Abstract
MicroRNAs are small noncoding transcripts that posttranscriptionally regulate gene expression via base-pairing complementarity. Their role in cancer can be related to tumor suppression or oncogenic function. Moreover, they have been linked to processes recognized as hallmarks of cancer, such as apoptosis, invasion, metastasis, and proliferation. Particularly, one of the first oncomiRs found upregulated in a variety of cancers, such as gliomas, breast cancer, and colorectal cancer, was microRNA-21 (miR-21). Some of its target genes associated with cancer are PTEN (phosphatase and tensin homolog), PDCD4 (programmed cell death protein 4), RECK (reversion-inducing cysteine-rich protein with Kazal motifs), and STAT3 (signal transducer activator of transcription 3). As a result, miR-21 has been proposed as a plausible diagnostic and prognostic biomarker, as well as a therapeutic target for several types of cancer. Currently, research and clinical trials to inhibit miR-21 through anti-miR-21 oligonucleotides and ADM-21 are being conducted. As all of the evidence suggests, miR-21 is involved in carcinogenic processes; therefore, inhibiting it could have effects on more than one type of cancer. However, whether miR-21 can be used as a tissue-specific biomarker should be analyzed with caution. Consequently, the purpose of this review is to outline the available information and recent advances regarding miR-21 as a potential biomarker in the clinical setting and as a therapeutic target in cancer to highlight its importance in the era of precision medicine.
Main Text
MicroRNAs
In 1993, Ambros et al.1 discovered in Caenorhabditis elegans that the lin-4 gene (currently known as lin-4 microRNA [miRNA]) could decrease the levels of lin-14 protein through antisense complementary binding of the RNA transcripts. Later, other miRNA genes with the same mechanism of action were found in different species, including humans. Thus, miRNAs were established as novel, small regulatory RNA molecules. These noncoding genes are single-stranded structures of 19–25 nt that can be found in intergenic or intragenic regions of the genome.2
As regulatory biomolecules, miRNAs need to be only partially complementary to induce the regulation of a target gene.3 Hence, a single miRNA can regulate a variety of genes, and a single gene can be targeted by many miRNAs.4 As a result, the identification of target genes is a complex process.5 Moreover, due to their broad regulatory activity, miRNAs are involved in a large number of processes, including development,6,7 proliferation,8, 9, 10 apoptosis,11,12 metabolism,13,14 differentiation,6,15 and metastasis,16,17 among others.18 Despite their broad involvement in a variety of processes, miRNA functions can be divided into two types: homeostatic regulation of gene expression and robustness in cellular responses.19 The first type involves the regulation of gene expression through the precise adjustment of the cell’s requirements.20,21 As there are proteins that should be optimally expressed at low levels in certain cell types, miRNAs can fine-tune the expression of these. However, the second type of regulation is present on processes such as cell fate, its differentiation state, and stress responses,22, 23, 24 and its main objective is to dampen the protein production of the targeted messenger RNA (mRNA) almost completely.19,25 This regulation ensures accuracy and robustness by repressing the expression of mRNAs that linger from previous cell states or the products of leaky transcription.25
To synthesize a miRNA, a particular biogenesis pathway is followed. The biogenesis of miRNAs starts when RNA polymerase II (for intragenic or intergenic miRNAs)26, 27, 28 or RNA polymerase III (for some intergenic miRNAs)29, 30, 31 transcribes the miRNA gene and produces a primary transcript (pri-miRNA) with a 5′ cap and polyadenylation at the 3′ end.28 Subsequently, Drosha (an RNase III), assisted by DGCR8 (DiGeorge syndrome critical region 8), cleaves the pri-miRNA sequence, releasing it as a precursor loop of approximately 70 nt called a pre-miRNA.32, 33, 34 Furthermore, exportin 5 (XPO5) in the nuclear membrane is capable of exporting the precursor sequences out of the nucleus.35 Outside the nucleus, pre-miRNAs are cleaved by Dicer (an RNase III), aided by TRBP (transactivation response element RNA-binding protein), to become mature miRNAs of approximately 22 nt in length.36, 37, 38 Two mature miRNA sequences are produced, one of which is selected as a guide strand on the basis of a hydrogen bonding selection mechanism dependent on Argonaute (AGO) proteins.39 Finally, this complex, along with AGO proteins, forms the RNA-induced silencing complex (RISC) for gene silencing by base pairing to the 3′ untranslated region (UTR) of an mRNA.40
Alternatively, the translation repression mechanism is still the subject of debate. Mechanisms inhibiting translation include cap-dependent translation inhibition,41, 42, 43 cap-independent translation mechanisms,41,44,45 premature termination (the drop-off theory),42,46,47 and the recruitment of proteolytic enzymes that can degrade a polypeptide as it is being produced.42 mRNA decay is another mechanism of miRNA inhibition in which mRNAs bound to miRNAs accumulate in P-bodies inside the cytoplasm where they are degraded by the 5′ to 3′ exonuclease activity of Xrn1 (5′-3′ exoribonuclease 1).48, 49, 50 miRNA biogenesis and repression mechanisms are complex processes that are tightly regulated and have not yet been fully elucidated. Their dynamics and effects are of special interest to have a better understanding of many complex diseases, such as cancer.
miRNAs and Cancer
As reported previously, miRNAs are involved in a variety of processes inside the cell, some of which are considered hallmarks of cancer (e.g., metastasis,16,17 cell proliferation,9,51 apoptosis,11,12). Furthermore, it has been reported that more than 50% of miRNAs are located at fragile sites or regions where deletion or amplification tends to occur in human cancers.52 Accordingly, miRNA expression in cancer cells is dysregulated (more commonly upregulated) in comparison to normal cells.53, 54, 55 It has been found that miRNA expression profiles can classify poorly differentiated tumors more successfully than mRNA expression profiles, which highlights their importance in cancer.56, 57, 58 These findings were obtained after Lu et al.58 analyzed 17 poorly differentiated tumors with non-diagnostic histologic appearance. Overall, poorly differentiated tumors had lower levels of miRNAs compared with more differentiated tumors. Even at low levels, miRNA profiles could establish a better diagnosis by classifying tumors into 11 categories (including colon, ovary, lung, breast, lymphoblastic lymphoma), unlike the mRNA-based classification.
In particular, miRNAs involved in cancer are divided into two categories: oncogenes (oncomiRs) and tumor suppressors.59 OncomiRs act by promoting tumor development by inhibiting tumor suppressor genes; some examples of these small RNAs are homologous miRNA (miR)-221/222,60 miR-27a,61,62 and miR-21.63, 64, 65 In contrast, tumor suppressor miRNAs inhibit oncogenes, thereby suppressing tumor development. Some miRNAs that serve as tumor suppressors are miR-145,66 the let-7 family,67 and miR-205.68,69 In either role, miRNAs have been shown to be crucial for processes considered to be hallmarks of cancer.
miR-21
miR-21 was one of the first mammalian miRNAs identified.28,70 Regarding its structure, miR-21 is found on chromosome 17 (17q.23.1) in the 11th intron of the TMEM49 (transmembrane protein 49) gene, precursor of VMP1 (vacuole membrane protein 1).28,71 It has its own highly conserved promoter.72 In 2008, Fujita et al.73 described a putative promoter region (miPPR-21) containing TATA, GC, and CCAAT boxes, as well as binding sites for activator protein 1 (AP-1), E-26 transformation specific/PU.1 (Ets/PU.1), CCAAT/enhancer-binding protein α (C/EBPα), nuclear factor I (NFI), serum response factor (SRF), p53, and signal transducer activator of transcription 3 (STAT3)74 (Figure 1). When transcribed, the miR-21 gene produces a 3,433-nt long pri-miRNA (pri-miR-21). Subsequently, it is cleaved into a 72-nt-long pre-miRNA loop, which is the source of the miR-21-5p and miR-21-3p mature transcript (21 and 20 nt long, respectively).75
Figure 1.
The Genomic and Epigenomic Landscape of miR-21
(A) Genomic location of miR-21: miR-21 is located on chromosome 17 (17q.23.1) in the 10th intron of the TMEM49 gene precursor of the VMP1 protein. The site is recognized as a fragile site of the neuroblastoma histotype, where HPV16 integration events occur.52 (B) Epigenetic landscape of miR-21. H3K4me3 and H3K4m1 marks are associated with active transcription (in green), and chromatin immunoprecipitation sequencing (ChiP-seq) histograms (in blue) in the IMR90 cell line were retrieved from the Wash U Epigenome Browser (GRCh37).144 Chromatin marks such as H3K27me3 and H3K9me3 are completely absent from the miRNA promoter, whereas H3K4me3 and H3K4me1 marks, which are related to active transcription, are present on the promoter of miR-21. (C) Putative promoter region of miR-21 according to Fujita et al.73 (purple region). Transcription start sites for miR-21 described by Mudduluru et al.74 (blue arrow) and Fujita et al.73 (pink arrow) are found in the 10th intron of the VMP1 gene. Transcription start sites described by Cai et al.28 (orange arrow) and Löffler et al.145 (yellow arrow) are found on the 11th intron of the same gene. Transcription factor binding regions described by Fujita et al.73 at the putative promoter region are shown. The putative miRNA promoter region contains several binding sites for AP-1, Ets/PU.1, C/EBP, NFI, SRF, p53, and STAT3.
Alternatively, the epigenetic regulation of miR-21 has not been thoroughly studied, but in research conducted by Ferraro et al.,76 it was found that histone posttranslational modifications such as H3K27me3 and H3K9me2 were completely absent, whereas the marks related to active transcription, H3K9-14ac, H3K4me3, and H3K27ac, were higher on the promoter of miR-21 in cell lines with an epithelial-mesenchymal transition (EMT) phenotype.76 However, further studies are needed to understand the relationship between the miRNA promoter sequence, histone marks, and the expression of miRNAs, particularly miR-21.76
As reported previously, miR-21 is subject to transcriptional regulation, but the mechanisms involved in its posttranscriptional regulation have also been described. For example, in smooth muscle cells, bone morphogenic proteins (BMPs) and transforming growth factor β (TGF-β) stimulate the maturation of miR-21 via the recruitment of SMAD proteins (SMAD1 and SMAD5 are specific for BMPs, and SMAD-3 is specific for TGF-β) that stabilize the DROSHA microprocessor complex.77 In another study, miR-21 expression was found to be downregulated in MCF-7 cells when exposed to estradiol. These results indicate that the estrogen receptor is a negative regulator through the inhibition of DROSHA activity.78 Consequently, these studies show that the regulation of miR-21 is a complex process that involves transcriptional and posttranscriptional steps along the biogenesis pathway.
miR-21 Pathways
Cell Proliferation
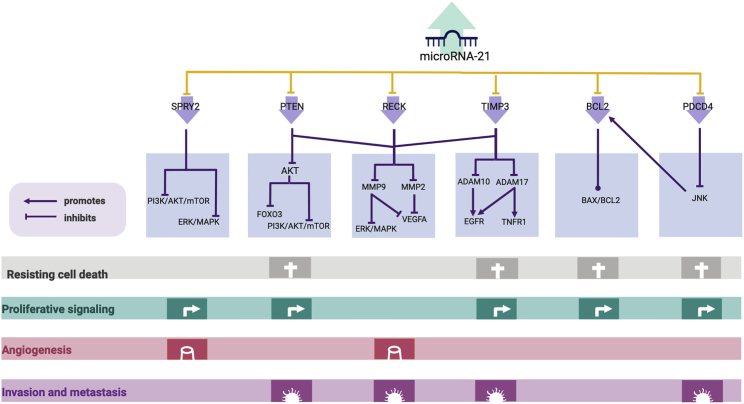
miR-21 has been linked to cell proliferation through some of its targets, such as programmed cell death protein 4 (PDCD4), sprouty RTK signaling antagonist 2 (SPRY2), phosphatase and tensin homolog (PTEN), and reversion-inducing cysteine-rich protein with Kazal motifs (RECK). In pancreatic cancer cells, miR-21 can promote epidermal growth factor (EGF)-induced proliferation by targeting SPRY2, which inhibits growth factor-induced cell proliferation.79 Additionally, mechanistic studies have revealed that the signaling pathways that miR-21 targets to modulate cell proliferation are mitogen-activated protein kinase/extracellular receptor kinase (MAPK/ERK) and phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT).79 As in pancreatic cancer, in non-small-cell lung cancer (NSCLC), miR-21 is associated with cell proliferation through PDCD480 and through PTEN and RECK in Gejiu squamous cell lung carcinoma (a type of NSCLC).81 Overall, miR-21 plays an important role in cell proliferation in diverse types of cancer, including NSCLC and pancreatic cancer, through pathways involved in the carcinogenic process (Figure 2).
Figure 2.
miR-21 Targets and Pathways Associated with the Hallmarks of Cancer
The upregulation of miR-21 can lead to the downregulation of target genes involved in the carcinogenic process, such as SPRY2,146 PTEN,97 RECK,147 TIMP3,64 BCL2,115 and PDCD4.84 In addition, SPRY2 can negatively regulate the PI3K/AKT/mTOR and ERK/MAPK pathways by controlling the trafficking of EGFR and HER2 through the endosome and by inhibiting Raf1, respectively.148 Therefore, in its absence, uncontrolled proliferation is observed, but angiogenesis is suppressed.149 PTEN, another miR-21 target, can also control the PI3K/AKT/mTOR pathway by inhibiting AKT activation. In this case, AKT can inhibit FOXO3, which regulates cell survival, growth, and differentiation by inducing the expression of proapoptotic BCL2 family proteins and Fas and TRAIL ligands or by enhancing cyclin-dependent kinase inhibitors (CDKIs).150,151 Additionally, AKT can activate mTORC1 by the phosphorylation and inactivation of TSC2 or by the phosphorylation of PRAS40,152 which affects cell growth, proliferation, survival, and motility.153 Alternatively, RECK can negatively control two matrix metallopeptidases, MMP-2 and MMP-9, which inhibit angiogenesis and invasion.154,155 In addition, TIMP3 negatively regulates ADAM10 and ADAM17, two metalloproteinases that are upregulated in cancer, and receptor substrates such as Notch receptors, transforming growth factor β (TGF-β), HER2, HER4, and VEGFR2, among others.156 In contrast, BCL2 expression can be induced by miR-21 binding to its 3′ UTR, which decreases apoptosis and increases proliferation115 by dysregulating the BAX/BCL2 ratio.157 Finally, PDCD4, a miR-21 target, can interfere with JNK activation and lead to the upregulation of BLC2-like proteins and affect apoptosis, proliferation, and migration.158
Migration and Invasion
miR-21 has been identified as a miRNA involved in metastasis and invasion (metasmiR).82,83 Particularly in breast, prostate, hepatocellular carcinoma, and colon cancer cell lines, it has been found to target genes linked to decreased metastatic potential, such as PDCD4,84 metalloproteinase inhibitor 3 (TIMP3),85,86 tropomyosin 1 (TPM1),87 serpin peptidase inhibitor/clade B (SERPINB5) coding for Maspin,88 and PTEN.89 In vitro, the overexpression of miR-21 is correlated with the downregulation of TIMP3 and an increase in the invasiveness of melanoma cell lines WM1552c and WM793b but not with migration potential.85 Regarding human hepatocellular carcinoma, it was found that miR-21 controls migration and invasion by targeting PTEN.63 PTEN can suppress the expression of MMP-9 and MMP-2 through FAK dephosphorylation. As seen here, miR-21 plays an important role in processes that lead to metastasis, such as migration and invasion, not only through the direct repression of its targets but also through the effectors of its targets.
Apoptosis
Another process that miR-21 has been associated with is apoptosis. In breast cancer, apoptosis is modulated through the miRNA targeting of B cell lymphoma 2 (BCL2); in addition, the targeting of PDCD4 by miR-21 has also been confirmed. An in vivo study also confirmed this association, which translates to the miR-21 targeting of various genes related to apoptosis in more than one cancer and demonstrated that miR-21 is a regulator of this process.90 In the previous study, it was found that miR-21 inhibits negative regulators of the Ras/MAPK kinase (MEK)/ERK pathways, and it was linked to the inhibition of apoptosis in a xenograft mouse model of NSCLC.90 Moreover, the expression of some miRNA targets associated with apoptosis was measured and found to be decreased, such as the case of PDCD4, apoptotic protease-activating factor 1 (APAF1), caspase 3 (CASP3), Fas ligand (FASLG), and Ras homolog family member B (RHOB).90 In summary, miR-21 can influence apoptotic processes through some of its targets and lead to their inhibition, thereby contributing to carcinogenic processes.
miR-21: A Biomarker for Diagnosis, Prognosis, and Prediction
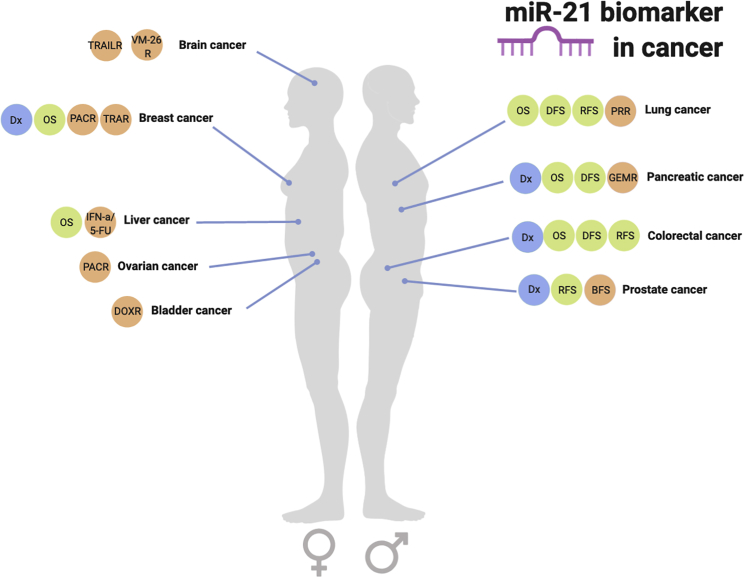
As described above, miR-21 is one of the oncomiRs identified that has been linked to carcinogenic processes. Additionally, it has been found to be upregulated in several cancers. These findings highlight the importance of miR-21 as a plausible molecular biomarker91, 92, 93 (Figure 3).
Figure 3.
The Plausible Applications of miR-21 as a Diagnostic, Prognostic, and Predictive Biomarker in Several Types of Cancer
Diagnostic biomarker applications (Dx) are shown in dark green and can be used in breast, pancreatic, colorectal, and prostate cancers. Plausible prognostic biomarkers are shown in light green, and their type is indicated by OS (overall survival), DFS (disease-free survival), BFS (biochemical-free survival), and RFS (recurrence-free survival). Prognosis parameters are found in breast, liver, lung, pancreatic, colorectal, and prostate cancers. Plausible predictive biomarkers are found in brain, breast, liver, ovarian, bladder, lung, pancreatic, and prostate cancers. The types of predictive biomarkers are also indicated in purple: TRAILR (tumor necrosis factor-related apoptosis-inducing ligand resistance), VM-26 R (teniposide resistance), PACR (paclitaxel resistance), TRAR (trastuzumab resistance), IFN-a/5-FY (interferon-α/5-fluorouracil resistance), DOXR (doxorubicin resistance), PRR (platinum resistance), GEMR (gemcitabine resistance).
The use of miRNAs as biomarkers is currently on the rise due to their stability and presence in bodily fluids, which makes them easy to assay.94, 95, 96 As previously mentioned, miR-21 is upregulated in many cancers, such as lung, ovarian, breast, stomach, prostate, colon, thyroid, and pancreatic cancers, as well as gliomas (Table 1). Furthermore, miR-21 targets important tumor suppressor genes as well as genes involved in carcinogenesis, such as PTEN,97 PDCD4,76 and RECK.98 As a result, various studies have proposed miR-21 as a potential molecular biomarker for diagnosis, prediction, and prognosis, as well as a new therapeutic target in various types of cancer.94,95,99
Table 1.
miR-21 as a Diagnostic, Prognostic, and Predictive Biomarker in Several Cancers
| Cancer | Expression | Function | Targets | Biomarker Type | References |
|---|---|---|---|---|---|
| Gastric cancer | downregulated in gastric juices but upregulated in gastric cancer tissues | promotes cell proliferation and invasion | PTEN | early diagnosis and prognosis of lymph node metastasis | 134,135 |
| PDCD4 | |||||
| RECK | |||||
| Colorectal cancer | upregulated | carcinoma-associated fibroblast formation, tumor formation, metastasis | SMAD7 | early diagnosis (circulating miR-21), prognosis adjuvant therapy (tissue miR-21), predictor of tumor relapse | 84,136 |
| SMAD 6 | |||||
| ITGβ4 | |||||
| PDCD4 | |||||
| Glioma | upregulated | cell growth, apoptosis cell proliferation, cancer stem cell differentiation | PTEN | diagnosis (screening) | 93 |
| RECK | |||||
| FasL | |||||
| PDCD4 | |||||
| Bcl2 | |||||
| Breast cancer | upregulated | cell survival, apoptosis, resistance to systemic therapy | PTEN | diagnosis triple-negative lymph metastasis and grade III | 137 |
| PDCD4 | |||||
| TPM1 | |||||
| RTN4 | |||||
| Lung cancer | upregulated | apoptosis, cell proliferation, survival, angiogenesis, inhibition of nuclear factor κB (NF-κB) activation | PTEN | prognosis and diagnosis (OS) | 95,138 |
| PDCD4 | |||||
| Smad7 | |||||
| Bcl2 | |||||
| EGFR | |||||
| Cas8 | |||||
| TGF-β | |||||
| Prostate cancer | upregulated | motility, invasion, apoptosis, androgen-independent growth | RECK | early diagnosis | 107 |
| MARCKS | |||||
| PDCD4 | |||||
| TPM1 | |||||
| PTEN |
miR-21 as a Diagnostic Biomarker
miR-21 has demonstrated its potential as a diagnostic biomarker for different types of cancer, such as breast, colorectal, and pancreatic cancers. In particular, Iorio et al.56 found that in breast cancer samples (76 primary tissue samples), miR-21 was progressively upregulated. It was also found to be a plausible sex-independent biomarker, as a study in male breast cancer demonstrated its overexpression.100 Despite several studies reporting the upregulation of this miRNA in breast cancer, its sensitivity was inconsistent. In 2016, Gao et al.101 reviewed 11 studies from 10 articles to evaluate this miRNA as a biomarker. They found a pooled sensitivity and specificity of 0.72 and 0.8, respectively. The area under the curve (AUC) of the summary receiving operating characteristic (SROC) was 0.8517. These results were higher than those of other markers, such as carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) (with a sensitivity of 0.48 and 0.39, respectively). Due to its relatively high specificity and sensitivity in comparison with other markers, miR-21 is recommended for further large-scale studies to validate its clinical application in diagnosis.
As in breast cancer, miR-21 dysregulation can also be used as a diagnostic biomarker in pancreatic cancer. In an article published in 2017, it was found that miR-21 is upregulated in the sera of pancreatic cancer patients, with a sensitivity of 0.77 and specificity of 0.8.102 In this case, the AUC of the SROC for the Asian population was 0.78. The same study recommended the use of circulating miR-21 alone or in combination with carbohydrate antigen 19-9 (CA-19-9) for a better diagnosis of pancreatic cancer, but the authors agreed that further studies are needed to unveil the clinical significance of miR-21.102 The overexpression of miR-21 is also found in serum samples from patients with colorectal cancer.103 In this study, the authors proposed a three-miRNA panel for the non-invasive diagnosis of colorectal cancer by the use of miR-21, miR-19a-3p, and miR-425-5p. The authors of this research refer to the high sensitivity and specificity in colorectal cancer serum samples (0.875 and 0.744, respectively) with an area under the ROC curve of 0.88, as found by Wang and Zhang.104 Furthermore, in another study, circulating miR-21 was analyzed as an early detection biomarker for colorectal cancer and was reported to have a sensitivity and specificity of 0.77 and 0.84, respectively,105 with an AUC of the ROC of 0.81.
Due to its relatively easy detection, miR-21 is proposed as a highly convenient diagnostic biomarker alongside prostate-specific antigen (PSA) and miR-141, with a high value of AUC when graphing sensitivity and specificity of 0.88.106 In prostate cancer, miR-21 expression, alongside other biomarkers, has been associated with the pathological stage, lymph node metastasis, and extracapsular extension.107 Analyses have revealed that miR-21 expression could be a predictor of biochemical-free survival (BFS) in prostate cancer patients.108 In this way, we hypothesize that the role of miR-21 as a diagnosis biomarker can be accompanied by its use as a prognosis biomarker.
The upregulation of miR-21 in several types of cancer, such as breast, pancreatic, prostate, and colorectal cancers, makes it possible to use miR-21 as a non-invasive diagnostic biomarker; however, its use might have to be broad and not for a particular type of cancer, as it is upregulated in more than one type of cancer. Further large-scale validation studies are needed before its clinical application in each type of cancer.
miR-21 as a Prognostic Biomarker
Although miR-21 has been found to be dysregulated in many types of cancer, its role as a prognostic biomarker has still not been elucidated, as the results have been inconsistent.101 Zhou et al.109 published an article that aimed to investigate the correlation between miR-21 and overall survival (OS) in general cancers. This work demonstrated that miR-21 correlated with poor survival in general carcinomas, with a pool hazard ratio (HR) of 1.91 for OS, 1.42 for disease-free survival (DFS), and 2.2 for recurrence-free survival (RFS) and cancer-specific survival. Interestingly, when divided into subgroups, miR-21 could predict poor OS in gastrointestinal tumors (HR = 1.68), pancreatic cancer (HR = 2.53), lung cancer (HR = 1.59), breast cancer (HR = 2.55), and liver cancer (HR = 1.93). Moreover, poor DFS was observed when miR-21 was elevated in pancreatic cancer (HR of 2.87)109,110 and lung cancer (HR = 2.05),97,109 and poor RFS and cancer-specific survival were detected in gastrointestinal tumors (HR = 2.5), lung cancer (HR = 2.25), and prostate cancer (HR = 2.04).109 These results prove that miR-21 plays an important role in general carcinomas, regardless of its origin. More studies have been conducted regarding the role of miR-21 as a prognostic biomarker for colorectal cancer, in which high expression of this miRNA in tissues correlates with poor OS and DFS.105
Although this miRNA has been linked to the prognosis of many cancers, specific studies are needed to confirm this general association and to offer additional information for each type of cancer through non-invasive but highly accurate testing.111, 112, 113
miR-21 as a Predictive Biomarker
The use of miR-21 as a biomarker is not limited to diagnosis and prognosis, as several studies have uncovered its usefulness as a predictive biomarker in breast, lung, and ovarian cancers, among others. This is particularly important due to the resistance to treatments that some cancers develop. Increased miR-21 levels were linked to resistance to platinum-based chemotherapy in patient samples with NSCLC (stages I–III).114 In this study, resistant responders had increased levels of miR-21 compared with sensitive responders. The resistance to platinum due to an increase in miR-21 was attributed to PTEN and BCL2 expression in this case. Although the molecular mechanism remains unclear, it is known that the loss of PTEN leads to increased activity of the AKT and mammalian target of rapamycin (mTOR) pathways. BCL2, which is thought to be indirectly regulated by miR-21, promotes a chemoresistant phenotype in regard to cisplatin.115
Additionally, miR-21 was also found to play a role in the sensitivity to gemcitabine treatment in patients with advanced pancreatic cancer (stages III and IV).116 A high level of serum miR-21 was associated with a poor response to gemcitabine in 177 patients because the time to progression after treatment was of only 80 days, compared with 161 days in patients whose expression of miR-21 was low. The authors of the research linked the decreased sensitivity to gemcitabine to apoptosis resistance after treatment, as miR-21 targets Fas/FasL. Moreover, serum miR-21 levels were also found to predict the outcome of HER2-positive breast cancer patients who had received neoadjuvant chemotherapy and trastuzumab.117 Increased levels of miR-21 led to worse clinical responses to chemotherapy during neoadjuvant treatment when combined with trastuzumab. In this way, it was found that miRNA levels distinguished between clinical and non-clinical responders; however, they could not distinguish between pathologic complete response and non-pathologic complete response in the same study. Therefore, more information and validation of miR-21 as a predictive biomarker for chemotherapy is needed, and multicenter studies will be convenient for this purpose.
miR-21 as a Therapeutic Target in Clinical Trials
In addition to its use as a potential biomarker in a diverse range of cancers, miR-21 has become of great interest in the field of miRNA therapeutics, an area that intends to use miRNA replacement therapy through miRNA mimics and the inhibition of miRNA function through antimiRs.118 The ability of miRNAs to target multiple genes that might be altered in a disease gives them an advantage as therapeutic molecules, either by the use of miRNA mimics or antimiRs. In the case of miR-21, antimiRs offer a new therapeutic approach for liver injury119 and cardiovascular disease.120 In particular, mouse models with kidney injury have shown that anti-miR-21 reduced reactive oxygen species (ROS) accumulation by rescuing the expression of peroxisome proliferator-activated receptor α (PPARα),121 and this effect resulted in decreased liver steatosis in mice. Nonetheless, these results are not conclusive, as PPARα might not be the only miR-21 target that has an impact on this network. Although miR-21 appears to be a promising alternative as an anticancer therapy, there are still some obstacles to address before its clinical application. One of the main concerns is the delivery of miRNA therapeutic molecules, as anti-miR-21 has not overcome the tumor microenvironment, and, as a consequence, in vivo targeting of this miRNA has not been completely successful in all cancers.122
In cancer therapeutics, miR-21 is an appealing candidate because of all of the carcinogenic processes it has been traced to. In particular, in breast cancer, Mei et al.91 demonstrated that the combination of paclitaxel (Taxol) and a miR-21 inhibitor enhanced chemotherapeutic effects in breast cancer cells (MCF7). However, this is not the only case where a miR-21 inhibitor has been shown to be efficient; anti-miR-21 has also been shown to reduce tumor size in xenograft mouse models of prostate cancer with an androgen-independent cell line, DU-145.123 Furthermore, in pancreatic cancer, miR-21 inhibition has been shown to stop tumor growth in an aggressive xenograft mouse model; additionally, in combination with gemcitabine, it can induce tumor regression. The authors hypothesized that an increase in angiogenesis due to the inhibition of miR-21 could enhance the delivery of gemcitabine to the tumor.124 In view of this information, miR-21 was introduced as a potential therapeutic target in breast cancer and other cancer types, such as prostate and pancreatic cancers, where the regulation of these genes plays an important role in cancer progression.
In fact, clinical trials for suppressing miR-21 are currently being conducted (Table 2). For example, Genzyme is conducting a phase 1 multicenter study where RG-012, a chemically modified oligonucleotide that can bind to miR-21, is being tested in patients with Alport syndrome (ClinicalTrials.gov: NCT03373786). The molecule has proven to decrease the rate of progression of renal fibrosis by inhibiting miR-21.125 Alternatively, ADM-21, a miR-21 inhibitor, is under assessment in a xenograft mouse model of bladder cancer.126 Results have shown that ADM-21 effectively decreases bladder cancer growth in vitro and in vivo by inhibiting the miR-21 regulation of the protein phosphatase 2 regulatory subunit Bα (PPP2R2A), a regulator of the AKT/mTOR pathway. After toxicity studies have concluded, testing this modified oligonucleotide in clinical phase 1 trials is needed.126 Finally, an interventional clinical trial is currently being conducted that involves the study of six miRNAs (including miR-21) to determine whether a patient with stage II colon cancer should not receive adjuvant chemotherapy (ClinicalTrials.gov: NCT02466113) according to OS and DFS measurements.127 Although trials are currently being conducted on miR-21 therapeutics, there are still many therapeutic and biomarker options that have not yet been explored, especially in cancer, where it is one of the most dysregulated miRNAs.
Table 2.
miR-21 in Clinical Trials and RNA-Based Therapeutics
| Method | Disease | Experimental Models | Status | Reference |
|---|---|---|---|---|
| Anti-miR-21 | Alport syndrome | Alport syndrome patients (18–65 years old) | clinical trial phase 1 multicenter study | 125 |
| Anti-miR-21 | bladder cancer | mouse xenograft | preclinical | 126 |
| Anti-miR-21 | cardiovascular disease | C57BL/6 male mice | preclinical | 139, 140, 141 |
| Anti-miR-21 | liver injury | bile duct ligation mouse model | preclinical | 142 |
| Anti-miR-21 | pancreatic cancer | Mia PaCa-2 Lucia F1 cells in mice (xenograft model) | preclinical | 124 |
| qRT-PCR | colon cancer stage II | patients with stage II colon cancer (18–65 years old) | interventional clinical trial | 127 |
| Anti-miR-21 | prostate cancer | DU145 cells in nude mice (xenograft model) | preclinical | 143 |
Challenges for miR-21 as a Biomarker and Therapeutic
Ironically, one of the greatest advantages of miR-21 for its use as a biomarker is also its most difficult challenge: miR-21 has been found to be dysregulated in more than one condition, including several types of cancer.128 Then, how can this plausible biomarker be specific enough to detect a condition and differentiate among others? This is particularly important because miR-21 has been proposed as a non-invasive circulating biomarker; however, if its dysregulation is so common in many different diseases, its expression pattern might follow broad stress responses in a cell instead of specific conditions. In other words, if miR-21 is involved in many types of cancer, it might confer robustness to the carcinogenic process by dampening the protein production of its targets. If this is the case, miR-21 would not be the best biomarker for a specific cancer. It would instead describe a carcinogenic process occurring in any organ or tissue showing elevated levels of miR-21 in cancer. Therefore, it would be more convenient to use miR-21 as part of a biomarker panel specific for each condition in combination with tissue-specific miRNAs to validate the occurring carcinogenic process and locate the affected tissue.
Another issue that has to be solved in order for miR-21 to become a biomarker is the standardization of methods. Recently, inconsistencies between the expression of several miRNAs measured under the same conditions have been found,129 such as the analysis made by Gao et al.101 for miR-21 in breast cancer. This finding was not understood, as miRNAs have become of great interest as biomarkers due to their stability. However, it has been proposed that preprocessing steps make a difference when measuring their expression.130
In addition, miR-21-based therapeutics also present challenges. The broad regulation of miRNAs within a cell has been discussed, and miRNAs can have many targets related to different functions. This can have a positive aspect when most targets follow the functions of oncomiRs or tumor suppressors, but when a target gene has functions that can either promote or suppress the carcinogenic process,131 such as the miR-21 target BCL2,132 the usefulness of a miRNA-based therapeutic is questioned. Furthermore, only some of the targets of a miRNA have been validated, and cancer is a complex disease. How can we be certain that miRNA therapies will have the results we expect from models? Finally, some other challenges faced by miRNA therapeutics are toxicity and long-term effects, both of which address the fact that antimiRs have to avoid the action of nucleases, but little is known about the toxic metabolites produced by the oligonucleotide that is degraded.133
Although miR-21-based biomarkers and therapeutics seem very plausible for use in the near future, there are still challenges to address before approving them. If these challenges are overcome, miR-21 could become one of the first miRNA biomarkers and therapy molecules used for complex and sometimes fatal diseases, such as cancer.
Conclusions
Experimental and clinical evidence points to miR-21 as a key element in cancer development, as it is involved in invasion, metastasis, cell proliferation, and apoptosis. The identification of some of its target genes and their downregulation effects in cancer remains to be fully elucidated. Despite this fact, miR-21 is a promising biomarker for diagnosis, prognosis, and prediction, although its broad function and dysregulation have been linked to more than one type of cancer. Furthermore, in vitro and in vivo studies have shown that its inhibition has positive effects on cancer therapy, which enhances its role as a plausible therapeutic target. Currently, clinical trials and toxicity pharmacokinetic evaluations are being conducted for miR-21 inhibitors, such as ADM-21, as therapeutic molecules.
However, there still exist some limitations for its use, such as the lack of knowledge about dose requirements and its administration. Nevertheless, work on these limitations is currently underway, and important issues, such as the administration and delivery of miRNA mimics and antimiRs, are being solved. These findings will bring a new era for therapeutics, RNA-based therapeutics, which is especially promising for miRNAs in cancer, where they are especially dysregulated. Low-cost therapy and diagnosis with almost no immune response complications could enhance the use of personalized therapies that could provide all cancer patients with the most effective and specific combination for their sickness. Therefore, to reach the era of RNA therapeutics, two main goals must be achieved: improve the delivery strategies and identify the side effects that these molecules could have on complex diseases.
In conclusion, further information and basic research are required before miR-21 can be used extensively as an approved biomarker and therapeutic target, but its upregulation in a large number of cancers, as well as its relationship with many processes that regulate cancer progression, make it a promising molecule in cancer and in RNA-based therapies.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We thank the National Cancer Institute of Mexico (INCan) for support. Figures were created with BioRender.com.
References
- 1.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V., Bartel B., Bartel D.P., Burge C.B., Carrington J.C., Chen X., Dreyfuss G., Eddy S.R., Griffiths-Jones S., Marshall M. A uniform system for microRNA annotation. RNA. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doench J.G., Sharp P.A. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seitz H. Issues in current microRNA target identification methods. RNA Biol. 2017;14:831–834. doi: 10.1080/15476286.2017.1320469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivey K.N., Srivastava D. MicroRNAs as developmental regulators. Cold Spring Harb. Perspect. Biol. 2015;7:a008144. doi: 10.1101/cshperspect.a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayed D., Abdellatif M. MicroRNAs in development and disease. Physiol. Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 8.Croce C.M., Calin G.A. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Bueno M.J., Malumbres M. MicroRNAs and the cell cycle. Biochim. Biophys. Acta. 2011;1812:592–601. doi: 10.1016/j.bbadis.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hydbring P., Wang Y., Fassl A., Li X., Matia V., Otto T., Choi Y.J., Sweeney K.E., Suski J.M., Yin H. Cell-cycle-targeting microRNAs as therapeutic tools against refractory cancers. Cancer Cell. 2017;31:576–590.e8. doi: 10.1016/j.ccell.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jovanovic M., Hengartner M.O. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 12.Su Z., Yang Z., Xu Y., Chen Y., Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6:8474–8490. doi: 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vienberg S., Geiger J., Madsen S., Dalgaard L.T. MicroRNAs in metabolism. Acta Physiol. (Oxf.) 2017;219:346–361. doi: 10.1111/apha.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottiers V., Näär A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012;13:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao S. MicroRNA biogenesis and their functions in regulating stem cell potency and differentiation. Biol. Proced. Online. 2016;18:8. doi: 10.1186/s12575-016-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou W., Liu J., Gao Y., Zhong G., Chen D., Shen J., Bao C., Xu L., Pan J., Cheng J. MicroRNAs in cancer metastasis and angiogenesis. Oncotarget. 2017;8:115787–115802. doi: 10.18632/oncotarget.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L. MicroRNA and metastasis. Adv. Cancer Res. 2016;132:165–207. doi: 10.1016/bs.acr.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Li S., Liang Z., Xu L., Zou F. MicroRNA-21: a ubiquitously expressed pro-survival factor in cancer and other diseases. Mol. Cell. Biochem. 2012;360:147–158. doi: 10.1007/s11010-011-1052-6. [DOI] [PubMed] [Google Scholar]
- 19.Bushati N., Cohen S.M. MicroRNA functions. Annu. Rev. Cell Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 20.Mihailovich M., Bremang M., Spadotto V., Musiani D., Vitale E., Varano G., Zambelli F., Mancuso F.M., Cairns D.A., Pavesi G. miR-17-92 fine-tunes MYC expression and function to ensure optimal B cell lymphoma growth. Nat. Commun. 2015;6:8725. doi: 10.1038/ncomms9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vian L., Di Carlo M., Pelosi E., Fazi F., Santoro S., Cerio A.M., Boe A., Rotilio V., Billi M., Racanicchi S. Transcriptional fine-tuning of microRNA-223 levels directs lineage choice of human hematopoietic progenitors. Cell Death Differ. 2014;21:290–301. doi: 10.1038/cdd.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner J.L., Jasiewicz K.L., Fahley A.F., Kemp B.J., Abbott A.L. Loss of individual microRNAs causes mutant phenotypes in sensitized genetic backgrounds in C. elegans. Curr. Biol. 2010;20:1321–1325. doi: 10.1016/j.cub.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez-Saavedra E., Horvitz H.R. Many families of C. elegans microRNAs are not essential for development or viability. Curr. Biol. 2010;20:367–373. doi: 10.1016/j.cub.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Cassidy J.J., Reinke C.A., Fischboeck S., Carthew R.W. A microRNA imparts robustness against environmental fluctuation during development. Cell. 2009;137:273–282. doi: 10.1016/j.cell.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartel D.P., Chen C.-Z. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y., Kim M., Han J., Yeom K.-H., Lee S., Baek S.H., Kim V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G., Wang Y., Shen C., Huang Y.W., Huang K., Huang T.H.M., Nephew K.P., Li L., Liu Y. RNA polymerase II binding patterns reveal genomic regions involved in microRNA gene regulation. PLoS ONE. 2010;5:e13798. doi: 10.1371/journal.pone.0013798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai X., Hagedorn C.H., Cullen B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borchert G.M., Lanier W., Davidson B.L. RNA polymerase III transcribes human microRNAs. Nat. Struct. Mol. Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 30.Canella D., Praz V., Reina J.H., Cousin P., Hernandez N. Defining the RNA polymerase III transcriptome: genome-wide localization of the RNA polymerase III transcription machinery in human cells. Genome Res. 2010;20:710–721. doi: 10.1101/gr.101337.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faller M., Guo F. MicroRNA biogenesis: there’s more than one way to skin a cat. Biochim. Biophys. Acta. 2008;1779:663–667. doi: 10.1016/j.bbagrm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denli A.M., Tops B.B.J., Plasterk R.H.A., Ketting R.F., Hannon G.J. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 33.Gregory R.I., Yan K.-P., Amuthan G., Chendrimada T., Doratotaj B., Cooch N., Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 34.Triboulet R., Chang H.-M., Lapierre R.J., Gregory R.I. Post-transcriptional control of DGCR8 expression by the Microprocessor. RNA. 2009;15:1005–1011. doi: 10.1261/rna.1591709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi R., Qin Y., Macara I.G., Cullen B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chendrimada T.P., Gregory R.I., Kumaraswamy E., Norman J., Cooch N., Nishikura K., Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson R.C., Tambe A., Kidwell M.A., Noland C.L., Schneider C.P., Doudna J.A. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol. Cell. 2015;57:397–407. doi: 10.1016/j.molcel.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koscianska E., Starega-Roslan J., Krzyzosiak W.J. The role of Dicer protein partners in the processing of microRNA precursors. PLoS ONE. 2011;6:e28548. doi: 10.1371/journal.pone.0028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz D.S., Hutvágner G., Du T., Xu Z., Aronin N., Zamore P.D. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 40.Klein M., Chandradoss S.D., Depken M., Joo C. Why Argonaute is needed to make microRNA target search fast and reliable. Semin. Cell Dev. Biol. 2017;65:20–28. doi: 10.1016/j.semcdb.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Thermann R., Hentze M.W. Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature. 2007;447:875–878. doi: 10.1038/nature05878. [DOI] [PubMed] [Google Scholar]
- 42.Morozova N., Zinovyev A., Nonne N., Pritchard L.-L., Gorban A.N., Harel-Bellan A. Kinetic signatures of microRNA modes of action. RNA. 2012;18:1635–1655. doi: 10.1261/rna.032284.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chendrimada T.P., Finn K.J., Ji X., Baillat D., Gregory R.I., Liebhaber S.A., Pasquinelli A.E., Shiekhattar R. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447:823–828. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 44.Mathonnet G., Fabian M.R., Svitkin Y.V., Parsyan A., Huck L., Murata T., Biffo S., Merrick W.C., Darzynkiewicz E., Pillai R.S. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science. 2007;317:1764–1767. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 45.Pillai R.S., Bhattacharyya S.N., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W. Inhibition of translational initiation by Let-7 microRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 46.Hendrickson D.G., Hogan D.J., McCullough H.L., Myers J.W., Herschlag D., Ferrell J.E., Brown P.O. Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol. 2009;7:e1000238. doi: 10.1371/journal.pbio.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen C.P., Bordeleau M.-E., Pelletier J., Sharp P.A. Short RNAs repress translation after initiation in mammalian cells. Mol. Cell. 2006;21:533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 48.Chang J.H., Xiang S., Xiang K., Manley J.L., Tong L. Structural and biochemical studies of the 5′→3′ exoribonuclease Xrn1. Nat. Struct. Mol. Biol. 2011;18:270–276. doi: 10.1038/nsmb.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones C.I., Zabolotskaya M.V., Newbury S.F. The 5′ → 3′ exoribonuclease XRN1/Pacman and its functions in cellular processes and development. Wiley Interdiscip. Rev. RNA. 2012;3:455–468. doi: 10.1002/wrna.1109. [DOI] [PubMed] [Google Scholar]
- 50.Jinek M., Coyle S.M., Doudna J.A. Coupled 5′ nucleotide recognition and processivity in Xrn1-mediated mRNA decay. Mol. Cell. 2011;41:600–608. doi: 10.1016/j.molcel.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chivukula R.R., Mendell J.T. Circular reasoning: microRNAs and cell-cycle control. Trends Biochem. Sci. 2008;33:474–481. doi: 10.1016/j.tibs.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calin G.A., Sevignani C., Dumitru C.D., Hyslop T., Noch E., Yendamuri S., Shimizu M., Rattan S., Bullrich F., Negrini M., Croce C.M. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calin G.A., Ferracin M., Cimmino A., Di Leva G., Shimizu M., Wojcik S.E., Iorio M.V., Visone R., Sever N.I., Fabbri M. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 55.Caldas C., Brenton J.D. Sizing up miRNAs as cancer genes. Nat. Med. 2005;11:712–714. doi: 10.1038/nm0705-712. [DOI] [PubMed] [Google Scholar]
- 56.Iorio M.V., Ferracin M., Liu C.-G., Veronese A., Spizzo R., Sabbioni S., Magri E., Pedriali M., Fabbri M., Campiglio M. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfeld N., Aharonov R., Meiri E., Rosenwald S., Spector Y., Zepeniuk M., Benjamin H., Shabes N., Tabak S., Levy A. MicroRNAs accurately identify cancer tissue origin. Nat. Biotechnol. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 58.Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B., Pan X., Cobb G.P., Anderson T.A. MicroRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 60.Garofalo M., Quintavalle C., Romano G., Croce C.M., Condorelli G. miR221/222 in cancer: their role in tumor progression and response to therapy. Curr. Mol. Med. 2012;12:27–33. doi: 10.2174/156652412798376170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guttilla I.K., White B.A. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J. Biol. Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang W., Zhu J., Su S., Wu W., Liu Q., Su F., Yu F. miR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS ONE. 2012;7:e51702. doi: 10.1371/journal.pone.0051702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng F., Henson R., Wehbe-Janek H., Ghoshal K., Jacob S.T., Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu J., Ni S., Cao Y., Zhang T., Wu T., Yin X., Lang Y., Lu H. The Angiogenic effect of microRNA-21 Targeting TIMP3 through the regulation of MMP2 and MMP9. PLoS ONE. 2016;11:e0149537. doi: 10.1371/journal.pone.0149537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valeri N., Gasparini P., Braconi C., Paone A., Lovat F., Fabbri M., Sumani K.M., Alder H., Amadori D., Patel T. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) Proc. Natl. Acad. Sci. USA. 2010;107:21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuse M., Nohata N., Kojima S., Sakamoto S., Chiyomaru T., Kawakami K., Enokida H., Nakagawa M., Naya Y., Ichikawa T., Seki N. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int. J. Oncol. 2011;38:1093–1101. doi: 10.3892/ijo.2011.919. [DOI] [PubMed] [Google Scholar]
- 67.Mizuno R., Kawada K., Sakai Y. The molecular basis and therapeutic potential of Let-7 microRNAs against colorectal cancer. Can. J. Gastroenterol. Hepatol. 2018;2018:5769591. doi: 10.1155/2018/5769591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gulei D., Magdo L., Jurj A., Raduly L., Cojocneanu-Petric R., Moldovan A., Moldovan C., Florea A., Pasca S., Pop L.-A. The silent healer: miR-205-5p up-regulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly up-regulating E-cadherin expression. Cell Death Dis. 2018;9:66. doi: 10.1038/s41419-017-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Cola A., Volpe S., Budani M.C., Ferracin M., Lattanzio R., Turdo A., D’Agostino D., Capone E., Stassi G., Todaro M. miR-205-5p-mediated downregulation of ErbB/HER receptors in breast cancer stem cells results in targeted therapy resistance. Cell Death Dis. 2015;6:e1823. doi: 10.1038/cddis.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 71.Ribas J., Ni X., Castanares M., Liu M.M., Esopi D., Yegnasubramanian S., Rodriguez R., Mendell J.T., Lupold S.E. A novel source for miR-21 expression through the alternative polyadenylation of VMP1 gene transcripts. Nucleic Acids Res. 2012;40:6821–6833. doi: 10.1093/nar/gks308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ozsolak F., Poling L.L., Wang Z., Liu H., Liu X.S., Roeder R.G., Zhang X., Song J.S., Fisher D.E. Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008;22:3172–3183. doi: 10.1101/gad.1706508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujita S., Ito T., Mizutani T., Minoguchi S., Yamamichi N., Sakurai K., Iba H. miR-21 gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J. Mol. Biol. 2008;378:492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Mudduluru G., George-William J.N., Muppala S., Asangani I.A., Kumarswamy R., Nelson L.D., Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011;31:185–197. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 75.Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferraro A., Kontos C.K., Boni T., Bantounas I., Siakouli D., Kosmidou V., Vlassi M., Spyridakis Y., Tsipras I., Zografos G., Pintzas A. Epigenetic regulation of miR-21 in colorectal cancer: ITGB4 as a novel miR-21 target and a three-gene network (miR-21-ITGΒ4-PDCD4) as predictor of metastatic tumor potential. Epigenetics. 2014;9:129–141. doi: 10.4161/epi.26842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumarswamy R., Volkmann I., Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8:706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wickramasinghe N.S., Manavalan T.T., Dougherty S.M., Riggs K.A., Li Y., Klinge C.M. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Q., Chen S., Zhu Z., Yu L., Ren Y., Jiang M., Weng J., Li B. miR-21 promotes EGF-induced pancreatic cancer cell proliferation by targeting Spry2. Cell Death Dis. 2018;9:1157. doi: 10.1038/s41419-018-1182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Y., Meng H., Peng Q., Yang X., Gan R., Zhao L., Chen Z., Lu J., Meng Q.H. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Ther. 2015;22:23–29. doi: 10.1038/cgt.2014.66. [DOI] [PubMed] [Google Scholar]
- 81.Xu L.F., Wu Z.P., Chen Y., Zhu Q.S., Hamidi S., Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS ONE. 2014;9:e103698. doi: 10.1371/journal.pone.0103698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu Y., Song Y., Xiong Y., Wang X., Xu K., Han B., Bai Y., Li L., Zhang Y., Zhou L. MicroRNA-21 (Mir-21) promotes cell growth and invasion by repressing tumor suppressor PTEN in colorectal cancer. Cell. Physiol. Biochem. 2017;43:945–958. doi: 10.1159/000481648. [DOI] [PubMed] [Google Scholar]
- 83.Liu Z.-L., Wang H., Liu J., Wang Z.-X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell. Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 84.Asangani I.A., Rasheed S.A., Nikolova D.A., Leupold J.H., Colburn N.H., Post S., Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 85.Martin del Campo S.E., Latchana N., Levine K.M., Grignol V.P., Fairchild E.T., Jaime-Ramirez A.C., Dao T.-V., Karpa V.I., Carson M., Ganju A. miR-21 enhances melanoma invasiveness via inhibition of tissue inhibitor of metalloproteinases 3 expression: in vivo effects of miR-21 inhibitor. PLoS ONE. 2015;10:e0115919. doi: 10.1371/journal.pone.0115919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song B., Wang C., Liu J., Wang X., Lv L., Wei L., Xie L., Zheng Y., Song X. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J. Exp. Clin. Cancer Res. 2010;29:29. doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu S., Si M.-L., Wu H., Mo Y.-Y. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J. Biol. Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 88.Qiu F., Tong H., Wang Y., Tao J., Wang H., Chen L. Inhibition of miR-21-5p suppresses high glucose-induced proliferation and angiogenesis of human retinal microvascular endothelial cells by the regulation of AKT and ERK pathways via maspin. Biosci. Biotechnol. Biochem. 2018;82:1366–1376. doi: 10.1080/09168451.2018.1459179. [DOI] [PubMed] [Google Scholar]
- 89.Huang T.-H., Wu F., Loeb G.B., Hsu R., Heidersbach A., Brincat A., Horiuchi D., Lebbink R.J., Mo Y.-Y., Goga A., McManus M.T. Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion. J. Biol. Chem. 2009;284:18515–18524. doi: 10.1074/jbc.M109.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hatley M.E., Patrick D.M., Garcia M.R., Richardson J.A., Bassel-Duby R., van Rooij E., Olson E.N. Modulation of K-ras-dependent lung tumorigenesis by microRNA-21. Cancer Cell. 2010;18:282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mei M., Ren Y., Zhou X., Yuan X.B., Han L., Wang G.X., Jia Z., Pu P.Y., Kang C.S., Yao Z. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol. Cancer Res. Treat. 2010;9:77–86. doi: 10.1177/153303461000900109. [DOI] [PubMed] [Google Scholar]
- 92.Peng Q., Zhang X., Min M., Zou L., Shen P., Zhu Y. The clinical role of microRNA-21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:44893–44909. doi: 10.18632/oncotarget.16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qu K., Lin T., Pang Q., Liu T., Wang Z., Tai M., Meng F., Zhang J., Wan Y., Mao P. Extracellular miRNA-21 as a novel biomarker in glioma: evidence from meta-analysis, clinical validation and experimental investigations. Oncotarget. 2016;7:33994–34010. doi: 10.18632/oncotarget.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sazanov A.A., Kiselyova E.V., Zakharenko A.A., Romanov M.N., Zaraysky M.I. Plasma and saliva miR-21 expression in colorectal cancer patients. J. Appl. Genet. 2017;58:231–237. doi: 10.1007/s13353-016-0379-9. [DOI] [PubMed] [Google Scholar]
- 95.Zhao W., Zhao J.-J., Zhang L., Xu Q.-F., Zhao Y.-M., Shi X.-Y., Xu A.-G. Serum miR-21 level: a potential diagnostic and prognostic biomarker for non-small cell lung cancer. Int. J. Clin. Exp. Med. 2015;8:14759–14763. [PMC free article] [PubMed] [Google Scholar]
- 96.Weber J.A., Baxter D.H., Zhang S., Huang D.Y., Huang K.H., Lee M.J., Galas D.J., Wang K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xue X., Liu Y., Wang Y., Meng M., Wang K., Zang X., Zhao S., Sun X., Cui L., Pan L., Liu S. miR-21 and miR-155 promote non-small cell lung cancer progression by downregulating SOCS1, SOCS6, and PTEN. Oncotarget. 2016;7:84508–84519. doi: 10.18632/oncotarget.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markou A., Zavridou M., Lianidou E.S. miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer (Auckl.) 2016;7:19–27. doi: 10.2147/LCTT.S60341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corcoran C., Friel A.M., Duffy M.J., Crown J., O’Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin. Chem. 2011;57:18–32. doi: 10.1373/clinchem.2010.150730. [DOI] [PubMed] [Google Scholar]
- 100.Lehmann U., Streichert T., Otto B., Albat C., Hasemeier B., Christgen H., Schipper E., Hille U., Kreipe H.H., Länger F. Identification of differentially expressed microRNAs in human male breast cancer. BMC Cancer. 2010;10:109. doi: 10.1186/1471-2407-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao Y., Cai Q., Huang Y., Li S., Yang H., Sun L., Chen K., Wang Y. MicroRNA-21 as a potential diagnostic biomarker for breast cancer patients: a pooled analysis of individual studies. Oncotarget. 2016;7:34498–34506. doi: 10.18632/oncotarget.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qu K., Zhang X., Lin T., Liu T., Wang Z., Liu S., Zhou L., Wei J., Chang H., Li K. Circulating miRNA-21-5p as a diagnostic biomarker for pancreatic cancer: evidence from comprehensive miRNA expression profiling analysis and clinical validation. Sci. Rep. 2017;7:1692. doi: 10.1038/s41598-017-01904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu M., Huang Z., Zhu D., Zhou X., Shan X., Qi L.-W., Wu L., Cheng W., Zhu J., Zhang L. A panel of microRNA signature in serum for colorectal cancer diagnosis. Oncotarget. 2017;8:17081–17091. doi: 10.18632/oncotarget.15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang B., Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J. Cancer Res. Clin. Oncol. 2012;138:1659–1666. doi: 10.1007/s00432-012-1244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H., Li P., Ju H., Pesta M., Kulda V., Jin W., Cai M., Liu C., Wu H., Xu J. Diagnostic and prognostic value of microRNA-21 in colorectal cancer: an original study and individual participant data meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2014;23:2783–2792. doi: 10.1158/1055-9965.EPI-14-0598. [DOI] [PubMed] [Google Scholar]
- 106.Yaman Agaoglu F., Kovancilar M., Dizdar Y., Darendeliler E., Holdenrieder S., Dalay N., Gezer U. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 2011;32:583–588. doi: 10.1007/s13277-011-0154-9. [DOI] [PubMed] [Google Scholar]
- 107.Jackson B.L., Grabowska A., Ratan H.L. MicroRNA in prostate cancer: functional importance and potential as circulating biomarkers. BMC Cancer. 2014;14:930. doi: 10.1186/1471-2407-14-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Melbø-Jørgensen C., Ness N., Andersen S., Valkov A., Dønnem T., Al-Saad S., Kiselev Y., Berg T., Nordby Y., Bremnes R.M. Stromal expression of miR-21 predicts biochemical failure in prostate cancer patients with Gleason score 6. PLoS ONE. 2014;9:e113039. doi: 10.1371/journal.pone.0113039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou X., Wang X., Huang Z., Wang J., Zhu W., Shu Y., Liu P. Prognostic value of miR-21 in various cancers: an updating meta-analysis. PLoS ONE. 2014;9:e102413. doi: 10.1371/journal.pone.0102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vychytilova-Faltejskova P., Kiss I., Klusova S., Hlavsa J., Prochazka V., Kala Z., Mazanec J., Hausnerova J., Kren L., Hermanova M. miR-21, miR-34a, miR-198 and miR-217 as diagnostic and prognostic biomarkers for chronic pancreatitis and pancreatic ductal adenocarcinoma. Diagn. Pathol. 2015;10:38. doi: 10.1186/s13000-015-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Du J., Zhang L. Analysis of salivary microRNA expression profiles and identification of novel biomarkers in esophageal cancer. Oncol. Lett. 2017;14:1387–1394. doi: 10.3892/ol.2017.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yoshizawa J.M., Wong D.T.W. Salivary microRNAs and oral cancer detection. Methods Mol. Biol. 2013;936:313–324. doi: 10.1007/978-1-62703-083-0_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kutwin P., Konecki T., Borkowska E.M., Traczyk-Borszyńska M., Jabłonowski Z. Urine miRNA as a potential biomarker for bladder cancer detection—a meta-analysis. Cent. European J. Urol. 2018;71:177–185. doi: 10.5173/ceju.2018.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao W., Lu X., Liu L., Xu J., Feng D., Shu Y. miRNA-21: a biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer. Cancer Biol. Ther. 2012;13:330–340. doi: 10.4161/cbt.19073. [DOI] [PubMed] [Google Scholar]
- 115.Dong J., Zhao Y.-P., Zhou L., Zhang T.-P., Chen G. Bcl-2 upregulation induced by miR-21 via a direct interaction is associated with apoptosis and chemoresistance in MIA PaCa-2 pancreatic cancer cells. Arch. Med. Res. 2011;42:8–14. doi: 10.1016/j.arcmed.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 116.Wang P., Zhuang L., Zhang J., Fan J., Luo J., Chen H., Wang K., Liu L., Chen Z., Meng Z. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol. Oncol. 2013;7:334–345. doi: 10.1016/j.molonc.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu B., Su F., Lv X., Zhang W., Shang X., Zhang Y., Zhang J. Serum microRNA-21 predicted treatment outcome and survival in HER2-positive breast cancer patients receiving neoadjuvant chemotherapy combined with trastuzumab. Cancer Chemother. Pharmacol. 2019;84:1039–1049. doi: 10.1007/s00280-019-03937-9. [DOI] [PubMed] [Google Scholar]
- 118.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 119.Zhang J., Jiao J., Cermelli S., Muir K., Jung K.H., Zou R., Rashid A., Gagea M., Zabludoff S., Kalluri R., Beretta L. miR-21 inhibition reduces liver fibrosis and prevents tumor development by inducing apoptosis of CD24+ progenitor cells. Cancer Res. 2015;75:1859–1867. doi: 10.1158/0008-5472.CAN-14-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 121.Rodrigues P.M., Rodrigues C.M.P., Castro R.E. Modulation of liver steatosis by miR-21/PPARα. Cell Death Discov. 2018;4:9. doi: 10.1038/s41420-018-0076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iorio M.V., Croce C.M. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li T., Li R.-S., Li Y.-H., Zhong S., Chen Y.-Y., Zhang C.-M., Hu M.-M., Shen Z.-J. miR-21 as an independent biochemical recurrence predictor and potential therapeutic target for prostate cancer. J. Urol. 2012;187:1466–1472. doi: 10.1016/j.juro.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 124.Sicard F., Gayral M., Lulka H., Buscail L., Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol. Ther. 2013;21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.National Institutes of Health. A Study of RG-012 in subjects with Alport syndrome. https://clinicaltrials.gov/ct2/show/NCT03373786.
- 126.Drakaki A., Koutsioumpa M., O’Brien N.A., Vorvis C., Iliopoulos D., Slamon D.J. A chemically-modified miR-21 inhibitor (ADM-21) as a novel potential therapy in bladder cancer. J. Clin. Oncol. 2017;35(Suppl):335. [Google Scholar]
- 127.National Institutes of Health. A 6 microRNA tool for stratifying stage II colon cancer of receiving adjuvant chemotherapy. https://clinicaltrials.gov/ct2/show/NCT02466113.
- 128.Haider B.A., Baras A.S., McCall M.N., Hertel J.A., Cornish T.C., Halushka M.K. A critical evaluation of microRNA biomarkers in non-neoplastic disease. PLoS ONE. 2014;9:e89565. doi: 10.1371/journal.pone.0089565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Witwer K.W. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin. Chem. 2015;61:56–63. doi: 10.1373/clinchem.2014.221341. [DOI] [PubMed] [Google Scholar]
- 130.Tiberio P., Callari M., Angeloni V., Daidone M.G., Appierto V. Challenges in using circulating miRNAs as cancer biomarkers. BioMed Res. Int. 2015;2015:731479. doi: 10.1155/2015/731479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Svoronos A.A., Engelman D.M., Slack F.J. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76:3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buscaglia L.E.B., Li Y. Apoptosis and the target genes of microRNA-21. Chin. J. Cancer. 2011;30:371–380. doi: 10.5732/cjc.011.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rupaimoole R., Han H.-D., Lopez-Berestein G., Sood A.K. MicroRNA therapeutics: principles, expectations, and challenges. Chin. J. Cancer. 2011;30:368–370. doi: 10.5732/cjc.011.10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ren J., Kuang T.-H., Chen J., Yang J.-W., Liu Y.-X. The diagnostic and prognostic values of microRNA-21 in patients with gastric cancer: a meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2017;21:120–130. [PubMed] [Google Scholar]
- 135.Sekar D., Krishnan R., Thirugnanasambantham K., Rajasekaran B., Islam V.I.H., Sekar P. Significance of microRNA 21 in gastric cancer. Clin. Res. Hepatol. Gastroenterol. 2016;40:538–545. doi: 10.1016/j.clinre.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 136.Xu P., Zhu Y., Sun B., Xiao Z. Colorectal cancer characterization and therapeutic target prediction based on microRNA expression profile. Sci. Rep. 2016;6:20616. doi: 10.1038/srep20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Medimegh I., Omrane I., Privat M., Uhrhummer N., Ayari H., Belaiba F., Benayed F., Benromdhan K., Mader S., Bignon I.-J., Elgaaied A.B. MicroRNAs expression in triple negative vs non triple negative breast cancer in Tunisia: interaction with clinical outcome. PLoS ONE. 2014;9:e111877. doi: 10.1371/journal.pone.0111877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bica-Pop C., Cojocneanu-Petric R., Magdo L., Raduly L., Gulei D., Berindan-Neagoe I. Overview upon miR-21 in lung cancer: focus on NSCLC. Cell. Mol. Life Sci. 2018;75:3539–3551. doi: 10.1007/s00018-018-2877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gu G.-L., Xu X.-L., Sun X.-T., Zhang J., Guo C.-F., Wang C.-S., Sun B., Guo G.-L., Ma K., Huang Y.-Y. Cardioprotective effect of microRNA-21 in murine myocardial infarction. Cardiovasc. Ther. 2015;33:109–117. doi: 10.1111/1755-5922.12118. [DOI] [PubMed] [Google Scholar]
- 140.Yang L., Wang B., Zhou Q., Wang Y., Liu X., Liu Z., Zhan Z. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 2018;9:769. doi: 10.1038/s41419-018-0805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yuan J., Chen H., Ge D., Xu Y., Xu H., Yang Y., Gu M., Zhou Y., Zhu J., Ge T. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell. Physiol. Biochem. 2017;42:2207–2219. doi: 10.1159/000479995. [DOI] [PubMed] [Google Scholar]
- 142.Afonso M.B., Rodrigues P.M., Simão A.L., Gaspar M.M., Carvalho T., Borralho P., Bañales J.M., Castro R.E., Rodrigues C.M.P. miRNA-21 ablation protects against liver injury and necroptosis in cholestasis. Cell Death Differ. 2018;25:857–872. doi: 10.1038/s41418-017-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Namekawa T., Ikeda K., Horie-Inoue K., Inoue S. Application of prostate cancer models for preclinical study: advantages and limitations of cell lines, patient-derived xenografts, and three-dimensional culture of patient-derived cells. Cells. 2019;8:E74. doi: 10.3390/cells8010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou X., Wang T. Using the Wash U Epigenome Browser to examine genome-wide sequencing data. Curr. Protoc. Bioinformatics. 2012;40 doi: 10.1002/0471250953.bi1010s40. 10.10.1–10.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Löffler D., Brocke-Heidrich K., Pfeifer G., Stocsits C., Hackermüller J., Kretzschmar A.K., Burger R., Gramatzki M., Blumert C., Bauer K. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 146.Kwak H.-J., Kim Y.-J., Chun K.-R., Woo Y.M., Park S.-J., Jeong J.-A., Jo S.H., Kim T.H., Min H.S., Chae J.S. Downregulation of Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene. 2011;30:2433–2442. doi: 10.1038/onc.2010.620. [DOI] [PubMed] [Google Scholar]
- 147.Leite K.R.M., Reis S.T., Viana N., Morais D.R., Moura C.M., Silva I.A., Pontes J., Jr., Katz B., Srougi M. Controlling RECK miR21 promotes tumor cell invasion and is related to biochemical recurrence in prostate cancer. J. Cancer. 2015;6:292–301. doi: 10.7150/jca.11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao M., Patel R., Ahmad I., Fleming J., Edwards J., McCracken S., Sahadevan K., Seywright M., Norman J., Sansom O., Leung H.Y. SPRY2 loss enhances ErbB trafficking and PI3K/AKT signalling to drive human and mouse prostate carcinogenesis. EMBO Mol. Med. 2012;4:776–790. doi: 10.1002/emmm.201100944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wietecha M.S., Chen L., Ranzer M.J., Anderson K., Ying C., Patel T.B., DiPietro L.A. Sprouty2 downregulates angiogenesis during mouse skin wound healing. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H459–H467. doi: 10.1152/ajpheart.00244.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang X., Tang N., Hadden T.J., Rishi A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 151.Moriishi T., Kawai Y., Komori H., Rokutanda S., Eguchi Y., Tsujimoto Y., Asahina I., Komori T. Bcl2 deficiency activates FoxO through Akt inactivation and accelerates osteoblast differentiation. PLoS ONE. 2014;9:e86629. doi: 10.1371/journal.pone.0086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Memmott R.M., Dennis P.A. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell. Signal. 2009;21:656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou H., Huang S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr. Protein Pept. Sci. 2011;12:30–42. doi: 10.2174/138920311795659407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oh J., Takahashi R., Kondo S., Mizoguchi A., Adachi E., Sasahara R.M., Nishimura S., Imamura Y., Kitayama H., Alexander D.B. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi C., Sheng Z., Horan T.P., Kitayama H., Maki M., Hitomi K., Kitaura Y., Takai S., Sasahara R.M., Horimoto A. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc. Natl. Acad. Sci. USA. 1998;95:13221–13226. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miller M.A., Sullivan R.J., Lauffenburger D.A. Molecular pathways: receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin. Cancer Res. 2017;23:623–629. doi: 10.1158/1078-0432.CCR-16-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campbell K.J., Tait S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8:180002. doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen L., Ling M., Li Y., Xu Y., Zhou Y., Ye J., Pang Y., Zhao Y., Jiang R., Zhang J., Liu Q. Feedback regulations of miR-21 and MAPKs via Pdcd4 and Spry1 are involved in arsenite-induced cell malignant transformation. PLoS ONE. 2013;8:e57652. doi: 10.1371/journal.pone.0057652. [DOI] [PMC free article] [PubMed] [Google Scholar]