Abstract
Neurotrophic keratitis is a rare corneal disease that is challenging to treat. Corneal neurotization (CN) is among the developing treatments that uses the supraorbital (SON) or supratrochlear (STN) nerve as a donor. Therefore, the goal of this study was to provide the detailed anatomy of these nerves and clarify their feasibility as donors for ipsilateral CN. Both sides of 10 fresh-frozen cadavers were used in this study, and the SON and STN were dissected using a microscope intra- and extraorbitally. The topographic data between the exit points of these nerves and the medial and lateral angle of the orbit were measured, and nerve rotation of these nerves toward the ipsilateral cornea were attempted. The SON and STN were found on 19 of 20 sides. The vertical and horizontal distances between the exit point of the SON and that of the STN, were 7.3±2.1 mm (vertical) and 4.5±2.3 mm, respectively. The mean linear distances between the medial angle and the exit points of each were 22.2±3.0 mm and 14.5±1.9 mm, respectively, and the mean linear distances between the lateral angle and the exit points of the SON and STN were 34.0±2.7 mm and 36.9±2.5 mm, respectively. These nerves rotated ipsilaterally toward the center of the orbit easily. A better understanding of the anatomy of these nerves can contribute to the development and improvement of ipsilateral CN.
Keywords: Supraorbital nerve, Supratrochlear nerve, Neurotrophic keratopathy, Nerve transfer, Ipsilateral, Cadaver
Introduction
Neurotrophic keratitis (NK) is a rare corneal disease that such pathological conditions as ocular surface diseases, systemic diseases, and central or peripheral nervous damages induce. NK may develop corneal infections, ulcerations and are at risk of losing vision [1,2]. Corneal neurotization (CN), which moves the supraorbital nerve (SON) or supratrochlear nerve (STN) to the cornea directly or indirectly, is one of the surgical approaches used to treat NK [3,4,5,6]. The contralateral SON or STN are used commonly as a donor for CN; however, loss of contralateral forehead sensation and constriction of the scalp with bicoronal incisions can occur by this quite invasive procedure with a large incision on the contralateral forehead. Autografts for NK also can fail to supply accurate innervation and cause neuromas, scarring, and permanent loss of nerve function [7,8,9]. Recently, use of ipsilateral nerve transfer has been explored for successful CN [10,11]. If the ipsilateral frontal nerve is healthy, and NK induced by local damage to the long ciliary nerves is isolated, ipsilateral SON and STN may be ideal donors [10,11]. From an anatomical perspective, the SON and STN have been studied well. However, anatomical data available for ipsilateral CN is still lacking. Therefore, this study's objective was to clarify the feasibility of the SON and STN as donor transfer nerves for ipsilateral CN.
Materials and Methods
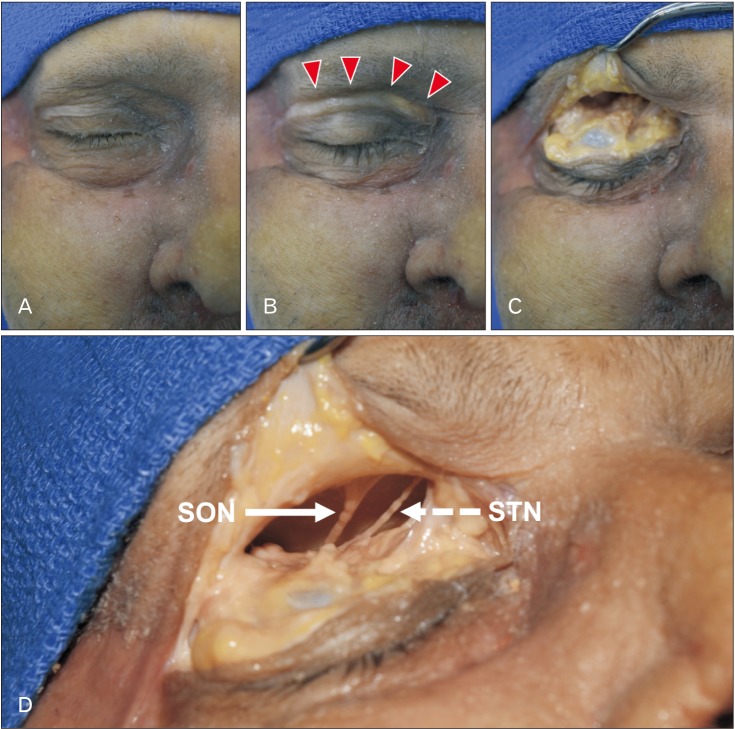
Both sides of 10 fresh-frozen, Caucasian cadaveric heads (4 males and 6 females) were dissected in this study. The mean age at death was 75.0±9.3 years (range, 61 to 88 years). The skin incision was made along the upper eyelid crease from the lateral angle of the eye to the midline at the glabella (Fig. 1). Intra- and extraorbitally, the SON and STN and each exit point (SONOUT and STNOUT) were observed via a surgical microscope (OPMI CS NC31, Carl Zeiss, Oberkochen, Germany). The location where SON and STN appear on the surface of the forehead from the orbit was defined as “the exit point.” Then, the branches that supply the lateral and medial forehead and arise from the frontal nerve inside the orbit were identified as the SON and STN, respectively. The skin flap including the periosteum and muscles located at the forehead was elevated 20 mm superior to the SON margin in order to expose the SON and STN.
Fig. 1. Step-by-step photos showing the skin incision used for locating the SON and STN. SON, supraorbital nerve; STN, supratrochlear nerve. (A) Before incision. (B) After incision (arrowheads). (C) Extra- and intraorbital dissection of the upper eyelid. (D) SON (arrow) and STN (dashed arrow).
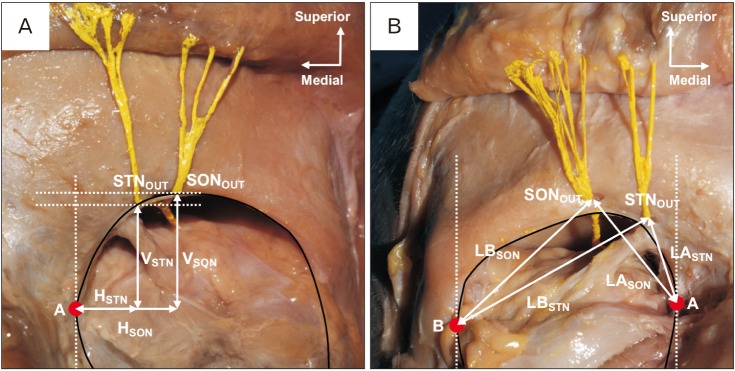
The orbit's medial and lateral angles were indicated as point A and B, respectively. The horizontal and vertical distances from point A to the exit points of the SON and STN were defined as HSON and HSTN, VSON and VSTN. The topographic relation between the SON and STN was measured as (HSON−HSTN) and (VSON−VSTN) (Fig. 2A). The linear distance from point A and B to the exit points of the SON and STN was recorded as LASON and LASTN, LBSON and LBSTN, respectively (Fig. 2B).
Fig. 2. (A) Frontal view of the left orbit. Shows the horizontal and vertical distances from point A to the STN and SON. (B) Front view of the right orbit. Shows the linear distances between each exit point and point A and B. A, medial angle of the orbit; B, lateral angle of the orbit; SON, supraorbital nerve; STN, supratrochlear nerve; HSON, horizontal distance between SONOUT and point A; HSTN, horizontal distance between STNOUT and point A; LASON, linear distance between SONOUT and point A; LASTN, linear distance between STNOUT and point A; LBSON, linear distance between SONOUT and point B; LBSTN, linear distance between STNOUT and point B; SONOUT, exit point of the supraorbital nerve; STNOUT, exit point of the supratrochlear nerve; VSON, vertical distance between SONOUT and point A; VSTN, horizontal distance between STNOUT and point A.
The number of SON and STN just after emerging from the orbit was counted. Finally, after cutting these nerves 20 mm above the SON margin, lateral and medial nerve rotation toward the ipsilateral cornea was attempted to confirm their mobility as donors for nerve transfer. When either the SON or STN had multiple exit points from the orbit, the exit point with a thicker nerve was used in this study. No previous scar was observed in the dissected area on any cadaver.
All quantitative measurements were performed using a microcaliper (Mitutoyo, Kanagawa, Japan), and recorded as the mean±SD. The study was performed according to the requirements of the Declaration of Helsinki (64thWMA General Assembly, Fortaleza, Brazil, October 2013) and the AQUA checklist [12,13].
Results
Both the SON and STN were found on nineteen of twenty sides. On one side, the frontal nerve exited from the orbit without any branches, and thus, this side was excluded in the study.
The mean distances of HSON and VSON were 13.7±2.1 mm (range, 11.4–19.9) and 17.5±3.1 mm (range, 13.0–25.7), respectively. The mean distances of HSTN and VSTN were 6.4±2.1 mm (range, 4.4–10.8) and 13.0±2.7 mm (range, 9.1–18.6), respectively. The mean distances (HSON−HSTN) and (VSON−VSTN) were 7.3±2.1 mm (range, 3.6–11.3) and 4.5±2.3 mm (range, 0.3–11.0), respectively. The mean distances of LASON and LBSON were 22.2±3.0 mm (range, 18.2–28.9) and 34.0±2.7 mm (range, 30.8–39.4), respectively. The mean distances of LASTN and LBSTN were 14.5±1.9 mm (range, 10.9–17.7) and 36.9±2.5 mm (range, 32.2–42.3), respectively (Table 1).
Table 1. Topographic relationship between the SON/STN and each landmarks.
| Supraorbital nerve | Supratrochlear nerve | Relationship | |||
|---|---|---|---|---|---|
| Horizontal distance | HSON | HSTN | HSON–HSTN | ||
| 13.7±2.1 (11.4–19.9) | 6.4±2.1 (4.4–10.8) | 7.3±2.1 (3.6–11.3) | |||
| Vertical distance | VSON | VSTN | VSON–VSTN | ||
| 17.5±3.1 (13.0–25.7) | 13.0±2.7 (9.1–18.6) | 4.5±2.3 (0.3–11.0) | |||
| Linear distance | LASON | LBSON | LASTN | LBSTN | |
| 22.2±3.0 (18.2–28.9) | 34.0±2.7 (30.8–39.4) | 14.5±1.9 (10.9–17.7) | 36.9±2.5 (32.2–42.3) | ||
SON, supraorbital nerve; STN, supratrochlear nerve.
The number of SON outside the orbit ranged from 1 to 7 with a mean of 2.9±1.4. One branch was found in 10.5% (2/19), two branches in 31.6% (6/19), and most commonly, three branches (42.1%, 8/19). Four, six, and seven branches were found in 5.3% (1/19), respectively. The number of STN outside the orbit ranged from 1 to 3 with a mean of 1.3±0.5. One branch was found in 78.9% (15/19) of cases, two branches in 15.8% (3/19), and three branches in 5.3% (1/19).
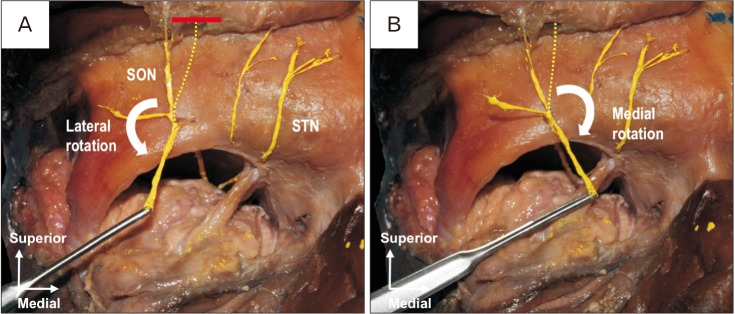
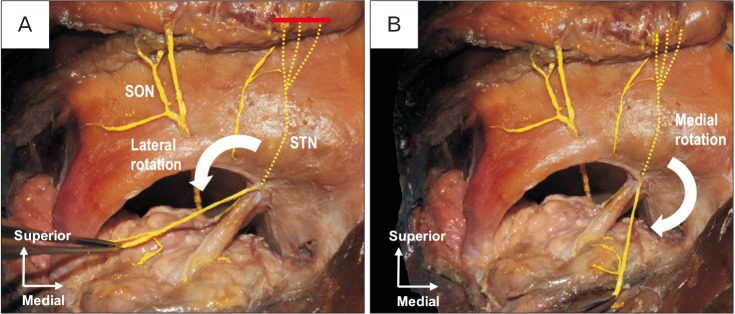
Finally, the mobility of each nerve as a donor for CN was confirmed by its medial and lateral rotation (Figs. 3, 4). In all specimens, the SON and STN could be rotated to the center of the orbit without tension.
Fig. 3. Frontal view of the right orbit. Red solid line is the location where the SON was cut. (A) Lateral rotation of the SON toward the orbit. (B) Medial rotation of the SON toward the orbit. SON, supraorbital nerve; STN, supratrochlear nerve; red solid line.
Fig. 4. Frontal view of the right orbit. Red solid line is the location where the STN was cut. (A) Lateral rotation of the STN toward the orbit. (B) Medial rotation of the STN toward the orbit. SON, supraorbital nerve; STN, supratrochlear nerve.
Discussion
CN is a comparatively novel treatment for NK and largely entails two different procedures: direct and indirect neurotization [3,4,5,6]. The former procedure harvests donor SON and STN from the contralateral (healthy) side by a bicoronal incision and transplants them around the limbus of the anesthetized eye [3,4]. The latter procedure anastomoses a sural nerve autograft and the STN, transplanting the graft to the anesthetized eye [5,6]. This method protects forehead sensation and avoid contralateral forehead and scalp contractions due to the sizeable bicoronal incision [5,6]. However, interpositional nerve grafts sometimes fail to give adequate nerve supply [7,9] and require additional invasion to harvest a new nerve graft. To solve these problems, Jacinto et al. [10] developed ipsilateral SON transfer, which was effective in a patient with NK attributable to local damage to the long ciliary nerves. If the sensation of the frontal nerve on the anesthetized side remains, ipsilateral nerve transfer is useful because of its close proximity. When the SON or STN has multiple branches, preserving some might help maintain forehead sensation. In a feasibility study, Leyngold et al. [11] proposed a novel invasive technique using endoscopy for ipsilateral SON transfer. Although these ipsilateral transfer techniques improved corneal health and sensitivity in clinical studies, anatomical studies remain limited [3,4,5,6]. Andersen et al. [14] reported that the mean distance between the exit of the most medial SON and the most lateral STN branches was 15.3 mm (3–38). In our study, the horizontal distance between the exit points of the SON and STN was 7.3±2.1 mm (3.6–11.3). When the notch can be palpated, ours and Andresen et al.'s results might help determine the topographic relation of these nerves [15].
The linear distances between each exit point and the orbit's medial and lateral canthi were measured to clarify the length required for ipsilateral SON/STN transfer. All nerves measured in this study were found to have sufficient length for transfer to the ipsilateral cornea. It is known well that the fibrous bands and fascial inscriptions compress peripheral nerves [16,17,18]. If the SON and STN pass through the bony foramen and/or notch bound by a distinct fibrous band [14,16,19], these structures might compress the nerve when the nerve transfer is attempted [16,19]. Accordingly, better anatomical knowledge of the SON and STN, as well as their related anatomical variation is essential to success of ipsilateral CN [20,21].
In conclusion, the ipsilateral CN that is performed on patients currently seems to lack supporting anatomical evidence. The results of this study can provide essential knowledge to improve the procedure.
Acknowledgements
The authors thank those who donated their bodies for anatomical study and research.
Footnotes
- Conceptualization: SK, JI, RST.
- Data acquisition: SK, JI.
- Data analysis or interpretation: SK, BY, JK.
- Drafting of the manuscript: SK, KW.
- Critical revision of the manuscript: JI, JK, RST.
- Approval of the final version of the manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–579. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, Di Iorio R, Costagliola C. Neurotrophic keratitis. Ophthalmologica. 2014;231:191–197. doi: 10.1159/000354380. [DOI] [PubMed] [Google Scholar]
- 3.Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009;123:112–120. doi: 10.1097/PRS.0b013e3181904d3a. [DOI] [PubMed] [Google Scholar]
- 4.Allevi F, Fogagnolo P, Rossetti L, Biglioli F. Eyelid reanimation, neurotisation, and transplantation of the cornea in a patient with facial palsy. BMJ Case Rep. 2014;2014:bcr2014205372. doi: 10.1136/bcr-2014-205372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: a new approach to a difficult problem. JAMA Ophthalmol. 2014;132:1289–1295. doi: 10.1001/jamaophthalmol.2014.2316. [DOI] [PubMed] [Google Scholar]
- 6.Bains RD, Elbaz U, Zuker RM, Ali A, Borschel GH. Corneal neurotization from the supratrochlear nerve with sural nerve grafts: a minimally invasive approach. Plast Reconstr Surg. 2015;135:397e–400e. doi: 10.1097/PRS.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 7.Höke A. Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans. Nat Clin Pract Neurol. 2006;2:448–454. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 8.Sabongi RG, Fernandes M, Dos Santos JB. Peripheral nerve regeneration with conduits: use of vein tubes. Neural Regen Res. 2015;10:529–533. doi: 10.4103/1673-5374.155428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomita K, Nishibayashi A, Yano K, Hosokawa K. Differential reanimation of the upper and lower face using 2 interpositional nerve grafts in total facial nerve reconstruction. Plast Reconstr Surg Glob Open. 2015;3:e544. doi: 10.1097/GOX.0000000000000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacinto F, Espana E, Padilla M, Ahmad A, Leyngold I. Ipsilateral supraorbital nerve transfer in a case of recalcitrant neurotrophic keratopathy with an intact ipsilateral frontal nerve: a novel surgical technique. Am J Ophthalmol Case Rep. 2016;4:14–17. doi: 10.1016/j.ajoc.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leyngold I, Weller C, Leyngold M, Espana E, Black KD, Hall KL, Tabor M. Endoscopic corneal neurotization: cadaver feasibility study. Ophthalmic Plast Reconstr Surg. 2018;34:213–216. doi: 10.1097/IOP.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 12.Henry BM, Marcinów A, PXMLLink_XYZkala P, Taterra D, Loukas M, Tubbs RS, Walocha JA, Tomaszewski KA. Polish translation of the Anatomical Quality Assurance (AQUA) checklist: new guidelines for reporting in original anatomical studies. Folia Med Cracov. 2017;57:105–116. [PubMed] [Google Scholar]
- 13.Tomaszewski KA, Henry BM, Kumar Ramakrishnan P, Roy J, Vikse J, Loukas M, Tubbs RS, Walocha JA. Development of the Anatomical Quality Assurance (AQUA) checklist: Guidelines for reporting original anatomical studies. Clin Anat. 2017;30:14–20. doi: 10.1002/ca.22800. [DOI] [PubMed] [Google Scholar]
- 14.Andersen NB, Bovim G, Sjaastad O. The frontotemporal peripheral nerves. Topographic variations of the supraorbital, supratrochlear and auriculotemporal nerves and their possible clinical significance. Surg Radiol Anat. 2001;23:97–104. doi: 10.1007/s00276-001-0097-8. [DOI] [PubMed] [Google Scholar]
- 15.Beer GM, Putz R, Mager K, Schumacher M, Keil W. Variations of the frontal exit of the supraorbital nerve: an anatomic study. Plast Reconstr Surg. 1998;102:334–341. doi: 10.1097/00006534-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Janis JE, Hatef DA, Hagan R, Schaub T, Liu JH, Thakar H, Bolden KM, Heller JB, Kurkjian TJ. Anatomy of the supratrochlear nerve: implications for the surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;131:743–750. doi: 10.1097/PRS.0b013e3182818b0c. [DOI] [PubMed] [Google Scholar]
- 17.Dellon AL. Musculotendinous variations about the medial humeral epicondyle. J Hand Surg Br. 1986;11:175–181. doi: 10.1016/0266-7681(86)90254-8. [DOI] [PubMed] [Google Scholar]
- 18.Iwanaga J, Simonds E, Patel M, Oskouian RJ, Tubbs RS. Anatomic study of superior cluneal nerves: application to low back pain and surgical approaches to lumbar vertebrae. World Neurosurg. 2018;116:e766–e768. doi: 10.1016/j.wneu.2018.05.087. [DOI] [PubMed] [Google Scholar]
- 19.Fallucco M, Janis JE, Hagan RR. The anatomical morphology of the supraorbital notch: clinical relevance to the surgical treatment of migraine headaches. Plast Reconstr Surg. 2012;130:1227–1233. doi: 10.1097/PRS.0b013e31826d9c8d. [DOI] [PubMed] [Google Scholar]
- 20.Iwanaga J, Watanabe K, Henry B, Tomaszewski KA, Walocha JA, Oskouian RJ, Tubbs RS. Anatomical study of the internal nasal branch of the infraorbital nerve: application to minimizing nerve damage with surgery in and around the nose. Clin Anat. 2017;30:817–820. doi: 10.1002/ca.22927. [DOI] [PubMed] [Google Scholar]
- 21.Iwanaga J, Watanabe K, Kusukawa J, Fisahn C, Alonso F, Oskouian RJ, Tubbs RS. A novel treatment for keratitis sicca (dry eye): anatomical feasibility study. Clin Anat. 2017;30:839–843. doi: 10.1002/ca.22946. [DOI] [PubMed] [Google Scholar]