.
As a result of stratification, median M value was clearly different between the low, mid, and high insulin sensitivity tertiles (Table 3). BMI was highest in the low and lowest in the high insulin sensitivity group, and the difference in BMI between the high and low tertiles reached significance. GFR was significantly higher in the low insulin sensitivity tertile compared with the two other tertiles (Table 3 and Fig. 1). This was also true for FF, although only the difference between the low and high tertile reached significance (Table 3 and Fig. 1). Lastly, although it was within normal range and nonsignificant, the low insulin sensitivity group did show the highest urinary albumin excretion. This tertile also included three people with microalbuminuria (30–300 mg/day) and one with macroalbuminuria (>300 mg/day) compared with two people with microalbuminuria in the mid and one in the high insulin sensitivity group. There were no differences for ERPF, ERBF, RVR, body weight, SBP, and MAP between the tertiles (Table 3).
Table 3.
Comparison between insulin sensitivity tertiles
| Low IS (n = 14) | Mid IS (n = 15) | High IS (n = 14) | P value | |||
|---|---|---|---|---|---|---|
| Low vs. mid | Mid vs. high | Low vs. high | ||||
| M value, mg/kglean/min | 2.6 ± 0.9 | 5.2 ± 0.6 | 9.0 ± 2.0 | <0.001 | <0.001 | <0.001 |
| BMI, kg/m2 | 33.3 ± 3.8 | 30.7 ± 3.9 | 29.5 ± 3.3 | n.s. | n.s. | 0.027 |
| SBP, mmHg* | 145 ± 14 | 141 ± 17 | 150 ± 18 | n.s. | n.s. | n.s. |
| DBP, mmHg | 89 ± 8 | 83 ± 8 | 82 ± 6.1 | n.s. | n.s. | n.s. |
| GFR, mL/min | 129 ± 26 | 100 ± 20 | 103 ± 21 | 0.003 | n.s. | 0.010 |
| ERPF, mL/min* | 551 ± 105 | 464 ± 108 | 488 ± 107 | n.s. | n.s. | n.s. |
| FF, % | 23.3 ± 2.7 | 22.3 ± 2.4 | 20.7 ± 2.8 | n.s. | n.s. | 0.039 |
| ERBF, mL/min* | 953 ± 193 | 786 ± 207 | 807 ± 189 | n.s. | n.s. | n.s. |
| RVR, mmHg/L/min* | 117 ± 24 | 139 ± 37 | 136 ± 34 | n.s. | n.s. | n.s. |
| Albuminuria, mg/day*# | 13.5 (9.0–64.5) | 8.0 (3.3–13.3) | 11.0 (6.5–18.1) | n.s. | n.s. | n.s. |
Values are expressed as mean ± SD or median (IQR) when nonparametric. Clinical characteristics and renal measures compared across tertiles of insulin sensitivity (IS) (lean M value). When one-way ANOVA was significant, individual tertiles were compared using the Bonferroni post hoc test. n.s., nonsignificant.
One-way ANOVA test nonsignificant, and individual tertiles were thus not compared.
Analyzed after ln-transformation. Significant differences indicated in boldface type.
Conclusions
This is the first study to investigate the relationship between insulin sensitivity and renal hemodynamics measured with gold standard methodologies in adults with T2D and preserved renal function. We observed strong inverse associations between insulin sensitivity and both GFR and FF. Additionally, the participants in the tertile with the least insulin sensitivity had the highest GFR, FF, and albumin excretion rate. These results suggest that impaired insulin sensitivity is associated with renal hemodynamic dysfunction, including hyperfiltration, and raise the possibility that insulin resistance contributes to the development of DKD in adult T2D.
Glomerular hyperfiltration is a recognized risk factor for the development and progression of DKD (2). Although hyperfiltration is classically defined as elevated whole-kidney GFR, the phenotype is subject to misclassification bias in adults with T2D, who likely hyperfiltrate at the single-nephron level despite having a normal or even reduced whole-kidney GFR due to functional nephron loss. Therefore, it has been proposed that increased FF is useful to assess hyperfiltration at the single-nephron level (2). Evidence to support the importance of (single-nephron) hyperfiltration as a risk factor for progression of DKD is derived from pharmacological interventions that modulate renal hemodynamics. As an example, RAS blockers have been described to improve DKD progression by lowering glomerular pressure and hyperfiltration through postglomerular vasodilatation (2,15,16).
Although the mechanisms of glomerular hyperfiltration are not entirely clear, it has been hypothesized that hyperfiltration is the result of obesity and diabetes-induced changes in structural and hemodynamic factors that determine GFR (2). According to the vascular theory, an imbalance of vasoactive factors that control pre- and postglomerular arteriolar tone causes preglomerular vasodilatation and postglomerular vasoconstriction leading to glomerular hypertension and hyperfiltration (2,5). Impaired insulin signaling due to insulin resistance for instance reduces nitric oxide (NO) production and NO-dependent vascular relaxation (17). Insulin also promotes sodium retention, and the tubular theory proposes that a reduced delivery of sodium and chloride to the macula densa dilates the preglomerular arteriole by inhibition of tubuloglomerular feedback to induce hyperfiltration (2). Lastly, it has been suggested that impaired insulin sensitivity diminishes mitochondrial function leading to a loss of nephrons possibly related to hypoxic stress and as a result of which the remaining nephrons hyperfilter to sustain whole-kidney function (18,19). Collectively, these mechanisms can lead to altered renal hemodynamics that may eventually cause hyperfiltration. Subsequently, glomerular hyperfiltration can inflict glomerular damage, albuminuria, and hypoxia, which could further drive renal impairment (20).
Previous reports have related insulin sensitivity to hyperfiltration and renal health (9,10,21). However, these studies were based on estimated GFR, estimated insulin sensitivity, or both, whereas our study directly measured both insulin sensitivity and renal hemodynamics. Filtration fraction can only be calculated in humans by directly measuring GFR and ERPF. Our findings confirm those of a study in obese people without diabetes in whom eGFR was inversely related to clamp-measured insulin sensitivity (22). Interestingly, insulin sensitivity was more closely related to eGFR than BMI or blood pressure in that study (22). Furthermore, Tsuda et al. (10) recently demonstrated that FF and estimated glomerular pressure are higher in individuals with prediabetes than in healthy individuals. Together, these findings suggest that insulin sensitivity may contribute to hyperfiltration in early (pre)diabetes. In contrast, insulin-mediated increments in GFR were blunted by reduced insulin sensitivity in healthy individuals (23), indicating that this relationship may differ with varying degrees of insulin sensitivity. Insulin sensitivity also tended to be associated with albuminuria in our trial, and we found that albuminuria was highest in the low insulin sensitivity group, confirming findings previously demonstrated in prediabetes (10). This reinforces the notion that insulin sensitivity may be implicated in DKD development and progression.
There is some controversy on how to express whole-body insulin sensitivity (21,23–25). We evaluated different measures (e.g., whole-body M value, M value corrected for lean body mass, and M/I ratio). The results were similar when different measures were used, and we chose to use the M value corrected for lean body mass. In multivariable analysis, we demonstrated that insulin sensitivity remained inversely associated with FF after multivariable adjustments for age, sex, and SBP. We did not adjust for BMI, since insulin sensitivity was already corrected for lean body mass, which would introduce collinearity (10,26).
The current study has some strengths and limitations. To our knowledge, we are the first to measure both insulin sensitivity and renal hemodynamics with gold standard methods in the same study. Also, our study was performed in adults with T2D, while previous work has focused on prediabetes, youth-onset T2D, or healthy people. Due to the comprehensive and cumbersome study procedures, our sample size is relatively small, which does not allow subgroup analyses and limits stratification by GFR categories, i.e., hyperfiltration, normofiltration, and impaired GFR. To increase homogeneity, we made our eligibility criteria relatively strict, which limits the generalizability of our findings to patients with different characteristics. For instance, only metformin was allowed as a glucose-lowering agent, and eGFR had to be >60 mL/min/1.73 m2. We therefore cannot translate our findings to patients with poorly controlled T2D requiring insulin and polypharmacy and/or overt kidney disease. Also, despite the fact that the albuminuria data are interesting, they have to be interpreted with caution, since only seven participants had abnormal urinary albumin excretion. Lastly, this study is limited by its cross-sectional design. We are therefore not able to imply causality between insulin sensitivity and hyperfiltration. Follow-up data would be important, since both insulin resistance and DKD progress over time.
Since we demonstrated that impaired insulin sensitivity is associated with renal hemodynamic dysfunction in adults with T2D, it is interesting that the insulin-sensitizing agent rosiglitazone was found to reduce glomerular hyperfiltration and urinary albumin excretion in patients with early T2D with microalbuminuria (27). Rosiglitazone is assumed to reduce urinary albumin excretion by improving glomerular endothelial function, thereby reducing leakiness of the endothelium (27). Bariatric surgery also improves insulin sensitivity and renal outcomes, including glomerular hyperfiltration, in patients with and without diabetes (28,29). Longitudinal studies that delineate whether improvements in insulin sensitivity drive renoprotection by these interventions in the setting of T2D are warranted. Moreover, we measured renal hemodynamic function during a hyperinsulinemic-euglycemic clamp while participants were fasted for >12 h. In modern society, we are, however, more often in the postprandial than in the fasted state. We know that a protein load exacerbates glomerular hyperfiltration in T2D, while it does not affect GFR or ERPF in healthy people (30). Future studies should investigate whether insulin sensitivity is a determinant of the susceptibility to this protein-induced hyperfiltration. If this is true, greater dietary protein restrictions could help improve renal outcome in less insulin-sensitive people with T2D.
In conclusion, we demonstrate that insulin resistance measured by the hyperinsulinemic-euglycemic clamp technique relates to renal hemodynamic function measured by inulin and PAH clearance in adults with T2D and preserved kidney function.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the help of the assistants and technicians who were indispensable in the process of data collection: Jeanette Boerop, Ingrid Knufman, Renée de Meijer, and Sandra Gassman (Diabetes Center, Department of Internal Medicine, Amsterdam University Medical Centers, location VUmc, Amsterdam, the Netherlands); Adele Dijk and Nel Willekes-Koolschijn (Department of Nephrology and Hypertension, University Medical Center, Utrecht, the Netherlands); and Hiltjo Kuiper (Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, the Netherlands). The authors are very grateful for the patients who volunteered to participate in the study.
Funding. P.B. receives salary and research support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (K23 DK116720-01), in addition to research support from JDRF (JDRF 2-SRA-2019-845-S-B, 2-SRA-2018-627-M-B), NIDDK/DiaComp, Thrasher Research Fund, International Society of Pediatric and Adolescent Diabetes, Colorado Clinical & Translational Sciences Institute, and Center for Women’s Health Research at the University of Colorado.
Duality of Interest. This trial was funded by AstraZeneca as an investigator-initiated study. M.H.A.M. is a consultant and speaker for Eli Lilly & Company, Sanofi, and Novo Nordisk, with all honoraria paid to his employer (Amsterdam University Medical Center, location VUmc). M.N. received an unrestricted investigator-initiated grant from AstraZeneca on sodium–glucose cotransporter 2 inhibitor and lipid fluxes. P.B. has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk, and Horizon Pharma. P.B. serves on the advisory board of XORTX Therapeutics. D.H.v.R. has acted as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Novo Nordisk, Sanofi, and AstraZeneca and has received research operating funds from Boehringer Ingelheim–Lilly Diabetes Alliance, AstraZeneca, Merck, and Novo Nordisk, with all honoraria paid to his employer (Amsterdam University Medical Center, location VUmc). No other potential conflicts of interest relevant to this article were reported.
The funder had no role in the study design, the analyses or interpretation of the data, or drafting of the manuscript. The funder had no role in the decision to submit this manuscript for publication.
Author Contributions. E.J.M.v.B. and D.R. performed statistical analysis. E.J.M.v.B., D.R., P.B., and D.H.v.R. wrote the first draft of the manuscript, and the submitted version was approved by all authors. E.J.M.v.B., M.H.A.M., and M.J.B.v.B. were involved in sample collection and/or analysis. E.J.M.v.B., M.H.A.M., M.H.H.K., M.N., J.A.J., and D.H.v.R. designed and set up the trial. E.J.M.v.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02682563, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1651/-/DC1.
References
- 1.Tuttle KR, Bakris GL, Bilous RW, et al. . Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonneijck L, Muskiet MH, Smits MM, et al. . Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017;28:1023–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melsom T, Nair V, Schei J, et al. . Correlation between baseline GFR and subsequent change in GFR in Norwegian adults without diabetes and in Pima Indians. Am J Kidney Dis 2019;73:777–785 [DOI] [PubMed] [Google Scholar]
- 4.Melsom T, Mathisen UD, Ingebretsen OC, et al. . Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 2011;34:1546–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012;8:293–300 [DOI] [PubMed] [Google Scholar]
- 6.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon) 2014;42:698–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32 (Suppl. 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornstad P, Maahs DM, Cherney DZ, et al. . Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care 2014;37:3033–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornstad P, Nehus E, El Ghormli L, et al.; TODAY Study Group . Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the TODAY clinical trial. Am J Kidney Dis 2018;71:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuda A, Ishimura E, Uedono H, et al. . Association of albuminuria with intraglomerular hydrostatic pressure and insulin resistance in subjects with impaired fasting glucose and/or impaired glucose tolerance. Diabetes Care 2018;41:2414–2420 [DOI] [PubMed] [Google Scholar]
- 11.van bommel EJM, van Baar MJB, Tonneijck L, et al. . Renal hemodynamic effects of SGLT2 inhibitor dapagliflozin compared with sulfonylurea gliclazide in metformin-treated people with type 2 diabetes: a 12-week, randomized, doubleblind trial (RED). Kidney Int. 10 October 2019 [Epub ahead of print]. DOI: 10.1016/j.kint.2019.09.013
- 12.Brown SC, O’Reilly PH. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol 1991;146:675–679 [DOI] [PubMed] [Google Scholar]
- 13.Tonneijck L, Smits MM, Muskiet MH, et al. . Renal effects of DPP-4 inhibitor sitagliptin or GLP-1 receptor agonist liraglutide in overweight patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2016;39:2042–2050 [DOI] [PubMed] [Google Scholar]
- 14.Tonneijck L, Muskiet MHA, Smits MM, et al. . Postprandial renal haemodynamic effect of lixisenatide vs once-daily insulin-glulisine in patients with type 2 diabetes on insulin-glargine: an 8-week, randomised, open-label trial. Diabetes Obes Metab 2017;19:1669–1680 [DOI] [PubMed] [Google Scholar]
- 15.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest 1986;77:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtkamp FA, de Zeeuw D, Thomas MC, et al. . An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011;80:282–287 [DOI] [PubMed] [Google Scholar]
- 17.Whaley-Connell A, Sowers JR. Insulin resistance in kidney disease: is there a distinct role separate from that of diabetes or obesity? Cardiorenal Med 2017;8:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 2015;4:R1–R15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Che R, Yuan Y, Huang S, Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 2014;306:F367–F378 [DOI] [PubMed] [Google Scholar]
- 20.De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant 2013;28:29–36 [DOI] [PubMed] [Google Scholar]
- 21.Bjornstad P, Maahs DM, Cherney DZ, et al. . Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care 2014;37:3033–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naderpoor N, Lyons JG, Mousa A, et al. . Higher glomerular filtration rate is related to insulin resistance but not to obesity in a predominantly obese non-diabetic cohort. Sci Rep 2017;7:45522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ter Maaten JC, Bakker SJ, Serné EH, ter Wee PM, Donker AJ, Gans RO. Insulin’s acute effects on glomerular filtration rate correlate with insulin sensitivity whereas insulin’s acute effects on proximal tubular sodium reabsorption correlation with salt sensitivity in normal subjects. Nephrol Dial Transplant 1999;14:2357–2363 [DOI] [PubMed] [Google Scholar]
- 24.Voytovich MH, Simonsen C, Jenssen T, Hjelmesaeth J, Åsberg A, Hartmann A. Short-term treatment with rosiglitazone improves glucose tolerance, insulin sensitivity and endothelial function in renal transplant recipients. Nephrol Dial Transplant 2005;20:413–418 [DOI] [PubMed] [Google Scholar]
- 25.Pratt SE, Geor RJ, McCutcheon LJ. Repeatability of 2 methods for assessment of insulin sensitivity and glucose dynamics in horses. J Vet Intern Med 2005;19:883–888 [DOI] [PubMed] [Google Scholar]
- 26.Cheng YH, Tsao YC, Tzeng IS, et al. . Body mass index and waist circumference are better predictors of insulin resistance than total body fat percentage in middle-aged and elderly Taiwanese. Medicine (Baltimore) 2017;96:e8126journal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pistrosch F, Herbrig K, Kindel B, Passauer J, Fischer S, Gross P. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes 2005;54:2206–2211 [DOI] [PubMed] [Google Scholar]
- 28.Chang AR, Chen Y, Still C, et al. . Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int 2016;90:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 2003;14:1480–1486 [DOI] [PubMed] [Google Scholar]
- 30.Tuttle KR, Bruton JL. Effect of insulin therapy on renal hemodynamic response to amino acids and renal hypertrophy in non-insulin-dependent diabetes. Kidney Int 1992;42:167–173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.