A recent retrospective cohort study found that new use of dipeptidyl peptidase 4 inhibitors (DPP4i) of 1.6 years was associated with an increased risk of inflammatory bowel disease (IBD) compared with other antidiabetes medications (hazard ratio 1.75 (95% CI 1.22, 2.49) (1). We previously demonstrated a weak association in an analysis of 86 prevalent and incident cases of IBD among DPP4i users using the U.S. Food and Drug Administration's Adverse Event Reporting System (2). In contrast, a cohort study suggested DPP4i initiation reduces risk of autoimmune diseases including IBD (3). Because the link between DPP4i and IBD remains uncertain, we performed a meta-analysis of randomized controlled trials (RCTs) to evaluate this potential association among patients with type 2 diabetes (T2D).
This meta-analysis is registered with the international prospective register of systematic reviews (PROSPERO) (no. CRD42018095206). We systematically searched PubMed, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from inception to 28 April 2018 to identify DPP4i trials in T2D patients that explicitly reported IBD events. The large-scale cardiovascular trial for linagliptin (CARMELINA trial [4]) was published 7 months after our search; we therefore included this study. Two reviewers independently performed study selection, data extraction, and quality assessment. The primary outcome was IBD, including both Crohn disease (CD) and ulcerative colitis (UC). IBD events were strictly identified using preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA version 21.0). We examined a secondary end point that included unspecified colitis in addition to CD and UC cases. Quality assessment was assessed by the Cochrane risk of bias tool.
We estimated relative risk (RR) with 95% CI using random-effects models. Statistical heterogeneity between studies was measured using the I2 statistic and Cochran Q test. We conducted sensitivity analyses using person-years as the denominator and number of events as the numerator to test the robustness of our primary analysis and calculated the number needed to harm for the primary outcome. All analyses were performed using Stata 14.
Of the 4,669 studies retrieved from the electronic databases, 13 eligible RCTs (8 placebo-controlled and 5 active-controlled) involving 54,719 patients and 39 events were identified. The mean age, diabetes duration, baseline HbA1c, and follow-up were 60.9 years, 9.3 years, 7.8% (62 mmol/mol), and 1.5 years, respectively. The risk of bias for included trials was judged as high because IBD was not a predefined outcome.
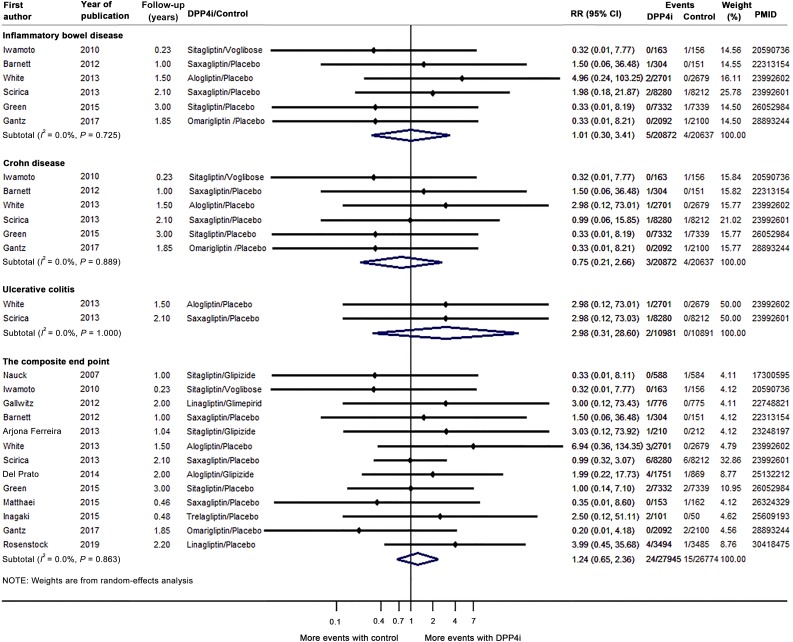
Overall, IBD risk was similar between DPP4i users and control subjects (RR 1.01 [95% CI 0.30, 3.41]) (Fig. 1). DPP4i use may reduce CD risk (RR 0.75 [0.21, 2.66]) and increase UC risk (RR 2.98 [0.31, 28.60]). For the composite end point, the RR was 1.24 (0.65, 2.36). No evidence for statistical heterogeneity across studies was observed (I2 = 0.0%, P > 0.05). The sensitivity analysis was consistent with primary analysis. The number needed to harm for IBD was 21,868 over an average of 2.3 years.
Figure 1.
Results of the meta-analysis of DPP4i use on the risk of IBD. The results of the CARMELINA randomized clinical trial were published in November 2018 (4). We incorporated data from this large trial, and our final analysis included 13 studies (4,5,7–17).
To our knowledge, this is the first meta-analysis of RCTs to evaluate the risk of IBD with DPP4i use. We used rigorous inclusion criteria to minimize misclassification bias and observed no association between DPP4i and IBD. The absolute IBD risk in the included trials was low; 21,868 patients had to be treated with DPP4i, over 2.3 years, to lead to one additional case of IBD. In contrast, only 12 T2D patients require treatment with DPP4i, over 2.1 years, for one patient to achieve the HbA1c <7% (53 mmol/mol) goal (5); thus, the potential benefits of DPP4i treatment appear to outweigh any associated IBD risk. However, while we identified no significant association between DPP4i and IBD, we acknowledge that this analysis may have been underpowered to detect such an association due to the limited number of included trials and events and the statistical imprecision of our effect estimates.
Several experimental studies have shown that DPP4i may decrease IBD activity through inhibition of T-cell proliferation and cytokine production and decrease IBD severity through the restoration of gut mucosal damage (6). However, human studies have reported lower DPP4 concentrations in tissue and plasma from patients with IBD versus healthy subjects, suggesting that lower DPP4 concentrations may be associated with higher IBD activity (6). Hypothesized mechanisms for this link might relate to DPP4’s immunoregulatory function, including signal transduction, chemotaxis, and T-cell activation (6). More work is needed to explore the association and possible mechanisms linking DPP4i and IBD.
In conclusion, our meta-analysis of 13 RCTs found no association between DPP4i use and IBD risk among T2D patients. However, given the relatively low number of trials and events as well as potential trial bias, we cannot definitively exclude the possibility of a weak association. Additional real-world studies are needed to investigate IBD risk among DPP4i users.
Article Information
Acknowledgments. The authors thank Lulu Sun (School of Pharmaceutical Sciences, Peking University) for helping extract data from more trials when revising the manuscript.
Funding. M.J.C. is supported by a career development award from Veterans Affairs Health Services Research and Development (CDA 13-261).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.L., M.J.C., H.T., J.Y.Y., and T.W. contributed to data interpretation. G.L. and H.T. identified and selected trials, extracted data, performed all data analyses, checked for statistical consistency, interpreted results, and drafted the report. H.T. and T.W. contributed to study idea conception and led the study design. All authors critically reviewed the report and saw and approved the submitted manuscript. G.L. and T.W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Abrahami D, Douros A, Yin H, et al. Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. BMJ 2018;360:k872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T, Lu W, Li D, et al. Assessing the association between dipeptidyl peptidase 4 inhibitor use and inflammatory bowel disease through drug adverse event reporting. Diabetes Care 2019;42:e89–e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis 2015;74:1968–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenstock J, Perkovic V, Johansen OE, et al. ; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 2019;321:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 6.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009;30:600–607 [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto Y, Tajima N, Kadowaki T, et al. Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind trial. Diabetes Obes Metab 2010;12:613–622 [DOI] [PubMed] [Google Scholar]
- 8.Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin 2012;28:513–523 [DOI] [PubMed] [Google Scholar]
- 9.White WB, Cannon CP, Heller SR, et al. ; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 10.Green JB, Bethel MA, Armstrong PW, et al. ; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 11.Gantz I, Chen M, Suryawanshi S, et al. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2017;16:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group . Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007;9:194–205 [DOI] [PubMed] [Google Scholar]
- 13.Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 2012;380:475–483 [DOI] [PubMed] [Google Scholar]
- 14.Arjona Ferreira JC, Marre M, Barzilai N, et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care 2013;36:1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Prato S, Camisasca R, Wilson C, Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes Obes Metab 2014;16:1239–1246 [DOI] [PubMed] [Google Scholar]
- 16.Matthaei S, Catrinoiu D, Celiński A, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care 2015;38:2018–2024 [DOI] [PubMed] [Google Scholar]
- 17.Inagaki N, Onouchi H, Maezawa H, Kuroda S, Kaku K. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol 2015;3:191–197 [DOI] [PubMed] [Google Scholar]