Highlights
-
•
MEG may aid in the localization of the epileptogenic zone in the parietal lobe.
-
•
SAMepi – a novel kurtosis beamformer – results in localizations similar to those of the ECD analysis.
-
•
A unifocal result in both the ECD and the SAMepi analysis is associated with a good clinical outcome.
Keywords: Epilepsy surgery, Magnetoencephalography, Interictal, Parietal lobe, Equivalent current dipole, Beamformer
Abstract
Objective
To evaluate a novel analysis method (SAMepi) in the localization of interictal epileptiform magnetoencephalographic (MEG) activity in parietal lobe epilepsy (PLE) patients in comparison with equivalent current dipole (ECD) analysis.
Methods
We analyzed the preoperative interictal MEG of 17 operated PLE patients utilizing visual analysis and: (1) ECD with a spherical conductor model; (2) ECD with a boundary element method (BEM) conductor model; and (3) SAMepi – a kurtosis beamformer method. Localization results were compared between the three methods, to the location of the resection and to the clinical outcome.
Results
Fourteen patients had an epileptiform finding in the visual analysis; SAMepi detected spikes in 11 of them. A unifocal finding in both the ECD and in the SAMepi analysis was associated with a better chance of seizure-freedom (p = 0.02). There was no significant difference in the distances from the unifocal MEG localizations to the nearest border of the resection between the different analysis methods.
Conclusions
Localizations of unifocal interictal spikes detected by SAMepi did not significantly differ from the conventional ECD localizations.
Significance
SAMepi – a novel semiautomatic analysis method – is useful in localizing interictal epileptiform MEG activity in the presurgical evaluation of parietal lobe epilepsy patients.
1. Introduction
Patients with parietal lobe epilepsy (PLE) are relatively rare in epilepsy surgery series (Salanova, 2012; Francione et al., 2015), which may partly be explained by the difficulty of correctly localizing the epileptogenic zone in the parietal lobe, especially when there is no visible lesion in the magnetic resonance imaging (MRI) (Salanova, 2012). Major reasons for the difficulty of localizing the epileptogenic zone include the variable spreading patterns of the ictal epileptic activity, which may cause the parietal lobe seizures to present with a variety of symptoms, and that the symptomatogenic zone can be distant to the epileptogenic zone (Francione et al., 2015). Especially, very rapid spreading of the epileptic activity to temporal and frontal lobes is common (Ristić et al., 2012).
Furthermore, the localization of neurophysiological abnormalities in PLE has proven difficult. The few EEG studies in operated PLE patients reported quite a poor localization of both interictal and ictal activity. In a study of 40 operated PLE patients, a localizing interictal EEG finding was seen in only 25% of the patients (Francione et al., 2015). In these patients, interictal EEG was falsely localizing in 28% and falsely lateralizing in 5% of the patients. The ictal EEG was localizing in only 25% of the 32 patients in whom ictal recording was available. Falsely localizing and falsely lateralizing ictal EEG findings were seen in one patient (3%) each. Another study demonstrated that patients with PLE showed more scatter of interictal epileptiform discharges outside the epileptogenic lobe and a lower localizing value of ictal EEG compared to patients with frontal or temporal epilepsy (Ristić et al., 2012).
In addition to EEG, magnetoencephalography (MEG) is another established method in localizing the sources of interictal epileptic activity in epilepsy surgery candidates (Kharkar and Knowlton, 2015, Bagić, 2016). However, so far only few studies with small numbers of patients have examined its use in posterior cortex epilepsies: one study (Badier et al., 2015) compared the interictal MEG localizations to the interictal and ictal findings of stereotactic EEG (SEEG) and found that the MEG localizations were concordant with the SEEG findings in four of the five cases who had a focal irritative zone in SEEG. In the nine patients with a widespread irritative zone in the SEEG, MEG typically localized only part of the irritative zone. Another study (Harroud et al., 2017) described six patients with precuneal epilepsy. Interictal MEG was recorded in three of these patients. The MEG localizations were concordant with the resected area in two patients, whereas one patient had the MEG localization in the ipsilateral temporal lobe (the interictal scalp EEG findings also localized to the ipsilateral frontotemporal area in this patient). There are no previous studies specifically examining the use of MEG in the presurgical evaluations of patients with PLE.
Equivalent current dipole (ECD) analysis remains perhaps the most utilized method in the source localization of interictal epileptiform MEG discharges. It is, however, subjective and the results depend on the visual selection of the epileptic activity to be analysed as well as on several parameters of the dipole modelling itself.
Currently, several other MEG source localization methods are available, but not yet fully established in the every-day clinical use. A study (Tenney et al., 2014) comparing several of these methods found no significant differences between them in their concordance with the seizure-onset zone determined by intracranial EEG. Synthetic aperture magnetometry (SAM) – a beamforming method – with excess kurtosis (SAM(g2)) has been utilized as a semi-automatic and user-independent method for localizing interictal spikes in MEG (e.g., Kirsch et al., 2006). This method, however, has the problem of being biased towards detecting infrequent epileptiform discharges. Harpaz et al. (2015) recently suggested an improvement on this methodology, named ”SAMepi”, to increase its sensitivity to frequent spike activity. Scott et al. (2016) further studied the optimal parameters of the SAMepi method in seven epilepsy patients and five healthy volunteers.
One of the factors potentially affecting the source modelling is the choice of a conductor model: whether a single-shell spherical conductor model or one of the more realistic models is used. A single-shell boundary element method (BEM) conductor model of the inner surface of the skull is probably the most popular of these realistic models (Stenroos et al., 2014). Although there remains no clear evidence of its superiority over the spherical conductor model in clinical ECD analysis, there is some implication that the use of more realistic conductor models may improve the performance of kurtosis beamformers: a study with simulated epileptic activity by Prendergast et al (Prendergast et al., 2013) concluded that the original implementation of SAM(g2) with a spherical conductor model was prone to mislocalizing the epileptic activity in some cases because the radial components of the sources could not be modelled. In previous publications the SAMepi analysis was performed with a BEM conductor model.
The aim of our current study was to investigate different analysis methods, namely (1) ECD analysis with a single-shell spherical conductor model; (2) ECD analysis with a single-shell boundary element method (BEM) conductor model; and (3) SAMepi analysis utilizing the same BEM conductor model, to localize interictal epileptiform MEG activity in operated PLE patients. More specifically, we (a) compared the localizations by ECD modelled with a BEM conductor model and by SAMepi to conventional ECD modelling with a spherical conductor model; and (b) compared the localization results of all three analysis methods to the location of the resection and the clinical outcome.
2. Methods
The study had a prior approval of the HUS Medical Imaging Center, University of Helsinki and Helsinki University Hospital. All data were collected retrospectively from the patient histories and from the studies which had been carried out as part of the routine preoperative evaluation.
2.1. Patients
Patients with resective parietal lobe epilepsy surgery and a minimum of two years of post-surgery follow-up were identified from the epilepsy surgery register of Helsinki University Hospital. The search included operations performed between the years of 1991 and 2016, and identified 25 patients, of whom 18 had undergone a preoperative MEG examination. One of these patients was excluded from the study because the MEG data could not be retrieved (See Tables 1 and 2 describing the characteristics of the operated PLE patients included and not included in this study).
Table 1.
Description of the patients included in the study.
| Patient | Sex | Age at operation(s) | Age at the onset of epilepsy | iiEEG epileptiform localizationsa | MRI lesion | Etiology/Histology | AEDs at MEG | Resection | Outcome at two years post-op. (Engel) | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 7 y | 2 y 6 m | RPO | RP ischaemic lesion (white matter lesion only) | Ischaemia + FCD IIId | OXC, LEV, TPM | RP lateral and mesial | Ia | |
| 2 | M | 11 y | 7 y | RF | Right gyrus angularis DNT | DNT | OXC, VPA, CZP | RP lateral | Ia | |
| 3 | M | 24 y | 3.5 y | RP | – | FCD IIb | OXC, TPM | RTP lateral | Ia | |
| 4 | F | 22 y | 12 y | RP | – | FCD IIb | OXC, LEV, CZP, AZA, FPHT, VPAb | RP lateral | Ia | |
| 5 | M | 15 y | 9 y | LT | LP ischaemic | Perinatal infarction + FCD IIId | OXC, VPA | LP lateral | Ia | |
| 6 | F | 16 y | 5 y | midline P | Left SPL parasagittal cortical dysplasia | FCD IIb | OXC | LP lateral and mesial | Ib | |
| 7 | F | 1. 6 y; 2. 7 y | 3 m | 1. LP, LC, (midline F); 2. left, bilateral | Left SPL cortical dysplasia | FCD I | 1. OXC, LTG, TPM; 2. OXC, LTG | LP lateral (both operations) | IIb | Two operations of the same lesion; MEG recording prior to both operations |
| 8 | F | 9 y | 8 y | Bilateral, (left) | Right posterior cingulum DNT | DNT | OXC, TPM, CLB | Right posterior cingulum | IIb | |
| 9 | M | 34 y | 24 y | RCP, RantT | Right widespread parieto-temporal lesion: thick cortex, abnormal white matter signal | Histology inconclusive | VPA, LEV, LTG, PGB | RP lateralc | IIb | |
| 10 | M | 17 y | 8 y | LCP | – | FCD IIa | 1. TPM, LTG, CLB; 2. CBZ, LTG, LEV | LP lateral and mesial | IIb | |
| 11 | F | 5 y | 1 y 4 m | midline P, (RantT) | – | FCD I (uncertain) | OXC, VPA | Right posterior cingulum | IIb | |
| 12 | M | 16 y | 5 y 6 m | LP | Left parieto-insular ischaemic lesion | Perinatal infarction | OXC, VPA, ZNS | LP lateralc | IIIa | |
| 13 | F | 4 y 5 m | 6 m | midline/right CP, bilateral | Right pre- and post-central gyrus cortical dysplasia | FCD I | VPA, LTG | RP laterald | IIIa | |
| 14 | F | 7 y | 1 week | midline/right multifocal | Right PVL (extensive) | Perinatal IVH | VPA, STM, CLB | RP lateral and mesialc | IIIae | |
| 15 | M | 13 y | 6 y | Bilateral multifocal | – | FCD I | OXC, VPA | LP operculum | IVb | |
| 16 | M | 11 y | 1 y | midline C, LFT | Bilateral: multiple tubers | TSC | OXC, VPA, LCM | LPO lateral and mesial | IVb | Previous operation of the same tuber; no MEG before that operation |
| 17 | F | 14 y | 3–4 y | midline FCP | – | FCD I | OXC, ZNS, CLB | RP lateral and mesiald | IVb |
a: localizations in parenthesis indicate foci with few spikes.
b: MEG study during frequent seizures (focal status epilepticus); fosphenytoin and valproic acid given as loading doses.
c: extent of the resection limited by the proximity of the primary sensorimotor area.
d: extent of the resection limited by the proximity of the primary motor cortex (resection involves sensory cortex).
e: based on the effect on cognition; no significant reduction in seizures.
Abbreviations:
iiEEG: interictal electroencephalogram; C: central; CP: centro-parietal; FCP: fronto-centro-parietal; LCP: left centro-parietal; LFT: left fronto-temporal; LP: left parietal; LPO: left parieto-occipital;
LT: left temporal; P: parietal; RantT: right anterior temporal; RCP: right centro-parietal; RF: right frontal; RP: right parietal; RPO: right parieto-occipital; RTP: right temporo-parietal;
SPL: superior parietal lobulus; DNT: dysembryoplastic neuroepithelial tumor; FCD: focal cortical dysplasia; IVH: intraventricular hemorrhage; PVL: periventricular leukomalacia;
TSC: tuberous sclerosis complex; AZA: acetazolamide; CBZ: carbamazepine; CLB: clobazam; CZP: clonazepam; DZP: diazepam; FPHT: fosphenytoin; LCM: lacosamide; LTG: lamotrigine;
LEV: levetiracetam; OXC: oxcarbazepine; PGB: pregabalin; STM: sultiame; TPM: topiramate; VPA: valproic acid; ZNS: zonisamide.
Table 2.
Description of the operated patients excluded from the study because of the lack of preoperative MEG data.
| Patient | Age at operation | Etiology/Histology | MRI lesion | Outcome at two years post-op. (Engel) |
|---|---|---|---|---|
| 18 | 14 y | Histology inconclusive | + | Ia |
| 19 | 15 y | FCD IIb | + | Ic |
| 20 | 15 y | FCD IIa | + | Id |
| 21 | 15 y | FCD Ia | + | IIb |
| 22 | 34 y | Perinatal infarction | + | III |
| 23 | 6 y 10 m | FCD I (uncertain) | + | IVb |
| 24 | 1 y 5 m | FCD I | + | IVb |
| 25b | 26 y | FCD IIb | – | Ia |
a: histological diagnosis, MRI and ECoG findings suggested FCD II,
b: MEG data not found,
Abbreviations:
FCD: focal cortical dysplasia; ECoG: electrocorticography.
Of the seventeen patients with an MEG study, fifteen had a resection strictly confined to the parietal lobe and two had a resection mostly involving the parietal lobe but slightly extending to the occipital lobe in one patient and to the posterior temporal lobe in the other patient. Two of the patients had a resection limited to the posterior cingulate gyrus. In five patients, the proximity of the primary sensorimotor area restricted the extent of the resection. The ages of the patients ranged from 4.5 to 34 years (median 13 years) at the time of the operation. Nine were female. Thirteen patients had a preoperative intracranial EEG study: nine patients underwent a subdural grid electrode recording, three patients had an SEEG study, and one patient had two SEEG studies and a subdural grid recording. One of the patients had two resections of the same lesion and MEG was performed prior to both operations. The operations were performed less than two years apart, and therefore, the clinical outcome was determined based on the follow-up after the second operation. Another patient had undergone previous resective surgery without a preoperative MEG examination. Additionally, one patient had been examined twice with MEG prior to surgery.
2.1.1. Clinical outcome
Six (35%) of the seventeen patients were seizure-free at two years after the operation (five class Ia and one class Ib based on the Engel classification (Engel et al., 1993)): these patients had DNT, ischemic lesions or FCD IIb. In fact, three of the four (75%) patients with an FCD type II became seizure-free, whereas none of the five patients with an FCD type I were seizure-free. Two of the six (33%) MRI-negative patients were seizure-free. The outcomes of the non-seizure-free patients were as follows: five (29%) Engel II, three (18%) Engel III and three (18%) Engel IV. One patient had infrequent seizures even before the operation and the indication for epilepsy surgery was the developmental stagnation due to epileptic encephalopathy. In this patient (#14), the outcome was defined to be IIIa based on the improvement of cognition rather than on seizure frequency.
2.1.2. MRI findings and etiologies
Eleven patients had a parietal lesion on MRI (Table 1). Three of the histologically verified focal cortical dysplasias (FCD) were detected on MRI (two FCD I and one FCD II), whereas six were not visualized on MRI (three FCD I and three FCD II). Other etiologies included dysembryoplastic neuroepithelial tumor (DNT), tuberous sclerosis complex (TSC) and perinatal vascular incidents.
2.1.3. Interictal EEG
The localizations of interictal epileptiform phenomena in EEG were obtained using the clinical reports of the latest long-term monitoring study before the operation (See Table 1). All patients had an epileptiform finding in the interictal EEG.
2.2. MEG recordings
All recordings were performed with an Elekta Vectorview™ 306-channel neuromagnetometer (MEGIN (Elekta Oy), Helsinki, Finland) with one magnetometer and two orthogonal planar gradiometers at each sensor location. A sampling frequency of 600 Hz and an online digital band-pass filter of 0.1–200 Hz were used in all recordings. Head position inside the MEG gantry was determined using four head position indicator (HPI) coils, whose positions in respect to three anatomical fiducial points (nasion, left and right preauricular points) were recorded before the MEG study. Twelve patients had continuous head position tracking (cHPI) during the recording. One of them had two MEG studies, but cHPI was utilized only in the second study.
2.3. MEG analysis
All MEG and MRI analysis was performed using the FieldTrip toolbox (Oostenveld et al., 2011) for MATLAB (The MathWorks, Inc., Natick, MA, USA) unless otherwise noted. Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) and 3D Slicer (https://www.slicer.org/) were used in one patient (#4) to obtain and to visualize cortical reconstruction for illustrative purposes only.
2.3.1. MEG-MRI coregistration and conductor models
Individual anatomical T1 MR images from a study closest in time to the MEG examination (range ± 7 months) were selected for conductor model preparation and the visualisation of the MEG localizations. They were transformed to the MEG coordinate system by first marking the three fiducial points to the surface reconstruction of the head and then further refining the transformation by utilizing additional head surface points (for example EEG electrode locations) in the patients where such points were present in the measurement.
An individual single-shell BEM conductor model of the inner surface of the skull with 3000 vertices was constructed for each patient. Additionally, an individual single-shell spherical conductor model with the best fit to the intracranial volume was also obtained.
2.3.2. Data selection and preprocessing
Thirty minutes of interictal data with no seizures were selected for the analysis except in four patients who each had a total recording duration of approximately twenty minutes. If sleep or drowsiness were present in the recording, a set of consecutive segments (measurement files) of data was selected to include both awake state and sleep (or drowsiness). In the patients with no sleep, data from the first 30 min of the recording session were used.
Before further analysis, an artefact removal procedure based on temporally extended signal space separation (tSSS, Maxfilter by MEGIN (Elekta Oy), (Taulu and Simola, 2006)) was utilized. In each patient, the first data file was selected as a head position reference file and the following data files were processed with the Maxfilter software to match the reference head position in order to make the different measurement files comparable on the sensor level. In one patient, the head position transfer was not possible due to too large a difference of the head positions between two measurement files. However, in this patient, epileptiform activity was only observed in one of these files, and therefore, the sensor level analysis was not affected by the head position difference between the files. Continuous HPI information was utilized in this process in the cases where it was available.
2.3.3. MEG visual analysis
Visual analysis of the MEG data was conducted using BESA Research 6.1 (BESA GmbH, Gräfelfing, Germany). Classification of spikes to one (“unifocal”) or multiple (“multifocal”) types was based on their localization and morphology on the sensor level and was performed by an expert in clinical MEG analysis (author J.W.). Interictal epileptiform phenomena were classified as unifocal if over 90% of the spikes were from one focus (thus occasional single spikes from different foci did not change the classification). In addition to spikes with multiple distinct foci, widespread non-localizing discharges were also categorized as multifocal. Spikes of each type were tagged using the pattern search function of BESA Research and the results were checked visually. In the patients with few spikes, all recorded spikes were included in the analysis whereas in the patients with frequent spikes (>20 spikes per type) this was not considered critical as long as a representative sample was obtained.
Further ECD analysis was based on the visual classification of different spike types described above. The SAMepi analysis was independent of the visual analysis except that in the patients who had no epileptiform activity in the visual analysis the SAMepi analysis was not performed (because the detection of epileptic activity in SAMepi was based on comparing the results to the original recorded MEG signals).
2.3.4. ECD analysis
The timestamps of the tagged spikes were exported from BESA Research to MATLAB where the rest of the analysis was conducted utilizing the FieldTrip toolbox. Spikes of each type were averaged and the resulting averaged spikes were visualized on the sensor level both by superimposing the signals of all MEG channels (“a butterfly plot”) and by plotting the signals of each channel separately corresponding to their positions in the sensor array (“a topographical plot”). Single-dipole scanning of the intracranial volume was performed using a three-dimensional grid with a seven millimeter resolution. The earliest spike component that could be clearly distinguished – i.e. not necessarily the component with the largest amplitude – was analysed. The time interval for dipole fitting was selected to start at the onset and to end at the earliest peak of the spike as a compromise between modelling the earliest possible component and a good signal-to-noise ratio. Channel selection was used only if there was significant MEG activity unrelated to the epileptiform activity, which was typically the case in patients with few epileptiform phenomena and therefore low signal-to-noise ratio of the averaged spikes. We did not reject dipoles based on goodness-of-fit or other similar measures, but the localization results were visually compared to the measured field patterns and deemed to be reasonable in all cases. Dipole fitting was performed separately using the BEM and the spherical conductor models with all the other modelling parameters remaining the same.
2.3.5. SAMepi analysis
We used SAM beamforming and calculated the excess kurtosis statistics from the virtual sensor signals using a method named SAMepi described by Harpaz et al. (2015). The MEG data were first band-pass-filtered with a pass-band of 20 to 70 Hz (zero-phase finite impulse response (FIR) filter, passband ripple of ± 0.015 dB, stopband attenuation of −60 dB, transition bands from 0 to 20 Hz and from 70 to 90 Hz). A noise covariance estimate was calculated based on a visually selected 30 s data segment with no epileptic activity. In four patients, interictal discharges were continuous or almost continuous, and a segment with the least discharges was selected for the noise covariance calculation. SAM beamforming was then utilized to calculate virtual sensors in the whole intracranial volume using the same seven-millimeter grid as in the ECD analysis. BEM conductor model was used for the SAMepi analysis.
For each of the virtual sensor signals, a sum of excess kurtosis over the whole dataset was calculated using 0.5 s segments of data with 50% overlap between adjacent segments for each kurtosis calculation. Segments with negative excess kurtosis were omitted from the sum kurtosis calculation. In the case of datafiles containing electric somatosensory stimuli, a –25 ms to +25 ms window in respect to the stimulus trigger was excluded from the kurtosis calculation because the stimulus artefact was discovered to cause a significant artefact in the virtual sensor signals. A custom-made script utilizing MATLAB’s internal kurtosis function was used to calculate the sum kurtosis. Local maxima, i.e. the virtual sensor locations where none of the orthogonally or diagonally adjacent 26 virtual sensors had a higher sum kurtosis, were determined.
The ten local maxima with the highest kurtosis values (“hotspots”) were selected for further analysis. SAM virtual sensors were calculated again for these hotspots otherwise using the same methodology as above, but without the initial band-pass filtering. These virtual sensor signals were then visually compared against original sensor level MEG data to verify whether they contained epileptiform activity, and also whether different hotspots represented the same or different epileptiform phenomena. The artefactual hotspots with no epileptiform activity were excluded from further analysis. When several hotspots were deemed to represent the same epileptiform phenomenon, the location with the highest sum kurtosis was selected. Therefore, if all the hotspots represented the same phenomenon, the SAMepi result was considered to be “unifocal” (uniregional) despite many local kurtosis maxima with the strongest kurtosis hotspot selected as the localization result. In these cases, we also verified that no other hotspot with the same phenomenon showed consistently earlier latency compared to the strongest hotspot, which would have suggested that the strongest hotspot is caused by the spreading of the epileptic activity. However, the latency check did not change the localization result in any of the patients.
2.3.6. Comparison of MEG localizations between the analysis methods
MEG localizations by the ECD BEM method and by the SAMepi method were compared to the localizations by the ECD with a spherical conductor model. Patients with multifocal findings were included in this analysis with each focus treated as a separate localization result. The comparison between ECD localizations with BEM and spherical conductor models was straightforward, since each localization result had an analogous result with the other conductor model. Each SAMepi localization was compared to the nearest ECD localization in multifocal cases.
Localizations were compared using three parameters in a spherical coordinate system based on the individual spherical conductor model of each patient: (1) “Laterality“, defined as the angle between the y-z plane and the line between the localization and the origin (both left and right positive); (2) “Frontality”, the angle between the x-z plane and the line between the localization and the origin (anterior positive and posterior negative); and (3) “Depth”, distance from the surface of the spherical conductor divided by the radius of the conductor (negative values are possible, if the source is outside the sphere). X, y and z refer to the three axes of the individual spherical conductor model that are parallel to the axes of the RAS (Right-Anterior-Superior) head coordinate system based on the three fiducial points (nasion, left and right preauricular points). Additionally, the Euclidean distances between the corresponding localizations were also calculated.
2.3.7. Comparison of MEG localizations to the resected area
In the patients with a unifocal finding in the ECD or the SAMepi analysis, the localizations were visually compared to the location of the resection using a postoperative MRI. A localization result was considered to be “concordant” on the quadrant level if the MEG localized to the posterior cortex (parietal, occipital or posterior temporal) in the same hemisphere as the resection. Similarly, a “false” MEG localization was defined to be a unifocal localization to any other brain region. Sensitivity of each analysis method was calculated as follows: “concordant” localizations as defined above were considered true positive findings, whereas multifocal bilateral localizations and recordings with no epileptiform activity were considered false negative findings. Additionally, the Euclidean distance was measured from the MEG localization to the nearest border of the resection. In cases where the MEG localization was inside the resected area, the distance was defined to be zero. Patients with an Engel IV outcome were excluded from all the aforementioned analyses, because it is doubtful whether the resected area represents the true epileptogenic zone in these patients. Additionally, one patient (#2) was excluded from the analysis utilizing the distances because no postoperative MRI had been obtained.
2.3.8. Comparison of the MEG results to clinical outcome
The association between a unifocal MEG finding and a seizure-free outcome (Engel I) at two years after the operation was examined. This analysis was performed separately to both the ECD and the SAMepi results. We also examined whether a unifocal finding in both the ECD and the SAMepi analysis was associated with seizure-freedom. Additionally, the distances from the MEG localizations of all three analysis methods to the resection were compared between the seizure-free patients and the patients with an Engel II or III outcome.
2.4. Statistical analysis
Wilcoxon signed-rank test was used for the pair-wise comparison of the Laterality, Frontality and Depth parameters, and for the comparison of the distances of the localizations from the resection between the different MEG analysis methods. Wilcoxon rank sum test was utilized to test for a difference in the median distance of the MEG localizations from the resection between the seizure-free and the non-seizure-free patients. Fisher’s exact test was used to test for an association between seizure freedom and unifocal MEG findings.
3. Results
3.1. MEG visual and ECD analysis
Fourteen of the analyzed seventeen patients showed interictal epileptiform activity in the visual analysis of MEG signals. In the patient with two operations and an MEG study prior to both operations, the MEG recorded after the first operation showed no epileptiform activity. In the other patient who had undergone two MEG studies prior to a single operation, the first study showed no epileptiform findings.
The number of detected spikes ranged from two to over three thousand (the exact number is not determined, since all spikes were not tagged in the patients with frequent spikes, as mentioned in Methods). Ten patients showed a unifocal finding (Table 3). Four patients had a multifocal finding, and in all of them at least one spike type was localized to the hemisphere contralateral to the resection in both the analysis with BEM and the spherical conductor models (a bilateral multifocal finding). Notably, all the patients with an FCD type II had a unifocal ECD finding.
Table 3.
MEG localization results and clinical outcome.
| Patient | ECD (spherical) | ECD (BEM) | SAMepi | Outcome (Engel) | Notes |
|---|---|---|---|---|---|
| 1 | Unifocal, concordant quadrant | Unifocal, midline posterior | Unifocal, concordant quadrant | Ia | |
| 2 | Unifocal, false frontal midlinea | Unifocal, false frontal midlinea | Unifocal, concordant quadranta | Ia | |
| 3 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Ia | |
| 4 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Ia | |
| 5 | No spikes | No spikes | No spikes | Ia | |
| 6 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Ib | |
| 7 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | No spikes | IIb | Second MEG study: no spikes. |
| 8 | Bilateral multifocal | Bilateral multifocal | Bilateral multifocal | IIb | |
| 9 | No spikes | No spikes | No spikes | IIb | |
| 10 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | No spikes | IIb | First MEG study: no spikes. |
| 11 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Unifocal, concordant quadrant | IIb | |
| 12 | Bilateral multifocal | Bilateral multifocal | Bilateral multifocal | IIIa | |
| 13 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | No spikes | IIIa | |
| 14 | Unifocal, concordant quadrant | Unifocal, concordant quadrant | Unifocal, concordant quadrant | IIIab | |
| 15 | Bilateral multifocal | Bilateral multifocal | Unifocal | IVb | |
| 16 | No spikes | No spikes | No spikes | IVb | |
| 17 | Bilateral multifocal | Bilateral multifocal | Unifocal | IVb |
a: no post-operative MRI.
b: based on the effect on cognition; no significant reduction in seizures.
3.2. SAMepi analysis
Of the 14 patients who had an epileptiform finding in the visual analysis, 11 showed epileptiform activity in the SAMepi analysis. Two of the three patients who had a negative SAMepi result despite having shown spikes in the visual analysis had very few (two and nine) spikes, but one patient had a relatively abundant finding (approximately 30 spikes) in the visual analysis. Nine patients showed a unifocal finding. Two of the four patients with a bilateral multifocal finding in the ECD analysis also showed a bilateral multifocal result in the SAMepi analysis whereas the other two patients had a unifocal finding. One of the latter had the localization in the same posterior quadrant as the resection while the other showed the localization in the ipsilateral temporal lobe. Both of these patients had an Engel class IV outcome, and were therefore not classified as “concordant” or “false” localizations.
Ten of the eleven patients who had an epileptiform finding in the SAMepi analysis showed artefactual hotspots. In four of these patients, the hotspot with the highest sum kurtosis was artefactual.
3.3. Comparison of MEG localizations between the analysis methods
Median distance between the corresponding localizations by the ECD analysis utilizing BEM and by the analysis utilizing the spherical conductor model was 13 mm (range 0 to 46 mm). There was a statistically near-significant difference in the Depth parameter with a median difference of 0.017 (BEM deeper than spherical model), which would correspond to 1.0–1.3 mm taking into account the radii of the spherical conductors in the patients included in the analysis (p = 0.06; Wilcoxon signed-rank test). There were no significant differences in the Laterality or the Frontality parameters (Laterality: p = 0.20; Frontality: p = 0.31; Wilcoxon signed-rank test).
Median distance between the SAMepi localizations and the ECD (spherical conductor model) localizations was 20 mm (range 6.8 to 90 mm). There were no significant differences in the Laterality, Frontality or Depth parameters (Laterality: p = 0.97; Frontality: p = 0.32; Depth: p = 0.58; Wilcoxon signed-rank test).
3.4. Comparison of MEG localizations to the resected area
3.4.1. ECD analysis
Of the ten patients with a unifocal finding in the ECD analysis and an Engel I-III outcome, eight had their ECD localizations in the concordant posterior quadrant in both the analysis with a spherical conductor model and the BEM analysis (Table 3). Additionally, one patient had a non-lateralizing (midline) unifocal posterior localization in the BEM analysis, but a localization to the concordant posterior quadrant in the analysis utilizing the spherical conductor model. Only one patient (#2) had a false frontal midline localization (with both conductor models). Sensitivity of localizing to the concordant posterior quadrant was 64% with the spherical conductor model and 57% in the BEM analysis. The median distance of the ECD localization from the nearest border of the resection was 1.0 cm (range 0–5.8 cm) in the BEM analysis and 0.5 cm (range 0–5.8 cm) in the spherical conductor model analysis (see Fig. 1); the difference between the BEM and the spherical conductor model was not statistically significant (p = 0.19; Wilcoxon signed-rank test).
Fig. 1.
Distances from the unifocal MEG localizations to the nearest border of the resection. Zero indicates a localization inside the resected area. Patients with an Engel class IV outcome are excluded.
3.4.2. SAMepi analysis
All seven patients with a unifocal SAMepi finding and an Engel I-III outcome had their SAMepi localizations in the concordant posterior quadrant. Notably, the seizure-free patient (#2) who had a false midline frontal finding in the ECD analysis had a unifocal SAMepi localization in the concordant posterior quadrant. Sensitivity of localizing to the concordant posterior quadrant was 50%. The median distance from the SAMepi localization to the nearest border of the resection was 0 cm (range 0–3.7 cm).
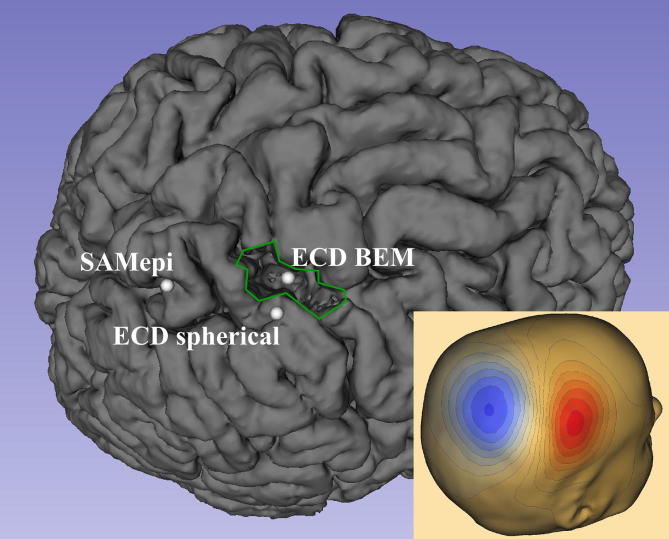
Table 3 shows the localization results of both the ECD and the SAMepi analysis as well as the clinical outcomes. Fig. 1 shows the distances from the MEG localizations to the resection in patients with a unifocal MEG finding and an Engel class I-III outcome. Fig. 2 shows an example of the MEG localizations in one patient (#4) compared to the resected area.
Fig. 2.
MEG localizations in one seizure-free patient (#4). Localizations by the three different analysis methods illustrated on the postoperative MRI showing the resection of the right postcentral gyrus (outlined in green). Bottom right: MEG field pattern of the averaged spikes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Comparison of the MEG results to clinical outcome
Five of the ten (50%) patients with a unifocal ECD finding and five of the nine (56%) patients with a unifocal SAMepi finding became seizure-free, whereas none of the patients with a multifocal finding in either the ECD or the SAMepi analysis achieved seizure-freedom. However, the association between seizure-freedom and a unifocal MEG finding was not statistically significant (ECD p = 0.22; SAMepi p = 0.45; Fisher’s exact test). Seven patients had a unifocal finding in both the ECD and in the SAMepi analysis. Five of them (71%) were seizure-free compared to none of the patients with a non-unifocal finding in either analysis. This association was statistically significant (p = 0.02; Fisher’s exact test).
The median distance between the MEG localization and the resection tended to be smaller in the seizure-free than in the non-seizure-free patients with all the analysis methods (ECD with BEM: 0.5 vs 1.4 cm; ECD with spherical conductor: 0.45 vs 1.3 cm; SAMepi: 0 vs 1.9 cm). However, these differences were not statistically significant (ECD BEM: p = 0.52; ECD spherical: p = 0.57; SAMepi p = 0.67; Wilcoxon rank sum test).
4. Discussion
The majority of the patients with parietal lobe epilepsy included in this study showed epileptiform phenomena in MEG even in a short interictal recording of 30 min. In most of these patients, the MEG localization result was unifocal in both the ECD and the SAMepi analysis. Patients with a unifocal finding in both of these analyses had a higher probability of seizure-freedom at two years after the operation than the other patients. In fact, none of the patients with a multifocal MEG finding in either the ECD or the SAMepi analysis became seizure-free.
The unifocal MEG findings were predominantly localized to the same posterior quadrant as the resection with all analysis methods. No falsely lateralizing unifocal findings were seen with any analysis method. Only one false frontal midline finding was observed in the ECD analysis. We used this crude categorization of the MEG localizations on the quadrant level in addition to the more accurate distances from the resection because false localizations to other brain areas would be potentially misleading in the clinical evaluation, and therefore, particularly interesting. The SAMepi method resulted in very similar localization results compared to the current clinical standard - the ECD analysis with a spherical conductor model - in most of the patients. This is in line with previous findings: for example Robinson et al. (2004) found that the kurtosis beamformer (SAM(g2)) localizations were equivalent to the ECD localizations especially in patients with a single spike focus and a good signal-to-noise ratio of the MEG data. In our current study, all patients with a unifocal SAMepi result had the localization in the same posterior quadrant as the resection. Overall, SAMepi had a somewhat lower sensitivity in detecting interictal epileptiform activity compared to visual analysis: three of the fourteen patients with an interictal epileptiform finding in the visual analysis had a negative SAMepi result.
Previous studies have reported relatively common false lateralizations and localizations in interictal scalp EEG in parietal lobe epilepsy: Francione et al. (2015) reported false lateralization or localization in 33% of their patients. Another study (Kim et al., 2004b) reported a false frontal or temporal localization in 21% of the patients who became seizure-free. In our patient group, a false unifocal localization to frontal or temporal regions in the interictal EEG was only reported in two of the seventeen (12%) patients. Interestingly, these included the patient who had a false frontal localization both in the MEG ECD analysis and the interictal EEG, but a localization to the concordant posterior quadrant in the SAMepi analysis. The other patient had a false ipsilateral temporal localization in interictal EEG and had no epileptiform findings in MEG.
The use of a more realistic conductor model, the individual single-shell BEM of the inner surface of the skull, did not significantly improve the MEG ECD localizations with respect to the resection when compared to the spherical conductor model. There was a tendency of the BEM model resulting in slightly deeper localizations than the spherical conductor model. However, the median difference was very small, about 1 mm, making this finding clinically insignificant. The lack of a significant difference between the conductor models is not surprising given that the centro-parietal region represents a “best case scenario” for the spherical conductor model. For example Stenroos et al. (Stenroos et al., 2014) found that the relative localization error of the single-sphere approach compared to their reference BEM conductor model was the smallest in the centro-parietal area. It should be mentioned that the SAMepi analysis could also have been performed using the spherical conductor model. However, the reason for including the spherical conductor model in the ECD analysis was its status as the long-time clinical standard to compare the other methods to. This doesn’t apply to the SAMepi analysis, and therefore, we chose to omit it for simplicity.
A recent study (Hall et al., 2018) investigated the use of the Elekta SSS-Spikiness Beamformer in epilepsy surgery patients and compared the result to the ECD analysis. They emphasize the importance of visually checking the virtual sensor signals at the kurtosis “hotspots”. Although they utilized a different variant of the kurtosis beamformer, we fully agree with this conclusion based on our experiences from the current study. Artefacts in the virtual sensor signals can indeed result in high kurtosis values, and validation with comparison to sensor level MEG data is mandatory to find the “real hotspots” associated with actual epileptiform phenomena. This does add some effort to the analysis, but is still often less laborious than the visual analysis of the whole MEG signal data that is necessary in the traditional ECD analysis – a view also presented by Hall et al. More specifically, in addition to the rejection of the artefactual kurtosis hotspots, the only step in the SAMepi analysis requiring visual analysis of the MEG signals is the choice of the data segment for the noise covariance calculation – i.e. a segment of the data with preferably no epileptiform activity or as few epileptiform phenomena as possible in the case of continuous epileptiform activity. In contrast, the traditional ECD analysis requires the visual analysis of the whole or at least the majority of the measured MEG data, a visual selection of the epileptiform activity to be modelled and the classification of the epileptiform activity to one (“unifocal”) or several (“multifocal”) categories. In addition, there are steps in the dipole modelling itself, such as the choice of the time period and the channels used for the ECD analysis, that also require user input.
Thirty-five percent of our patients were seizure-free at two years after surgery. In previous studies, seizure-free outcomes have been reported in 35–75% of the patients with parietal lobe epilepsy (Salanova et al., 1995, Kim et al., 2004b, Kim et al., 2004a, Binder et al., 2009, Francione et al., 2015). As MEG was utilized selectively in the presurgical evaluation of our patients, there is probably a bias towards more complicated cases (especially those with no MR lesion) to be included in our study group. Additionally, in five of our patients the extent of the resection was limited by the proximity of the primary sensorimotor areas. These factors may explain the relatively low percentage of seizure-free patients in our series.
The limitations of this study include the small and retrospective patient sample. However, PLE patients being such a rare patient group, these limitations seem acceptable. Because of the retrospective nature of the study, MEG findings have had a significant effect on the clinical epilepsy surgery planning, and therefore, there is unavoidably some degree of interdependency between the MEG results and the location of the resection. Additionally, little is yet known about the optimal utilization of the SAMepi method in epilepsy surgery patients. For example, the time window length might have significant effect on the results. The choice of the 0.5 s time window in this study was based on the recommendation by Harpaz et al. (Harpaz et al., 2015) indicating that optimally there should only be one epileptic discharge per one window, and therefore, a short time window is required for patients with frequent discharges. On the other hand, they also stated that a very short time window – for example 0.25 s – may be too short to include a sufficient baseline in the case of discharges with long duration, such as polyspikes. The choice of different SAMepi parameters might therefore have resulted in significantly different localization results.
5. Conclusions
In parietal lobe epilepsy, interictal MEG seems to correctly localize the epileptogenic zone at the quadrant level in most of the patients with a unifocal finding. SAMepi is a novel kurtosis beamformer method that shows localization results comparable to the ECD analysis in most of the patients. Its main advantage compared to the ECD analysis is that it requires less input from the MEG operator saving time and reducing the subjectivity of the analysis. However, SAMepi does not find all spike foci. At this stage we believe that combined use of the different localizing methods is beneficial, which has also been suggested previously (e.g., de Gooijer-van de Groep et al., 2013). Especially, a unifocal finding in both the ECD and the SAMepi modelling seems to be associated with good clinical outcome.
Declaration of interest
None.
Funding
This work was supported by the University of Helsinki and Helsinki University Hospital. The sponsors didn’t influence the study design; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the article for publication.
References
- Badier J.-M., Bartolomei F., Chauvel P., Bénar C.-G., Gavaret M. Magnetic source imaging in posterior cortex epilepsies. Brain Topogr. 2015;28(1):162–171. doi: 10.1007/s10548-014-0412-4. [DOI] [PubMed] [Google Scholar]
- Bagić A. Look back to leap forward: the emerging new role of magnetoencephalography (MEG) in nonlesional epilepsy. Clin. Neurophysiol. 2016;127(1):60–66. doi: 10.1016/j.clinph.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Binder D.K., Podlogar M., Clusmann H., Bien C., Urbach H., Schramm J. Surgical treatment of parietal lobe epilepsy. J. Neurosurg. 2009;110(6):1170–1178. doi: 10.3171/2008.2.17665. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr, van Ness P.C., Rasmussen T.B., Ojemann L. Outcome with respect to epileptic seizures. In: Engel J., editor. Surgical Treatment of Epilepsies. Raven Press; New York: 1993. pp. 609–621. [Google Scholar]
- Francione S., Liava A., Mai R., Nobili L., Sartori I., Tassi L. Drug-resistant parietal epilepsy: polymorphic ICTAL semiology does not preclude good post-surgical outcome. Epileptic Disord. 2015;17(1):32–46. doi: 10.1684/epd.2015.0728. [DOI] [PubMed] [Google Scholar]
- de Gooijer-van de Groep K.L., Leijten F.S., Ferrier C.H., Huiskamp G.J. Inverse modeling in magnetic source imaging: comparison of MUSIC, SAM(g2), and sLORETA to interictal intracranial EEG. Hum. Brain Mapp. 2013;34(9):2032–2044. doi: 10.1002/hbm.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M.B.H., Nissen I.A., van Straaten E.C.W., Furlong P.L., Witton C., Foley E. An evaluation of kurtosis beamforming in magnetoencephalography to localize the epileptogenic zone in drug resistant epilepsy patients. Clin. Neurophysiol. 2018;129(6):1221–1229. doi: 10.1016/j.clinph.2017.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz Y., Robinson S.E., Medvedovsky M., Goldstein A. Improving the excess kurtosis (g2) method for localizing epileptic sources in magnetoencephalographic recordings. Clin. Neurophysiol. 2015;126(5):889–897. doi: 10.1016/j.clinph.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harroud A., Boucher O., Tran T.P.Y., Harris L., Hall J., Dubeau F. Precuneal epilepsy: clinical features and surgical outcome. Epilepsy Behav. 2017;73:77–82. doi: 10.1016/j.yebeh.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kharkar S., Knowlton R. Magnetoencephalography in the presurgical evaluation of epilepsy. Epilepsy Behav. 2015;46:19–26. doi: 10.1016/j.yebeh.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Chung C.-K., Lee S.K., Lee Y.K., Chi J.G. Parietal lobe epilepsy: surgical treatment and outcome. Stereotact. Funct. Neurosurg. 2004;82(4):175–185. doi: 10.1159/000082206. [DOI] [PubMed] [Google Scholar]
- Kim D.W., Lee S.K., Yun C.-H., Kim K.-K., Lee D.S., Chung C.-K. Parietal lobe epilepsy: the semiology, yield of diagnostic workup, and surgical outcome. Epilepsia. 2004;45(6):641–649. doi: 10.1111/j.0013-9580.2004.33703.x. [DOI] [PubMed] [Google Scholar]
- Kirsch H.E., Robinson S.E., Mantle M., Nagarajan S. Automated localization of magnetoencephalographic interictal spikes by adaptive spatial filtering. Clin. Neurophysiol. 2006;117(10):2264–2271. doi: 10.1016/j.clinph.2006.06.708. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G., Green G.G.R., Hymers M. A robust implementation of a kurtosis beamformer for the accurate identification of epileptogenic foci. Clin. Neurophysiol. 2013;124(4):658–666. doi: 10.1016/j.clinph.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Ristić A.J., Alexopoulos A.V., So N., Wong C., Najm I.M. Parietal lobe epilepsy: the great imitator among focal epilepsies. Epileptic Disord. 2012;14(1):22–31. doi: 10.1684/epd.2012.0484. [DOI] [PubMed] [Google Scholar]
- Robinson S.E., Nagarajan S.S., Mantle M., Gibbons V., Kirsch H. Localization of interictal spikes using SAM(g2) and dipole fit. Neurol Clin Neurophysiol. 2004;2004:1–7. [PMC free article] [PubMed] [Google Scholar]
- Parietal Salanova V., Epilepsy Lobe. J Clin Neurophysiol. 2012;29(5):392–396. doi: 10.1097/WNP.0b013e31826c9ebc. [DOI] [PubMed] [Google Scholar]
- Salanova V., Andermann F., Rasmussen T., Olivier A., Quesney L.F. Parietal lobe epilepsy Clinical manifestations and outcome in 82 patients treated surgically between 1929 and 1988. Brain. 1995;118(3):607–627. doi: 10.1093/brain/118.3.607. [DOI] [PubMed] [Google Scholar]
- Scott J.M., Robinson S.E., Holroyd T., Coppola R., Sato S., Inati S.K. Localization of interictal epileptic spikes with MEG: optimization of an automated beamformer screening method (SAMepi) in a diverse epilepsy population. J. Clin. Neurophysiol. 2016;33(5):414–420. doi: 10.1097/WNP.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenroos M., Hunold A., Haueisen J. Comparison of three-shell and simplified volume conductor models in magnetoencephalography. Neuroimage. 2014;94:337–348. doi: 10.1016/j.neuroimage.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51(7):1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Tenney J.R., Fujiwara H., Horn P.S., Rose D.F. Comparison of magnetic source estimation to intracranial EEG, resection area, and seizure outcome. Epilepsia. 2014;55(11):1854–1863. doi: 10.1111/epi.12822. [DOI] [PubMed] [Google Scholar]