Abstract
Various drug transporters are widely expressed throughout the intestine and play important roles in absorbing nutrients and drugs, thus providing high quality targets for the design of prodrugs or nanoparticles to facilitate oral drug delivery. In particular, intestinal carnitine/organic cation transporter 2 (OCTN2) and mono-carboxylate transporter protein 1 (MCT1) possess high transport capacities and complementary distributions. Therefore, we outline recent developments in transporter-targeted oral drug delivery with regard to the OCTN2 and MCT1 proteins in this review. First, basic information of the two transporters is reviewed, including their topological structures, characteristics and functions, expression and key features of their substrates. Furthermore, progress in transporter-targeting prodrugs and nanoparticles to increase oral drug delivery is discussed, including improvements in the oral absorption of anti-inflammatory drugs, antiepileptic drugs and anticancer drugs. Finally, the potential of a dual transporter-targeting strategy is discussed.
Keywords: Carnitine/organic cation transporter 2 (OCTN2), Monocarboxylate transporter protein 1 (MCT1), Transporter-targeting, Nanoparticle, Prodrug
Graphical abstract
In this review, we outline the recent developments in transporter-targeting oral drug delivery, especially on intestinal carnitine/organic cation transporter 2 (OCTN2) and monocarboxylate transporter protein 1 (MCT1).
1. Introduction
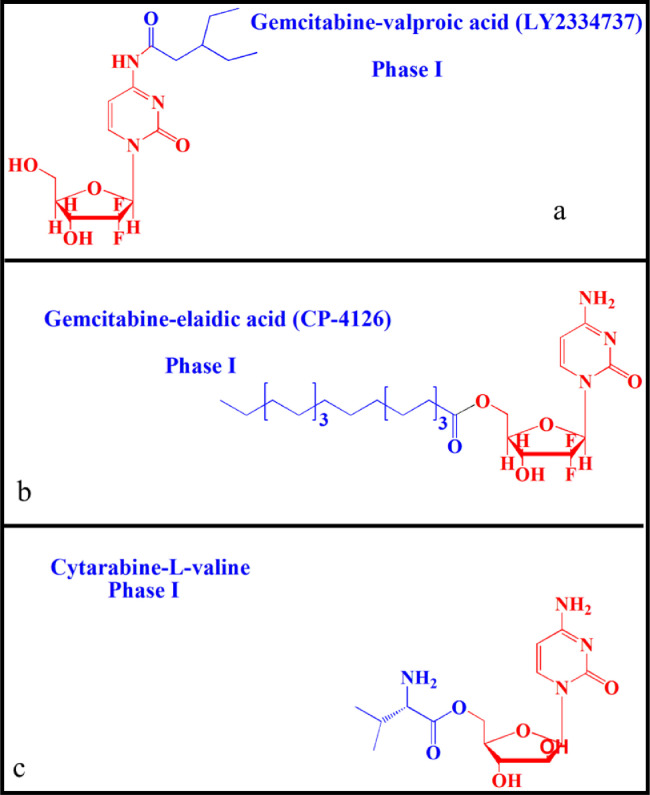
Oral drug delivery has long been considered a safe and convenient route for drug development. Unfortunately, not all drugs can be administered orally. Many factors, such as stability, solubility and permeability, affect oral bioavailability [1,2]. For example, oral insulin is of great significance to people with diabetes, but poor gastrointestinal tract (GI) stability leads to ineffectiveness [3]. In response, lipophilic prodrugs have been designed to overcome the barriers of oral drug delivery. Zhang et al. synthesized a valproic acid ester of gemcitabine (LY2334737, phase I) to treat solid tumors [4], [5], [6]. The prodrug had excellent stability in the GI, thus increasing the oral bioavailability by 1.4-fold compared to gemcitabine in rats (Fig. 1a). In addition, elaidic acid ester of gemcitabine greatly reduced tumor resistance originating from nucleoside transporter-dependent cellular uptake of gemcitabine [7,8] (Fig. 1b). These results revealed that lipophilic prodrug is an effective strategy to enhance oral absorption. However, the clinical results were far from satisfactory due to the high distribution on the membrane of epithelial cells and rapid degradation in the GI [9]. Additionally, nanoparticle-based drug delivery systems (nano-DDS) have been used to improve the oral bioavailability of drugs, with advantages of improved drug membrane permeability, stability and therapeutic efficacy [10], [11], [12]. However, the serious systemic toxicity of conventional nano-DDS caused by poor tissue selectivity significantly limits their clinical application [13,14]. By contrast, receptor-targeted nano-DDS have shown significant advantages. However, the variability and heterogeneity of membrane receptors limits their targeting efficacy [15], [16], [17]. Therefore, designing a high-efficiency delivery system to transport drugs with poor oral bioavailability has long been a focus of pharmacists.
Fig. 1.
Chemical structures of lipophilic prodrugs and carrier prodrugs in clinical trials: a: LY2334737, b: CP-4126 and c: Cytarabine-L-valine.
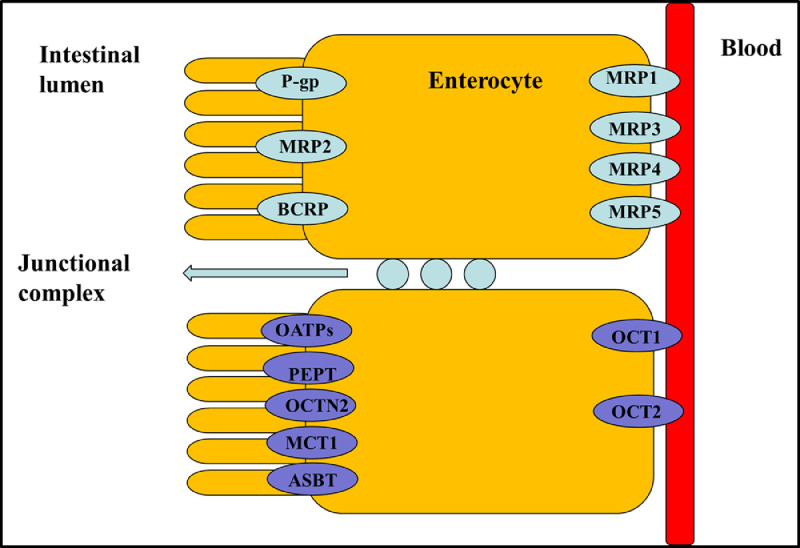
The human body consists of several physiological barriers that express a number of membrane transporters that are categorized into two families: solute carriers (SLC) and ATP-binding cassette (ABC) [18], [19], [20], [21], [22], [23]. Most ABC proteins play an important role in efflux of xenobiotics out of cells to sustain cell survival, and thus providing a target for inhibitor designing for disease treatment or drug delivery [24]. Meanwhile, SLC transporters transport nutrients as well as determine drug absorption and distribution. Therefore, a variety of transporter assays are development to test drug transporter interactions, transporter-mediated drug-drug interactions, and transporter-mediated toxicity [25]. Recently, intestinal nutritional transporter-based drug delivery strategies have attracted more attention (Fig. 2). In this strategy, the nutritional substrate is often coupled with parent drug or polymer materials to synthesize targeted prodrugs or nanoparticle-loading drugs. For instance, PEPT1-targeted prodrugs, such as zadovudine [26], acyclovir [26], ganciclovir [27], levovirin [28], and didanosine [29], showed significantly increased oral bioavailability of parent drugs. In particular, L-valine ester of cytarabine has entered into clinical evaluation [30] (Fig. 1c). In addition, bile acid transporter (ASBT) is highly expressed in the small intestine and has been used as a target to develop nanoparticles for oral-transporting insulin [31]. Recently, dipeptide-modified nanoparticles that target PEPT1 were proven to be a promising platform for the oral delivery of hydrophobic drugs [32]. By contrast, intestinal OCTN2 and MCT1 proteins have attracted attention due to their improved transport capacity compared with PEPT1 and ASBT proteins. OCTN2 is an integral membrane protein that absorbs carnitine in the small intestine. MCT1 is also located in the intestine and is known to facilitate the transfer of mono-carboxylates from the lumen into the circulatory system [33,34]. The wide distribution of the two transporters throughout the intestine provides a new possibility for designing an oral drug delivery system. Thus, substrates could be coupled to parent drugs or polymers to synthesize targeted prodrugs or nanoparticle-loading drugs that are recognized by OCTN2 or MCT1 protein [33,[35], [36], [37], [38]. Interestingly, we found that the transport activity of OCTN2 was higher in the duodenum and jejunum, while that of MCT1 was higher in the colon and rectum, although both proteins were expressed throughout the intestine. If a prodrug or nanoparticle was designed to target two transporters simultaneously, it would improve oral bioavailability compared with a mono-transporter targeted strategy. Accordingly, the present article summarizes development of the intestinal OCTN2 and MCT1 protein-targeted strategy. First, some basic information of the transporter, such as topological structure, characteristics and function, expression and key features of substrates, are reviewed in this article. Second, studies of prodrug- and nanoparticle-targeted intestinal OCNT2 and MCT1 protein are reviewed. Finally, a dual transporter-based drug targeted strategy is also discussed.
Fig. 2.
Diagram of major drug transporter proteins expressed by the intestinal epithelia, including intestinal uptake (purple) and efflux (green) transporters. Multidrug resistance protein (MDR1,P-glycoprotein), multidrug resistance associated protein (MRP), breast cancer resistance protein (BCRP), monocarboxylate transporter protein (MCT), peptide transporter protein (PEPT), organic anion transporting polypeptide (OATP), organic cation transporter (OCT), carnitine/organic cation transporter 2 (OCTN2), and bile acid transporter (ASBT) (Reproduced with permission from [23]. Copyright 2012 Elsevier B.V.).
2. OCTN2 transporter
2.1. Topological structure of OCTN2
Obtaining the topological structure of OCTN2, especially the orientation of the amino (N-) and carboxyl (C-) termini of a trans-membrane section, the number of TMD, and the position of the trans-membrane segments in the protein sequence, may contribute to revealing protein-substrate interactions and clarify the important biological functions of proteins [39]. To this end, hydropathy profiles were employed, and the results showed that OCTN2 possessed 12 TMD, with the intracellular C-terminus and N-terminus exposed to the cytosol (Fig. 3) [40]. To affirm the precise domain of the substrate binding site, site-directed mutagenesis was performed. Wang et al. found that the mutation of amino acid E452K, located in the hydrophilic loop between TMD 10 and 11, resulted in decreased affinity for Na+ but not carnitine [41]. Thus, this segment was a Na+ binding site. Moreover, Seth et al. reported that the mutation of S467C and P478L, located in TMD 11 in OCTN2, decreased the affinity of anions [42]. Therefore, this segment was a carboxyl moiety binding site of carnitine. Seth et al. also found that carnitine uptake was inhibited by TEA in the mutation model, whereas TEA did not show complete competitive inhibition for carnitine uptake, suggesting that the binding sites for TEA and carnitine partially overlap on OCTN2 [42]. However, the binding site of cations in OCTN2 has not been reported until now.
Fig. 3.
Proposed membrane topology of OCTN2 (Reproduced with permission from [31]. copyright 2008 American Chemical Society).
2.2. Characteristics and function of OCTN2
L-carnitine is an essential nutrient with various important physiological functions: (i) it is vital for β-oxidation of long-chain fatty acids in the mitochondria; (ii) it maintains cell membrane stability by acetylating membrane phospholipids; and (iii) it protects cells in metabolic disorders by antioxidant effects [43], [44], [45], [46]. In mammals, L-carnitine is absorbed from the small intestine by OCTN2 [47]. With its help, the concentration of intracellular carnitine is approximately 50 times higher than that in the extracellular space, indicating that OCTN2 is a protein with high transport capacity [48]. Only a few organs, such as the liver and kidneys, have the ability to biosynthesize L-carnitine [49]. Therefore, many tissues, such as skeletal and heart muscle, are highly dependent on OCTN2 protein for supplying L-carnitine.
OCTN2 is a specific Na+/carnitine co-transporter and contributes to carnitine homeostasis in humans. OCNT2 has been cloned from rat [50,51], human [52] and mouse [53]. The Michaelis constant of carnitine transport by OCTN2 is in the range of 10–20 µM, indicating that OCTN2 is a high affinity transporter. OCTN2 also transports drugs, such as TEA, ipratropium, prednisolone, and beta-lactam antibiotics [54], [55], [56], in a pH-dependent manner. Therefore, the cellular uptake of carnitine would inevitably be inhibited when taking ionic medicine, such as cephaloridine, levofloxacin and grepafloxacin, resulting in carnitine deficiency [57,58]. Although several reports have focused on drug-drug interactions mediated by OCTN2, few studies have provided key data, such as IC50/KI, which are important for drug development. Thus, we can utilize OCTN2 protein as a target for oral prodrugs or nanoparticle design.
2.3. The expression of OCTN2
OCTN2 transporters are widely distributed in various organs in human and mouse (Table 1) [59,60]. In humans, it is found in the liver, heart, placenta and muscle [61]. Recently, OCTN2 was discovered on hematopoietic cells [62]. The expression of OCTN2 can be regulated by some pathological conditions. For instance, intestinal OCTN2 was downregulated in inflammatory bowel disease (IBD) [63]. The mRNA level of OCTN2 was significantly reduced in dilated nonischemic cardiomyopathy (DCM) patients, while it was un-affected in patients with acute myocarditis (AMC) [64]. Additionally, therapeutic drugs also affect the expression of OCTN2 in tumors. Due to the high expression of proliferator-activated receptor alpha (PPARA) and gamma (PPARG), the expression of OCTN2 in tumors is downregulated. However, the decreased level of OCTN2 in tumor cells could be restored by decitabine, a demethylating reagent, resulting in the increased uptake of oxaliplatin [65].
Table 1.
Characteristics of the OCTN2 transporter (Reproduced with permission from [59]. Copyright 2012 John Wiley & Sons, Ltd.).
| Name | Gene | Tissue expression | Substrate | Km (L-carnitine) |
|---|---|---|---|---|
| OCTN2 (human) |
SLC22A5 | Brain, heart, intestine, kidney | D and L-carnitine, acetylcarnitine, TEA, γ-butyrobetaine, cephaloridine, imatinib, mildronate, oxaliplatin, pyrilamine, quinidine, spironolactone, valproate |
4.3 µM (D-carnitine) 22.1 µM (L-carnitine) 215 µM (TEA) |
| OCTN2 (mouse) |
SLC22A5 | Liver, lung, pancreas, placenta, thyroid, trachea, etc. |
In this article, we focus on the expression of OCNT2 in the intestine. Kato found that OCTN2 is mainly localized at the brush-border of apical membranes of intestinal epithelial cells [66]. By detecting the mRNA level of different intestinal segments, Meiery found that OCTN2 was expressed throughout the intestine, while PepT1 is only expressed in the anterior of the small intestine. These results indicate that the interaction time and absorptive area of substrate to transporter is greatly increased, which is suitable for transporter-based prodrug and nanoparticle design [67].
2.4. Key features of OCTN2 substrates
OCTN2 preferentially transports L-carnitine and cations, but the transportation mechanism is different. As shown in Fig. 2, the transport of L-carnitine is Na+-dependent, while the transport of cations is H+-dependent [68,69]. Interestingly, the L-carnitine transported out of cells is independent of OCTN2. In addition to TEA and L-carnitine, OCTN2 also accepts valproic acid. Therefore, Ohashi et al. proposed a model of substrate binding sites of OCTN2 protein, including a cation site, anion site and Na+ site (Fig. 4) [69].
Fig. 4.
Model of OCTN2-mediated transport of L-carnitine and drugs in intestinal epithelial cells. Carnitine is taken up by cells in a sodium ion-dependent manner, while drug transport is sodium ion-independent. Carnitine binding site is only functional when sodium ion is present on the same side. Carnitine/organic cation transporter 2 (OCTN2) (Reproduced with permission from [66]. Copyright 2006 American Society for Pharmacology and Experimental Therapeutics).
To optimize the derivative position of L-carnitine for prodrug design, 22 analogues were used [70]. The results suggested that the affinity of substrate for OCTN2 protein decreased when L-carnitine was esterified at the carboxylic position. Additionally, the affinity of substrate for OCTN2 also depends on the type of amine of L-carnitine. The binding ability follows the order of quaternary amine > tertiary amine > secondary amine> primary amine (Fig. 5). Accordingly, the amine group and carboxylate group of the L-carnitine are critical groups in the recognition of OCTN2 for substrates. By contrast, the 3′-OH shows a negligible effect and thus should be used as conjugate site for prodrug design. In response, L-carnitine was covalently coupled to prednisolone through the 3′-OH (PDSC) and carboxylate group (PDC) to synthesize OCTN2-targeted prodrugs. PDSC displayed a 1.79-fold increase in OCNT2-mediated cellular uptake than prednisolone, proving that 3′-OH can be used as a derivative site [56].
Fig. 5.
Effects of L-carnitine analogues on OCTN2-mediated L-carnitine transport. The values given are from competition experiments and show the percent of L-carnitine (2.5 µM) uptake when measured in the presence of the analogues; the concentration of L-carnitine analogues is 500 µM, and that of the two 3′‑hydroxyl derivatives is 100 µM. L-carnitine uptake measured in the absence of any competitors is taken as 100%. carnitine/organic cation transporter 2 (OCTN2) (Reproduced with permission from [70]. Copyright 2011 Wiley-Liss, Inc.).
2.5. Strategies to target OCTN2 for enhanced oral drug delivery
Despite many attempts, the current oral strategies are still far from satisfactory. Due to progress in molecular biology, many SLC transporters have been discovered in the intestine. The typical transporters are PEPT1, ASBT and OCTN2, which can accept both nutrients and therapeutic drugs. The molecular revolution of membrane transporters has spurred novel strategies to design transporter-targeted prodrugs and nanoparticles for increased intestinal permeability of drugs. Recently, great progress has been made, which will be discussed in the subsequent sections.
2.5.1. OCTN2-targeted oral prodrugs
Transporter-targeted prodrug strategies have obvious advantages, such as high permeability and appropriate water solubility, in which pro-moieties as ligands are covalently attached to drugs to selectively target certain membrane transporters. For example, OCTN2 protein was used as a target to design oral prodrugs for butyrate [63] and gemcitabine [33] (Fig. 6). Butyrate is a short-chain fatty, with a role in preventing ulcerative colitis. Butyrate is mainly absorbed via intestinal MCT1 protein [71], [72], [73], [74], [75]. However, its expression is downregulated in the intestinal mucosa of patients with IBD [76]. Additionally, L-carnitine has been shown to have protective effects in ulcerative colitis [77,78]. Accordingly, a butyrate ester of L-carnitine was synthesized by Srinivas et al. to treat gut inflammation (Fig. 6a). Butyrate ester of L-carnitine is taken up through OCTN2 or ATB0+ and then hydrolyzed to release butyrate and L-carnitine in epithelial cells for the treatment of gut inflammation. The cellular uptake experiment was performed in ATB0+ high expressing oocytes and hOCTN2-transfected HRPE cells. The results demonstrated that the butyrate ester of L-carnitine inhibited L-carnitine (IC50 = 0.3 µM) and glycine (IC50 = 4.6 mM) uptake, indicating that prodrug was a substrate of ATB0+ and OCTN2. Moreover, the uptake results of butyrate ester of L-carnitine in two cells showed different levels, suggesting that OCTN2 is a high affinity transporter (K0.5 = 0.04 µM), whereas ATB 0+ is a low affinity transporter (K0.5 = 1.4 mM).
Fig. 6.
L-carnitine conjugated prodrugs for OCTN2 targeting. L-carnitine is identified in pink, and the parent drug is identified in black. a: Butyrate ester of L-carnitine; b: L-carnitine ester of gemcitabine. GSC: L-carnitine-succinic-gemcitabine, GHC: L-carnitine-hexylic-gemcitabine, GOC: L-carnitine-octanedioic-gemcitabine, GDC: L-carnitine-decanedioic-gemcitabine. carnitine/organic cation transporter 2 (OCTN2).
Gemcitabine has been used as a first line drug in many solid tumors for chemotherapy, including pancreatic and non-small cell lung cancer [79], [80], [81]. However, hematological toxicity and other side effects significantly limit its clinical application by the intravenous route [6]. To develop high-efficacy, low-GI toxicity oral gemcitabine products, we proposed an OCTN2-targeting oral prodrug strategy. Four targeted prodrugs, differing in the length of linkage, were synthesized, as shown in Fig. 6b. L-carnitine was used as the ligand and was coupled with the N4-amino of gemcitabine. Compared to gemcitabine, the prodrugs displayed significantly improved stability (3-fold) and oral bioavailability (5-fold). Further, the cellular uptake of the prodrugs was Na+-dependent and temperature-dependent and was inhibited by L-carnitine. These results indicate that the prodrugs were absorbed into cells via OCTN2. In addition, a molecular docking study was performed to evaluate the affinity of the prodrugs for OCTN2. The hexane diacid-linked prodrug exhibited the highest affinity for OCTN2 among the prodrugs. Importantly, safety studies using hematoxylin and eosin (H&E) histological analysis suggested that there was no toxicity of the targeted prodrugs in mice at short exposure durations to the heart, liver, spleen, lung or kidney, likely due to low tissue distribution and rapid clearance in various tissues. These results suggest that L-carnitine ester of gemcitabine is a promising strategy to enhance the oral bioavailability of gemcitabine (Fig. 7).
Fig. 7.
Mechanism of OCTN2-targeting prodrug uptake by the intestine. carnitine/organic cation transporter 2 (OCTN2), hENT1: human equilibrative nucleoside transporter-1.
PEPT1 is a low affinity transporter with large Km and Vmax, indicating that this transporter is not easily saturated under high concentrations of substrate and thus is an ideal target for development of an oral prodrug [82]. By contrast, OCTN2 is a transporter with low Km and Vmax. Thus, it is easily saturated and is theoretically not suitable for the design of prodrugs. Amazingly, OCTN2 showed better transport capacity compared to PEPT1 in the above studies, likely due to its fast transport speed. Therefore, OCTN2 is also a good target for the design of oral prodrugs.
2.5.2. OCTN2-targeted oral nanoparticles
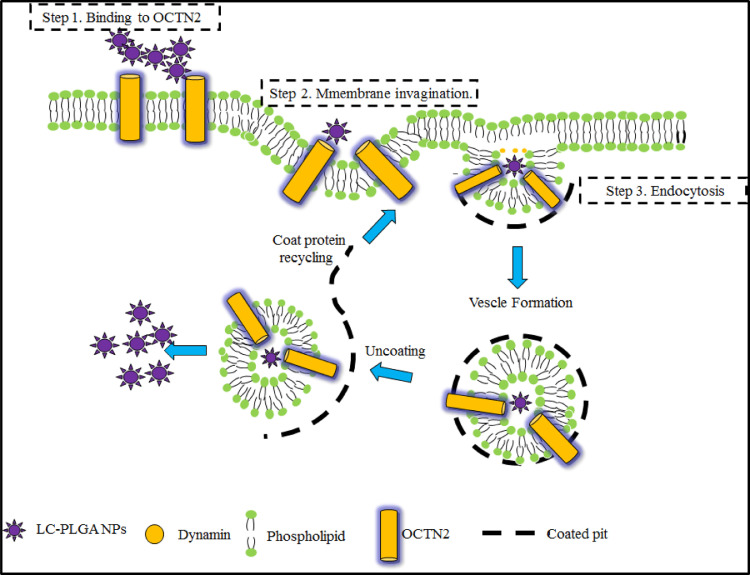
Although the transporter-targeted prodrug strategy has made great progress, poor stability and low activatable efficiency still limit its clinical application [18,20]. By contrast, transporter-based nano-DDS has shown obvious advantages in oral drug delivery, such as improved drug stability, permeability and safety. Paclitaxel (PTX) is widely used in the clinic for treating solid tumors [83]. However, no oral preparations have been developed for marketing due to its poor solubility and high GI toxicity. Based on the promise of OCTN2 as a target for oral drug delivery, we attempted to design and synthesize OCTN2-targeting PLGA nanoparticles loading paclitaxel [34]. All nanoparticles showed slower but prolonged release of paclitaxel compared to free taxol. L-carnitine-modified nanoparticles showed increased cellular uptake compared to non-targeted nanoparticles and was inhibited by L-carnitine, revealing that OCTN2 helps to increase uptake of the nanoparticles. Further, we evaluated the effect of Na+ and Cl− on the binding of OCTN2-targeted nanoparticles. The results showed that the binding of OCTN2-targeted nanoparticles was only dependent on Na+. However, these results could simply reveal the binding of nanoparticles to OCTN2 protein and not reflect the actual uptake mechanism since the size of the nanoparticle is larger than free L-carnitine by several orders of magnitude. Therefore, we further evaluated the endocytosis mechanism of the nanoparticle using various inhibitors, including indomethacin (an inhibitor of caveolin), chlorpromazine (an inhibitor of clathrin), colchicine (an inhibitor of macropinocytosis) and quercetin (an inhibitor of caveolin/clathrin). The results indicated that nanoparticles enter the cell via clathrin- and caveolae-mediated endocytosis. Accordingly, the specific transport process of OCTN2-targeted nanoparticles is summarized as follows (Fig. 8): (i) nanoparticles bind to OCTN2 in a Na+-dependent manner; (ii) after binding, OCTN2 transporter shifts from outward-facing to an occluded state, which induces membrane invagination; (iii) then, endocytosis occurs, with multipoint binding increasing the interaction and accelerating nanoparticle absorption; (iv) after endocytosis, some complexes may fuse with the lysosome, resulting in protein degradation, and some may go through the cytosol for transcytosis and release of nanoparticles to the basolateral side. The pharmacokinetics study exhibited that the oral bioavailability of paclitaxel loading in targeted nanoparticles increased by 3 times compared with non-targeted nanoparticles, confirming the contribution of OCTN2-targeted nanoparticles. Interestingly, the tmax of nanoparticles was three times longer than the parent drug, likely because the endocytosis process of nanoparticles is complex and time-consuming. These results suggest that OCTN2 is an ideal target for increasing the oral absorption of nanoparticle-loading drugs.
Fig. 8.
Mechanism of L-carnitine-conjugated nanoparticle uptake. LC-PLGA NPs bind to OCTN2 on the cell membrane in the presence of Na+, and OCTN2 then shifts conformation from outward-facing to an occulated state, forming a complex containing LC PLGA NPs, Na+ and OCTN2. The formed complex induces membrane invagination and subsequent endocytosis. Additionally, a multivalent binding connection between nanoparticles and the membrane further accelerates the absorption of nanoparticles. After endocytosis, some complexes might fuse with the lysosome, resulting in protein degradation, and some may go through the cytosol for transcytosis and release of nanoparticles to the basolateral side (Reproduced with permission from [34]. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim).
3. MCT1 transporter
3.1. Topological structure of MCT1
As shown in Fig. 9, the MCT1 transporter contains 12 TMD, which are predicted by hydropathy plots [84] using the Kyte–Doolittle algorithm [85] and the TMpred program [86]. To affirm the precise sites relevant to substrate binding, various technologies were used. Inhibitor and photo affinity-labeling studies suggest that the N-terminus of the protein is responsible for the energy coupling (e.g., H+ or Na+), membrane insertion and correct structure maintenance [87]. Mutagenesis studies revealed that some critical domains were related to the function of MCT1. For example, TMD 8 plays an essential role in proton and carboxylate binding [88]. The domain of the C-terminus of MCT1 is thought to help determine substrate specificity [89,90]. The loop between TMD 7 and 8 and the loop between TMD 11 and 12 are the substrate-binding sites [91]. The domain between TMD 4 and TMD 5 contribute to the conformational movements, protein stability, trafficking and stereo-selectivity [92]. However, no crystal structure of MCT1 has been reported to date.
Fig. 9.
Proposed membrane topology of MCT1 (Reproduced with permission from [64]. Copyright 2011 American Society for Investigative Pathology.). MCT1: mono-carboxylate transporter protein 1.
Another important factor for MCT1 is ancillary proteins with roles of proper intracellular trafficking, cell surface expression, and function. Previous studies have suggested that MCT1 protein requires the presence of CD147 or the related protein GP70, which contain one trans-membrane, two immunoglobulin and one carboxyl terminus cytoplasmic tail [93]. The interaction of the carboxyl terminus cytoplasmic tail and the fluorescent protein pulls the MCT1 protein from the synthetic site to the cell surface. Therefore, loss of the co-expression of ancillary proteins would result in the accumulation of MCT1 in the endoplasmic reticulum or Golgi body [94].
3.2. Characteristics and function of MCTs
Glycolysis is very important to muscle, red blood cells and tumor cells for maintaining normal function and proliferation [95]. Large amounts of lactic acid produced by glycolysis are rapidly pumped out of cells via proton-linked mono-carboxylate transporters (MCTs) [96], [97], [98]. Moreover, butyrate, the principal energy source for colonic epithelial cells originating from microbial fermentation of dietary carbohydrates, is also a substrate of MCT1 protein in the intestine [99]. Butyrate is present in the colon in millimolar concentrations and plays an important role in their proliferation, differentiation and apoptosis [99]. Furthermore, MCTs are essential for the transport of important mono-carboxylates, including pyruvate, ketone bodies acetoacetate, and β-hydroxybutyrate. Therefore, MCTs are vital transporters for cellular metabolism and intercellular signal transduction [84,100] (Fig. 10). As such, MCTs have attracted increased attention. MCTs belong to the SLC16A family and contain 14 family members, MCT1-MCT14. As shown in Table 2, MCTs are widely expressed at various tissues, including the intestine, brain, kidney, liver and tumor, delivering various substrates [101]. Among the MCT transporters, we focus on MCT1 in this review because it is a mono-carboxylate transporter mainly responsible for the proton-linked transport of several mono-carboxylate analogues and is also a potential target for oral drug delivery.
Fig. 10.
Metabolic pathways involving monocarboxylate transport across the mitochondrial and plasma membranes (Reproduced with permission from [84]. Copyright 1999 Biochemical Society.). Glc-1-P and Glc-6-P, glucose 1-phosphate and glucose 6-phosphate; AcβHB, acetoacetate plus β-hydroxybutyrate.
Table 2.
The MCT family of transporters (Reproduced with permission from [101]. Copyright 2016 The Authors).
| Human gene name | Protein name | Sequence accession ID | Main substrates | Tissue distribution |
|---|---|---|---|---|
| SLC16A1 | MCT1 | NM_003051 | Lactate, pyruvate, ketone bodies | Ubiquitous except βcells of the endocrine pancreas |
| SLC16A2 | MCT8 | NM_006517 | T2,rT3,T3,T4 | Ubiquitous |
| SLC16A3 | MCT4 | NM_004207 | Lactate, Ketone bodies | Skeletal muscle, chondrocytes, leukocytes, testis, lung, brain, ovary, placenta, heart |
| SLC16A4 | MCT5 | NM_004696 | – | Brain, muscle, liver, kidney, lung, ovary, placenta, heart |
| SLC16A5 | MCT6 | NM_004695 | Bumetanide probenecid nateglinide | Kidney, muscle, brain, heart, pancreas, prostate, lung, placenta |
| SLC16A6 | MCT7 | NM_004694 | Ketone bodies | Liver, brain, pancreas, muscle, prostate |
| SLC16A7 | MCT2 | NM_004731 | Pyruvate, lactate, ketone bodies | High expression in testis, moderate to low in spleen, heart, kidney, pancreas, skeletal muscle, brain and leukocytes |
| SLC16A | MCT | NM_ | ||
| SLC16A8 | MCT3 | NM_013356 | Lactate | Retinal pigment epithelium, choroid plexus |
| SLC16A9 | MCT9 | NM_194298 | Carnitine | Endometrium, testis, ovary, breast, brain, kidney, spleen, retina |
| SLC16A10 | MCT10/TAT1 | NM_018593 | Aromatic amino acids, T3, T4 | Kidney (basolateral), intestine, muscle, placenta, heart |
| SLC16A11 | MCT11 | NM_153357 | – | Skin, lung, ovary, breast, lung, pancreas, retinal pigment epithelium, choroid plexus |
| SLC16A12 | MCT12 | NM_213606 | – | Kidney, retina, lung, testis |
| SLC16A13 | MCT13 | NM_201566 | – | Breast, bone marrow stem cells |
| SLC16A14 | MCT14 | NM_152257 | – | Brain, heart, muscle, ovary, prostate, breast, lung, pancreas liver, spleen, thymus |
3.3. The expression of MCT1
As shown in Fig. 9, the expression of MCT1 is rather ubiquitous. In the heart, cardiomyocytes display high MCT1 expression [102], [103], [104]. In the liver, it is present on hepatocytes and is responsible for pumping lactic acid out of cells [102,103,105,106]. In the GI, different levels of MCT1 protein are widely distributed on the epithelial cells of the small and large intestines, contributing to the absorption of mono-carboxylates from the lumen to the blood [107], [108], [109], [110] (Fig. 9). Recently, a growing number of studies have shown that MCT1 protein is highly expressed in tumors to take up large amounts of lactic acid into cells as fuel for aerobic glycolysis [111]. The expression of MCT1 protein can be upregulated in human muscle by exercise training to extrude lactate and H+ to the interstitium. Further, the level of increased MCT1 expression is related to the intensity of training [112]. In addition, cerebral ischemia can upregulate the expression of MCT1 in astrocytes for transferring intracellular lactate to the extracellular space, which facilitates lactate utilization by injured neurons to improve neurological deficits [113]. In inflammatory bowel disease patients, the expression of MCT1 is strongly downregulated, leading to butyrate deficiency [76]. Beyond this, butyrate, a transporter substrate, has been shown to increase MCT1 expression at the mRNA and protein levels [114].
Due to the discussed potential of MCT1 protein in developing intestinal-targeted prodrugs or nanoparticles, we highlight the expression of MCT1 in the intestine. Shimoyama et al. detected the expression of intestinal MCT1 using western blotting and immunohistochemical staining. The results showed that MCT1 protein was observed on the basolateral membranes of epithelial cells throughout the intestines. The immuno-reactivity was greater in the large intestine than in the small intestine [115]. Furthermore, the mRNA of MCT1 increased by 20-fold in the large intestine compared with the small intestine [116]. These data indicate that the major transport site of MCT1 protein is the posterior segment of the small intestine and large intestine.
3.4. Key features of MCT1 substrates
MCT1 is responsible for the bidirectional transport of mono-carboxylates, such as pyruvic acid, lactic acid, acetoacetic acid and β-hydroxybutyric acid, in an H+-dependent manner [117]. As shown in Fig. 11, the affinity for pyruvic acid is the best among the four MCT1 substrates. Interestingly, mono-carboxylate transport by MCT1 is improved when the C-2 and C-3 positions are substituted [118], likely due to the increase in its binding affinity for the transporter. In addition, the transport level of L-lactic acid is higher than D-lactic acid, indicating that MCT1 has stereo-selectivity for substrate transport [117]. Beyond these, some therapeutic compounds could also be transported by MCT1, including β-lactam antibiotics, penicillins [119], nonsteroidal anti-inflammatory drugs, valproic acid [120], atorvastatin [121] and nateglinide [122]. Through structural analysis, we found that these drugs all contained a free carboxyl group. Thus, we conclude that the carboxyl group may be a vital factor for transport by MCT1.
Fig. 11.
Uptake mechanism of substrate via MCT1 and the affinity for different substrates (pyruvate, L-lactate, acetoacetate, D-β-hydroxybutyrate). The affinity of MCT1 for the substrates was judged by the Km: the smaller the Km, the higher the affinity. MCT1: mono-carboxylate transporter protein 1.
3.5. MCT1-targeting oral prodrug
The MCT1 transporter is overexpressed throughout the intestine and is responsible for the transport of short-chain fatty acids from the intestine to the blood. As such, MCT1 can provide a broadly oral absorption window compared with other intestinal transporters. Therefore, the MCT1 transporter has attracted increased attention for the rational design of oral prodrugs, such as gabapentin [123], 5-fluorouracil [124] and gemcitabine. Gabapentin is a GABA analogue that has been used for the treatment of anxiety disorders, restless legs syndrome and hot flashes [125], [126], [127]. However, low oral bioavailability, high inter-patient variability, nonlinear pharmacokinetics and short half-life have severely limited its clinical application. To overcome these shortcomings, XP13512, a novel prodrug of gabapentin, was synthesized to target intestinal MCT1 (Fig. 12a). The prodrug stability, cellular uptake and pharmacokinetics were evaluated. The stability results showed that the hydrolysis rate of XP13512 to gabapentin was slow in the plasma but rapid in liver homogenates, indicating that the primary bio-activation site is the liver followed by the plasma. To confirm the contribution of MCT1 transporter, MCT1-transfected HEK cells were used. As expected, the prodrug significantly inhibited the uptake of lactate (IC50 620 µM), but gabapentin did not. Moreover, lactate decreased the cellular uptake of prodrug by 50%. These results demonstrated that the prodrug is taken up by the MCT1 transporter. The oral bioavailability of gabapentin prodrug increased from 25% to 84% in monkeys. Presently, XP13512 is currently in clinical trials for the treatment of restless legs syndrome and neuropathic pain. This research indicates that intestinal MCT1 is an ideal target for enhancing the oral absorption of gabapentin.
Fig. 12.
Fatty acid-conjugated prodrugs for MCT1 targeting. Fatty acid is identified in blue, and the parent drug is identified in black; a: XP13512; b: hexanedioic acid (n = 1) and octanedioic acid (n = 2) ester of 5-fluorouracil; c: di-acid mono-amidation of gemcitabine.
Although MCT1 can accept various substrates, short-chain fatty acids are the appropriate high-affinity substrates. Accordingly, those are usually selected as ligands for MCT1-targeted prodrug design. We synthesized two MCT1-targeting prodrugs using hexanedioic acid and octanedioic acid as ligands to increase the oral bioavailability of 5-fluorouracil (Fig. 12b). The prodrugs were stable in phosphate buffers (pH1.2, 6.8 and 7.4) but rapidly released 5-fluorouracil in the plasma and liver homogenate, indicating that the prodrugs could be stable in the GI before interaction with MCT1 protein and were easily activated in the circulatory system. The in situ single-pass intestinal perfusion experiment showed that the permeability of the prodrugs increased by 3-fold compared with 5-fluorouracil. Further, the MCT1 competitive inhibitor (quercetin) and substrate (butyrate) significantly decreased the permeability of the prodrugs. These results indicate that the MCT1 transporter is involved in the absorption of the prodrugs. To further confirm the contribution of MCT1, an uptake mechanism study was performed using Caco-2 cells. The results demonstrated that the uptake process was saturable, temperature-dependent, and MCT1-mediated. An in vivo pharmacokinetics study exhibited that the bioavailability of the prodrugs increased by 1–4-fold over 5-fluorouracil. These results further indicate that the MCT1 transporter can be used as a target to design oral prodrugs.
An ideal prodrug should be rapidly converted to the parent drug in the blood. However, little attention has been paid to this aspect during transporter-targeting prodrug design. Therefore, we put forward a dual-function MCT1-targeted prodrug strategy to overcome this problem. Di-acid mono-amidation linkages with different carbon numbers were used as ligands and the immolative spacer, which were covalently linked to the N4-amino group of gemcitabine (Fig. 12c). The stability, cellular uptake mechanism, and pharmacokinetics were performed. Compared to gemcitabine, the prodrugs exhibited better MCT1 affinity, higher gastrointestinal tract stability (3-fold), improved oral bioavailability (8.8-fold), and low gastrointestinal toxicity. The uptake of the prodrugs in Caco-2 cells was significantly inhibited by 1 mM butyrate (decreased by 1.3- to 3.0-fold), while the cellular uptake of gemcitabine was not affected. In addition, the cellular uptake mechanism results exhibited that the uptake process was saturable, temperature-dependent, and MCT1-mediated. An in vivo pharmacokinetics experiment demonstrated that the prodrugs had significantly improved half-lives by 2-fold and oral bioavailability by 8.8-fold. Thus, the high membrane permeability and good gastrointestinal tract stability of the prodrugs significantly increased their pharmacokinetic behavior. It is worth noting that prodrug 2 with a 6-carbon linker exhibited the maximum oral bioavailability over the other prodrugs, indicating that the length of the di-acid linkage is vital in the performance of MCT1-targeted prodrugs. Moreover, the ratio of the prodrugs to gemcitabine in vivo increased with the extension of the carbon chain linkage, suggesting that the length of the linkage can modify the parent drug release rate. Therefore, we investigated the activation mechanism of prodrug 2 by incubation in phosphate buffers with the help of UPLC-MS/MS-Q-TOF. The molecular weight changed from 392.20612 [M Prodrug 2 + H]+ to 263.93022 [M Gem+H]+ in pH 7.4, but the amide hydrolysis intermediate molecular weight of 409.99214 [M Prodrug 2 + H2O+H]+ was not observed. This result indicated that the prodrugs were activated through a cyclization-activating pathway. The above research showed that the di-acid mono-amidation linkage improves the oral absorption via MCT1 and also modifies the drug release by cyclization-activation (Fig. 13).
Fig. 13.
The MCT1-targeting prodrug strategy and the cyclization-activating mechanism of the prodrugs. MCT1: mono-carboxylate transporter protein 1.
3.6. The MCT1-targeting oral nanoparticle
The widely expressed intestinal transporters afford new possibilities for oral nanoparticle design. Among them, PEPT1 and OCTN2 are two ideal targets for delivering antitumor drugs [32,34]. By contrast, the role of MCT1 as a target to facilitate nanoparticle internalization has been studied rarely until now, although it shows better transport capacity in small molecular prodrugs. Wu et al. linked short chain fatty acids butyrate on classical “mucus-inert” polyethylene glycol (PEG) nanoparticle loading insulin to overcome the mucus barrier, the acidic and enzymatic environment in GI tract. Finally, diabetic modified nanoparticle generated 2.87-fold higher oral bioavailability compared with bare PEG NPs in rats. Moreover, various MCT1 inhibitors, butyrate, proionic acid, lactic acid and pravastatin, significantly decreased the cellular uptake of diabetic modified nanoparticle in Caco-2 cells, indicating the MCT1-mediated endocytosis of nanoparticles [128]. In addition, we coupled di-carboxylic acids to the lipophilic polyoxyethylene stearate to synthesize MCT1-targeted polymers loading curcumin. This research is in progress, and we have achieved some preliminary results. Three curcumin-loaded targeted nanoparticles with different chain lengths of di-carboxylic acids as ligands were prepared by emulsion-solvent evaporation, with high encapsulation efficiency and drug loading. Then, the cellular uptake mechanism, endocytosis mechanism and permeability were evaluated to determine the role of MCT1-targeted nanoparticles. In the cellular uptake study, we found that the targeted nanoparticles showed significantly increased intracellular accumulation of coumarin 6 than those of solution in transfected hPepT1-hela cells than mock cells, indicating that MCT1 protein is involved in the enhanced cellular uptake of targeted nanoparticles. The nanoparticle using octanic acid as a ligand exhibited 2.2- to 3.1-fold increased cellular uptake than other nanoparticles, likely due to the improved interaction of the octanic acid linkage with the binding domain of MCT1. Then, the role of co-factor H+ on the interaction of nanoparticles to MCT1 was evaluated. The uptake of targeted nanoparticles was increased by 1.9-fold to 2.2-fold when the extracellular pH changed from 7.4 to 6.0, revealing the proton-dependent interaction of the nanoparticle with MCT1 protein. Moreover, the uptake of targeted nanoparticles was significantly decreased at 4 °C than 37 °C, while non-targeted nanoparticles were not affected. Accordingly, the interaction between nanoparticles and MCT1 protein was H+- and temperature-dependent. However, the size of the nanoparticle is too large to enter the cell directly through the channel of the MCT1 protein; thus, the endocytosis mechanism of the nanoparticle should be further evaluated by competitive inhibition experiments using various protein inhibitors. We found that the uptake of nanoparticles decreased in the presence of clathrin and caveolae protein inhibitors, suggesting that the nanoparticles were anchored to the cell membrane through MCT1 and then entered the cell through endocytosis-mediated by clathrin and caveolae proteins. To evaluate the permeability of targeted nanoparticles, in situ small intestinal perfusion was performed, and the Ka and Papp were determined. The targeted nanoparticles both had better absorption than curcumin and non-targeted nanoparticles. Compared to non-targeted nanoparticles, the Ka of octanic acid nanoparticles increased by 1.6–2.9-fold, and the Papp increased by 2.2–3.6-fold in the duodenum, jejunum, ileum, colon and rectum. Additionally, all the targeted nanoparticles showed the highest permeability in the rectum, consistent with the report that the main transport site of MCT1 is the large intestine. Thus, the targeted nanoparticles could enhance the permeability of curcumin due to MCT1. The results of the regulatory effect of the nanoparticles on MCT1, bio-distribution, pharmacokinetics, pharmacodynamics and safety have not been evaluated yet. The above research preliminarily shows that MCT1 can be used as a target to develop nanoparticles.
Pluronic-85, a tri-block copolymer with the form of PEP-PPO-PEO, consists of a hydrophilic chain of polypropylene oxide (PPO) and a hydrophobic chain of polyethylene oxide (PEO) [129]. Pluronic-85 is used in tablets, suppositories, emulsions and gels and is also used in micelles, resulting in increased solubility, metabolic stability and permeability for drugs [130]. In addition, pluronic-85 can be used in combination with an antitumor drug to overcome multidrug resistance due to the inhibition of p-glycoprotein (P-gp) and multidrug-resistant (MDR) proteins [130]. Recent research showed that poloxamer-85 is also a substrate of MCT1 protein. In cellular studies, the authors found that the uptake of 14C-lactate in bovine brain micro-vessel endothelial cells was abolished by poloxamer-85 at a dose of 0.01% wt. Meanwhile, the lactate levels in the extracellular medium were significantly increased in the presence of various concentrations of poloxamer-85 [131]. Furthermore, safety studies indicated no toxicity of pluronic-85 in the liver, kidney, or brain of mice. Therefore, poloxamer-85 can interact with MCT1 protein and can be used as a large molecular ligand for developing oral nanoparticles to increase the oral bioavailability of drugs with poor membrane permeability. However, no relevant studies have been reported.
4. Combination of intestinal OCTN2 and MCT1 for developing oral prodrugs or nanoparticles
The intestinal transporter-targeted strategy has opened a new method of developing oral prodrugs or nanoparticles. For drugs with low membrane permeability, ligands are introduced to parent drugs or carriers to increase the active transport capacity mediated by the intestinal transporter. Several studies have shown that intestinal OCTN2 protein and MCT1 protein are ideal targets for designing prodrugs or nanoparticles. However, a single transporter is easily saturated, thus limiting the transport level of drugs or nanoparticles and resulting in blood concentrations outside the treatment window. It is well known that multiple transporters are expressed in the intestine, and their distribution and function are different. In in situ single-pass perfusion studies, we found that the transport activity of OCTN2 was higher in the duodenum and jejunum, while that of MCT1 was higher in the colon and rectum, although both proteins were distributed throughout the intestine. If the ligands of two transporters are introduced simultaneously to parent drugs or carriers to synthesize double ligand-targeted prodrugs or nanoparticles, the oral bioavailability will be improved due to prolonged intestinal uptake time and increased uptake sites. Compared with the existing technology, a double ligand-targeted strategy has evident advantages: (i) it can achieve higher intestinal transport performance by efficient cooperation of double transporters; (ii) it is a versatile transport system to simultaneously solve the problems of poor permeability, low stability and strong toxicity of drugs; (iii) the system is stable and without immunogenicity. Based on the value of a double ligand-targeted strategy, we are carrying out relevant research on the development of oral prodrugs and nanoparticles.
5. Conclusion
Many factors limit the oral absorption of drugs, resulting in drug candidates that fail in preclinical and clinical trials. Transporters are expressed on intestinal epithelial cells and play a key role in the intestinal absorption of nutrients, thus providing an effective target for the design of oral prodrugs or nanoparticles. In this paper, we focus on intestinal OCTN2 and MCT1 transporters, summarizing the recent advances in transporter-targeting oral drug delivery. Transporter-targeting strategies have been proven successful by simultaneously modifying the permeability, stability and solubility of parent drugs, resulting in higher oral bioavailability.
Despite the rapid development of intestinal transporter-targeting strategies, some issues deserve further consideration. These include: (i) the expression of transporters is relatively ubiquitous, and how to decrease the non-targeting distribution of prodrugs or nanoparticles remains a problem; (ii) treatment of diseases often requires a combination of several different drugs, and how to solve the drug-drug interaction mediated by transporters is a big challenge; (iii) there are no crystal structures of transporters, and how to more accurately design transporter-targeting prodrugs remains a bottleneck. Continuous exploration of the underlying mechanisms will help us rationally design improved transporter-targeting strategies.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Guangxi Province (Nos. 2018JJB140325, 2018JJB140377), Science and Technology Base and Talents of Guangxi Province (Nos. 2018AD19035), Talents Project for Cultivating High-level Talent Teams in the Qi Huang Project of Guangxi University of Chinese Medicine (2018002), the specific subject of the dominant discipline construction of Chinese Pharmacy of Guangxi University of Chinese Medicine, Guang Xi Key Laboratory of Translational Medicine for Treating High-incidence Infectious Diseases with Integrative Medicine and School research projects (no. B170021, 2018MS003), and Scientific Research Projects of Guangxi University of Chinese Medicine (B170021, 2018MS003).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2020.02.002.
Appendix. Supplementary materials
References
- 1.Banker M. 2nd ed. Marcel Dekker; New York: 1990. Principles of drug absorption. [Google Scholar]
- 2.Gibaldi M. 4th ed. Lea and Lea Febiger; Philadelphia: 1991. Biopharmaceutics and clinical pharmacokinetics. [Google Scholar]
- 3.Luo Y.Y., Xiong X.Y., Tian Y. A review of biodegradable polymeric systems for oral insulin delivery. Drug Deliv. 2015;23(6):1882–1891. doi: 10.3109/10717544.2015.1052863. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Gao Y., Wen X. Current prodrug strategies for improving oral absorption of nucleoside analogues. Asian J Pharm Sci. 2014;9:65–74. [Google Scholar]
- 5.Faivre S.J., Olszanski A.J., Weigang-Köhler K. Phase i dose escalation and pharmacokinetic evaluation of two different schedules of LY2334737, an oral GEM prodrug, in patients with advanced solid tumors. Invest New Drugs. 2015;33:1206–1216. doi: 10.1007/s10637-015-0286-7. [DOI] [PubMed] [Google Scholar]
- 6.Bender D.M., Bao J., Dantzig A.H. Synthesis, crystallization, and biological evaluation of an orally active prodrug of gemcitabine. J Med Chem. 2009;52:6958–6961. doi: 10.1021/jm901181h. [DOI] [PubMed] [Google Scholar]
- 7.Adema A.D., Smid K., Losekoot N. Metabolism and accumulation of the lipophilic deoxynucleoside analogs elacytarabine and CP-4126. Investig New Drugs. 2012;30:1908–1916. doi: 10.1007/s10637-011-9756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman A.M., Adema A.D., Balzarini J. Antiproliferative activity, mechanism of action and oral antitumor activity of CP-4126, a fatty acid derivative of gemcitabine, in in vitro and in vivo tumor models. Investig New Drugs. 2011;29:456–466. doi: 10.1007/s10637-009-9377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuurman F.E., Voest E.E., Awada A. Phase I study of oral CP-4126, a gemcitabine derivative, in patients with advanced solid tumors. Investig New Drugs. 2013;31:959–966. doi: 10.1007/s10637-013-9925-z. [DOI] [PubMed] [Google Scholar]
- 10.Minko T., Rodriguez Rodriguez L., Pozharov V. Nanotechnology approaches for personalized treatment of multidrug resistant cancers. Adv Drug Deliv Rev. 2013;65:1880–1895. doi: 10.1016/j.addr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand N., Wu J., Xu X. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao J., Feng J., Chen J. External-stimuli responsive systems for cancer theranostic. Asian J Pharm Sci. 2016;11:585–595. [Google Scholar]
- 13.Luo C., Sun J., Sun B., He Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol Sci. 2014;35:556–566. doi: 10.1016/j.tips.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Mura S., Bui D.T., Couvreur P. Lipid prodrug nanocarriers in cancer therapy. J Control Release. 2015;208:25–41. doi: 10.1016/j.jconrel.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Lajoie J.M., Shusta E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev Pharmacol Toxicol. 2015;55:613–631. doi: 10.1146/annurev-pharmtox-010814-124852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazak R., Houri M., EI Achy S. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tambe V., Thakkar S., Raval N. Surface engineered dendrimers in sirna delivery and gene silencing. Curr Pharm Des. 2017;23:2952–2975. doi: 10.2174/1381612823666170314104619. [DOI] [PubMed] [Google Scholar]
- 18.Yan Z., Sun J., Chang Y. Bifunctional peptidomimetic prodrugs of didanosine for improved intestinal permeability and enhanced acidic stability: synthesis, transepithelial transport, chemical stability and pharmacokinetics. Mol Pharm. 2011;8(2):319–329. doi: 10.1021/mp100376q. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y., Sun J., Shi S. Synthesis, transport and pharmacokinetics of 5′-amino acid ester prodrugs of 1-β-D-arabinofuranosylcytosine. Mol Pharm. 2008;6(1):315–325. doi: 10.1021/mp800200a. [DOI] [PubMed] [Google Scholar]
- 20.Cao F., Gao Y., Wang M. Propylene glycollinked amino acid/dipeptide diester prodrugs of oleanolic acid for pept1-mediated transport: synthesis, intestinal permeability, and pharmacokinetics. Mol Pharm. 2013;10(4):1378–1387. doi: 10.1021/mp300647m. [DOI] [PubMed] [Google Scholar]
- 21.Trauner M., Boyer J.L. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83(2):633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 22.Swaan P.W., Hillgren K.M., Szoka F.C., Jr. Enhanced transepithelial transport of peptides by conjugation to cholic acid. Bioconjug Chem. 1997;8(4):520–525. doi: 10.1021/bc970076t. [DOI] [PubMed] [Google Scholar]
- 23.Margarida E., Jose G.M., Graca S. Intestinal drug transporters: an overview. Adv Drug Deliv Rev. 2013;65:1340–1356. doi: 10.1016/j.addr.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Guan X., Rodriguez-Cruz V., Marilyn E. Morris. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. 2019;21:13. doi: 10.1208/s12248-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krajcsi P. Drug-transporter interaction testing in drug discovery and development. World J Pharmacol. 2013;2(1):35–46. [Google Scholar]
- 26.Han H., de Vrueh R.L.A., Rhie J.K. 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998;15(8):1154–1159. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 27.Sugawara M., Huang W., Fei Y.J. Transport of valganciclovir, a ganciclovirprodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci. 2000;89(6):781–789. doi: 10.1002/(SICI)1520-6017(200006)89:6<781::AID-JPS10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Li F., Hong L., Mau C.I. Transport of levovirin prodrugs in the human intestinal Caco 2 cell line. J Pharm Sci. 2006;95(6):1318–1325. doi: 10.1002/jps.20434. [DOI] [PubMed] [Google Scholar]
- 29.Yan Z., Sun J., Chang Y. Bifunctional peptidomimetic prodrugs of didanosine for improved intestinal permeability and enhanced acidic stability: synthesis, transepithelial transport, chemical stability and pharmacokinetics. Mol Pharm. 2011;8(2):319–329. doi: 10.1021/mp100376q. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y., Sun J., Shi S. Synthesis, transport and pharmacokinetics of 5′-amino acid ester prodrugs of 1-β-D-arabinofuranosylcytosine. Mol Pharm. 2008;6(1):315–325. doi: 10.1021/mp800200a. [DOI] [PubMed] [Google Scholar]
- 31.Fan W., Xia D., Zhu Q. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials. 2018;151:13–23. doi: 10.1016/j.biomaterials.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Yuqian D., Chutong T., Menglin W. Dipeptide-modified nanoparticles to facilitate oral docetaxel delivery: new insights into PepT1-mediated targeting strategy. Drug Deliv. 2018;25(1):1403–1413. doi: 10.1080/10717544.2018.1480675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G., Chen H., Zhao D. Combination of l-carnitine with lipophilic linkage-donating gem derivatives as intestinal novel organic cation transporter 2-targeting oral prodrugs. J Med Chem. 2017;60:2552–2561. doi: 10.1021/acs.jmedchem.7b00049. [DOI] [PubMed] [Google Scholar]
- 34.Kou L., Yao Q., Sun M. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: a case study of high efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Adv Healthc Mater. 2017;6(17) doi: 10.1002/adhm.201700165. [DOI] [PubMed] [Google Scholar]
- 35.Zhang E.Y., Phelps M.A., Cheng C. Modeling of active transport systems. Adv Drug Deliv Rev. 2002;54(3):329–354. doi: 10.1016/s0169-409x(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 36.Cao F., Gao Y., Ping Q. Advances in research of PepT1-targeted prodrug. Asian J Pharm Sci. 2012;7(2):110–122. [Google Scholar]
- 37.Rautio J., Kumpulainen H., Heimbach T. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7(3):255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 38.Hu L. Prodrugs: effective solutions for solubility, permeability and targeting challenges. Invest Drugs J. 2004;7(8):736–742. [PubMed] [Google Scholar]
- 39.Tsirigos K.D., Govindarajan S., Bassot C. Topology of membrane proteins-predictions, limitations and variations. Curr Opin Struct Biol. 2017;50:9–17. doi: 10.1016/j.sbi.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi R., Tamai I., Inano A. Studies on functional sites of organic cation/carnitine transporter OCTN2 (SLC22A5) using a Ser467Cys mutant protein. J Pharmacol Exp Ther. 2002;302:1286–1294. doi: 10.1124/jpet.102.036004. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Meadows T.A., Longo N. Abnormal sodium stimulation of carnitine transport in primary carnitine deficiency. J Biol Chem. 2000;275:20782–20786. doi: 10.1074/jbc.M000194200. [DOI] [PubMed] [Google Scholar]
- 42.Seth P., Wu X., Huang W. Mutations in novel organic cation transporter (OCTN2), an organic cation/carnitine transporter, with differential effects on the organic cation transport function and the carnitine transport function. J Biol Chem. 1999;274:33388–33392. doi: 10.1074/jbc.274.47.33388. [DOI] [PubMed] [Google Scholar]
- 43.Muoio Deborah M., Noland Robert C., Kovalik J.P. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15(5):764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark P.M., Dawany N., Dampier W. Bioinformatics analysis reveals transcriptome and microRNA signatures and drug repositioning targets for IBD and other autoimmune diseases. Inflamm Bowel Dis. 2012;18(12):2315–2333. doi: 10.1002/ibd.22958. [DOI] [PubMed] [Google Scholar]
- 45.Adeva-Andany M.M., Calvo-Castro I., Fernández-Fernández C. Significance of L-carnitine for human health. IUBMB Life. 2017;69:578–594. doi: 10.1002/iub.1646. [DOI] [PubMed] [Google Scholar]
- 46.Mescka C.P., Guerreiro G., Hammerschmidt T. L-carnitine supplementation decreases DNA damage in treated VIPD patients. Mutation Res Fundam Mol Mech Mutagen. 2015;775:43–47. doi: 10.1016/j.mrfmmm.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Tamai I., Ohashi R., Nezu J.I. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273(32):20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 48.Bremer J. Carnitine–metabolism and functions. Physiol Rev. 1983;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 49.Rebouche C.J. Carnitine function and requirements during the life cycle. FASEB J. 1992;6:3379–3386. [PubMed] [Google Scholar]
- 50.Inazu M., Takeda H., Maehara K. Functional expression of the organic cation/carnitine transporter 2 in rat astrocytes. J Neurochem. 2006;97:424e34. doi: 10.1111/j.1471-4159.2006.03757.x. [DOI] [PubMed] [Google Scholar]
- 51.Schomig E., Spitzenberger F., Engelhardt M. Molecular cloning and characterization of two novel transport proteins from rat kidney. FEBS Lett. 1998;425:79e86. doi: 10.1016/s0014-5793(98)00203-8. [DOI] [PubMed] [Google Scholar]
- 52.Wu X., Prasad P.D., Leibach F. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem Biophys Res Commun. 1998;246:589e95. doi: 10.1006/bbrc.1998.8669. [DOI] [PubMed] [Google Scholar]
- 53.Tamai I., Ohashi R., Nezu J. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J Biol Chem. 2000;275:40064e72. doi: 10.1074/jbc.M005340200. [DOI] [PubMed] [Google Scholar]
- 54.Nakanishi T., Haruta T., Shirasaka Y. Organic cation transporter-mediated renal secretion of ipratropium and tiotropium in rats and humans. Drug Metab Dispos. 2011;39(1):117–122. doi: 10.1124/dmd.110.035402. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura T., Nakanishi T., Haruta T. Transport of ipratropium, an anti-chronic obstructive pulmonary disease drug, is mediated by organic cation /carnitine transporters in human bronchial epithelial cells: implications for carrier-mediated pulmonary absorption. Mol Pharm. 2009;7(1):187–195. doi: 10.1021/mp900206j. [DOI] [PubMed] [Google Scholar]
- 56.Mo J., Lim L.Y., Zhang Z.R. L-carnitine ester of prednisolone: pharmacokinetic and pharmacodynamic evaluation of a type I prodrug. Int J Pharm. 2014;475(1/2):123–129. doi: 10.1016/j.ijpharm.2014.08.049. [DOI] [PubMed] [Google Scholar]
- 57.Tune B.M., Hsu C.Y. Toxicity of cephaloridine to carnitine transport and fatty acid metabolism in rabbit renal cortical mitochondria: structure–activity relationships. J Pharmacol Exp Ther. 1994;270:873–878. [PubMed] [Google Scholar]
- 58.Hirano T., Yasuda S., Osaka Y. Mechanism of the inhibitory effect of zwitterionic drugs (levofloxacin and grepafloxacin) on carnitine transporter (OCTN2) in Caco-2 cells. Biochim Biophys Acta Biomembr. 2006;1758(11):1743–1750. doi: 10.1016/j.bbamem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Tamai I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and SLC22a21) Biopharm Drug Dispos. 2013;34:29–44. doi: 10.1002/bdd.1816. [DOI] [PubMed] [Google Scholar]
- 60.Tamai I., China K., Sai Y., Kobayashi D. Tsuji. Na+-coupled transport of L-carnitine via high-affinity carnitine transporter OCTN2 and its subcellular localization in kidney. Biochim Biophys Acta. 2001;1512:273–284. doi: 10.1016/s0005-2736(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 61.Koepsell H., Lips K., Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24(7):1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 62.Gong S., Lu X., Xu Y. Identification of OCT6 as a novel organic cation transporter preferentially expressed in hematopoietic cells and leukemias. Exp Hematol. 2002;30:1162–1169. doi: 10.1016/s0301-472x(02)00901-3. [DOI] [PubMed] [Google Scholar]
- 63.Srinivas S.R., Prasad P.D., Umapathy N.S. Transport of butyryl-L-carnitine, a potential prodrug, via the carnitine transporter OCTN2 and the amino acid transporter ATB0,+ Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1046–G1053. doi: 10.1152/ajpgi.00233.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grube M., Ameling S., Noutsias M. Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am J Pathol. 2011;178(6):2547–2559. doi: 10.1016/j.ajpath.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qu Q., Qu J., Zhan M. Different involvement of promoter methylation in the expression of organic cation/carnitine transporter 2 (OCTN2) in cancer cell lines. PLoS ONE. 2013;8(10):e76474. doi: 10.1371/journal.pone.0076474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kato Y., Sugiura M., Sugiura T., Wakayama T., Kubo Y., Kobayashi D. Organic cation/carnitine transporter OCTN2 (Slc22a5) is responsible for carnitine transport across apical membranes of small intestinal epithelial cells in mouse. Mol Pharmacol. 2006;70(3):829–837. doi: 10.1124/mol.106.024158. [DOI] [PubMed] [Google Scholar]
- 67.Meier Y., Eloranta J.J., Darimont J. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35(4):590–594. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- 68.Ohashi R., Tamai I., Yabuuchi H. Na(+)- dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther. 1999;291:778–784. [PubMed] [Google Scholar]
- 69.Ohashi R. Studies on functional sites of organic cation/carnitine transporter OCTN2 (SLC22A5) using a Ser467Cys mutant protein. J Pharmacol Exp Ther. 2002;302(3):1286–1294. doi: 10.1124/jpet.102.036004. [DOI] [PubMed] [Google Scholar]
- 70.Diao L., Polli J.E. Synthesis and in vitro characterization of drug conjugates of L-carnitine as potential prodrugs that target human Octn2. J Pharm Sci. 2011;100(9):3802–3816. doi: 10.1002/jps.22557. [DOI] [PubMed] [Google Scholar]
- 71.Miller S.J. Cellular and physiological effects of short-chain fatty acids. Mini Rev Med Chem. 2004;4:839–845. doi: 10.2174/1389557043403288. [DOI] [PubMed] [Google Scholar]
- 72.Scheppach W., Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care. 2004;7:563–567. doi: 10.1097/00075197-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Cavaglieri C.R., Nishiyama A., Fernandes L.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. doi: 10.1016/s0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- 74.Kinoshita M., Suzuki Y., Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation. Biochem Biophys Res Commun. 2002;293:827–831. doi: 10.1016/S0006-291X(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 75.Tong X., Yin L., Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004;317:463–471. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 76.Thibault R., De Coppet P., Daly K. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133:1916–1927. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 77.Cetinkaya A., Bulbuloglu E., Kantarceken B. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci. 2006;51:488–494. doi: 10.1007/s10620-006-3160-9. [DOI] [PubMed] [Google Scholar]
- 78.Giancaterini A., Mingrone G., De Gaetano A. Effects of propyonil-L-carnitine topical irrigation in distal ulcerative colitis: a preliminary report. Am J Gastroenterol. 2001;96:2275–2276. doi: 10.1111/j.1572-0241.2001.03988.x. [DOI] [PubMed] [Google Scholar]
- 79.Pasut G., Canal F., Dalla Via L. Antitumoral activity of PEG-gemcitabine prodrugs targeted by folic acid. J Control Release. 2008;127:239–248. doi: 10.1016/j.jconrel.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Fan W., Dai X. Enhanced tumor delivery of gemcitabine via PEG-DSPE/TPGS mixed micelles. Mol Pharm. 2014;11:1140–1150. doi: 10.1021/mp4005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Kim W.Y., Huang L. Systemic delivery of gemcitabine triphosphate via LCP nanoparticles for NSCLC and pancreatic cancer therapy. Biomaterials. 2013;34:3447–3458. doi: 10.1016/j.biomaterials.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Y., Sun J., Shi S. Synthesis, transport and pharmacokinetics of 5′-amino acid ester prodrugs of 1-beta-D-arabinofuranosylcytosine. Mol Pharm. 2009;6(1):315–325. doi: 10.1021/mp800200a. [DOI] [PubMed] [Google Scholar]
- 83.Kim B., Lee C., Lee E.S. Paclitaxel and curcumin co-bound albumin nanoparticles having antitumor potential to pancreatic cancer. Asian J Pharm Sci. 2016;11:708–714. [Google Scholar]
- 84.Halestrap A.P., Price N.T. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- 85.Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 86.Hofmann K., Stoel W. Tmbase-A database of membrane spanning protein segments. Biol Chem Hoppe Seyler. 1993;374:166. [Google Scholar]
- 87.Saier M.H.J. Computer-aided analyses of transport protein sequences: gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiol Rev. 1994;58:71–93. doi: 10.1128/mr.58.1.71-93.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahman B., Schneider H.P., Bröer A. Helix 8 and helix 10 are involved in substrate recognition in the rat monocarboxylate transporter MCT1. Biochemistry. 1999;38:11577–11584. doi: 10.1021/bi990973f. [DOI] [PubMed] [Google Scholar]
- 89.Kim C.M., Goldstein J.L., Brown M.S. cDNA cloning of MEV, a mutant protein that facilitates cellular uptake of mevalonate, and identification of the point mutation responsible for its gain of function. J Biol Chem. 1992;267(32):23113–23121. [PubMed] [Google Scholar]
- 90.Garcia C.K., Li X., Luna J. cDNA cloning of the human monocarboxylate transporter 1 and chromosomal localization of the SLC16A1 locus to 1p 13.2-p 12. Genomics. 1994;23:500–503. doi: 10.1006/geno.1994.1532. [DOI] [PubMed] [Google Scholar]
- 91.Poole R.C., Halestrap A.P. Identification and partial purification of the erythrocyte lactate transporter. Biochem J. 1992;283:855–862. doi: 10.1042/bj2830855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gali S., Schneider H.P., Br Er A. The loop between helix 4 and helix 5 in the monocarboxylate transporter MCT1 is important for substrate selection and protein stability. Biochem J. 2003;376(2):413–422. doi: 10.1042/BJ20030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schuster V.L., Lu R., Kanai N. Cloning of the rabbit homologue of mouse ‘basigin’ and rat ‘OX-47’: kidney cell type-specific expression, and regulation in collecting duct cells. Biochim Biophys Acta. 1996;1311(1):13–19. doi: 10.1016/0167-4889(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 94.Kirk P., Wilson M.C., Heddle C. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 96.Halestrap A.P. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life. 2012;64(1):1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 97.Dimmer K.S., Friedrich B., Lang F. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;3501(2):219–227. [PMC free article] [PubMed] [Google Scholar]
- 98.Chiche J., Fur Y.L., Vilmen C. In vivo pH in metabolic-defective Ras- transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int J Cancer. 2012;130(7):1511–1520. doi: 10.1002/ijc.26125. [DOI] [PubMed] [Google Scholar]
- 99.Cuff M.A., Lambert D.W., Shirazi-Beechey S.P. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol. 2002;539(2):361–371. doi: 10.1113/jphysiol.2001.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Halestrap A.P., Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflüg Arch Eur J Physiol. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 101.Pérez-Escuredo J., Van H. Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta Mol Cell Res. 2016;1863:2481–2497. doi: 10.1016/j.bbamcr.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garcia C.K., Brown M.S., Pathak R.K. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 103.Garcia C.K., Goldstein J.L., Pathak R.K. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 104.Johannsson E., Nagelhus E.A., McCullagh K.J. Cellular and subcellular expression of the monocarboxylate transporter MCT1 in rat heart: a high-resolution immunogold analysis. Circ Res. 1997;80:400–407. [PubMed] [Google Scholar]
- 105.Jackson V.N., Halestrap A.P. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2’,7’-bis(carboxyethyl)-5(6)-carboxyfluores cein. J Biol Chem. 1996;271:861–868. doi: 10.1074/jbc.271.2.861. [DOI] [PubMed] [Google Scholar]
- 106.Kirat D., Inoue H., Iwano H., Yokota H. Monocarboxylate transporter 1 (MCT1) in the liver of pre-ruminant and adult bovines. Vet J. 2007;173:124–130. doi: 10.1016/j.tvjl.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Ritzhaupt A., Wood I.S., Ellis A. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. J Cell Physiol. 1998;513:719–732. doi: 10.1111/j.1469-7793.1998.719ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Orsenigo M.N., Tosco M., Bazzini C. A monocarboxylate transporter MCT1 is located at the basolateral pole of rat jejunum. Exp Physiol. 1999;84:1033–1042. [PubMed] [Google Scholar]
- 109.Kirat D., Inoue H., Iwano H., Hirayama K. Monocarboxylate transporter 1 gene expression in the ovine gastrointestinal tract. Vet J. 2006;171:462–467. doi: 10.1016/j.tvjl.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 110.Welter H., Claus R. Expression of the monocarboxylate transporter 1 (MCT1) in cells of the porcine intestine. Cell Biol Int. 2008;32:638–645. doi: 10.1016/j.cellbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 111.Eilertsen M., Andersen S., Alsaad S. Monocarboxylate transporters 1-4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur J Appl Physiol. 2001;86(1):6–11. doi: 10.1007/s004210100516. [DOI] [PubMed] [Google Scholar]
- 113.Lu Y., Zhao H., Wang Y. Electro-acupuncture up-regulates astrocytic MCT1 expression to improve neurological deficit in middle cerebral artery occlusion rats. Life Sci. 2015;134:68–72. doi: 10.1016/j.lfs.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 114.Cuff M.A., Lambert D.W., Shirazi Beechey S.P. Substrate induced regulation of the human colnic monocarboxylate transporter, MCT1. J Physiol. 2002;539:361–371. doi: 10.1113/jphysiol.2001.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimoyama Y., Kirat D., Akihara Y. Expression of monocarboxylate transporter 1 (MCT1) in the dog intestine. J Vet Med Sci. 2007;69(6):599–604. doi: 10.1292/jvms.69.599. [DOI] [PubMed] [Google Scholar]
- 116.Welter H., Claus R. Expression of the monocarboxylate transporter 1 (MCT1) in cells of the porcine intestine. Cell Biol Int. 2008;32(6):599–604. doi: 10.1016/j.cellbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Halestrap A.P. The SLC16 gene family-structure, role and regulation in health and disease. Mol Aspects Med. 2013;34(2–3):337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 118.Doherty J.R., Yang C., Scott K.E. Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 2014;74(3):908–920. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Itoh T., Tanno M., Li Y.H. Transport of phenethicillin into rat intestinal brush border membrane vesicles: role of the monocarboxylic acid transport system. Int J Pharm. 1998;172:103–112. [Google Scholar]
- 120.Utoguchi N., Audus L.L. Carrier-mediated transport of valproic acid in BeWo cells, a human trophoblast cell line. Int J Pharm. 2000;195:115–124. doi: 10.1016/s0378-5173(99)00398-1. [DOI] [PubMed] [Google Scholar]
- 121.Wu X.C., Whitfield L.R., Stewart B.H. Atorvastatin transport in the Caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid cotransporter. Pharm Res. 2000;17:209–215. doi: 10.1023/a:1007525616017. [DOI] [PubMed] [Google Scholar]
- 122.Okamura A., Emoto A., Koyabu N. Transport and uptake of nateglinide in Caco-2 cells and its inhibitory effect on human monocarboxylate transporter MCT1. Br J Pharmacol. 2002;137:391–399. doi: 10.1038/sj.bjp.0704875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cundy K.C., Annamalai T., Bu L. XP13512 [(+/-)-1-([(alpha-isobutan oyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311(1):324–333. doi: 10.1124/jpet.104.067959. [DOI] [PubMed] [Google Scholar]
- 124.Sun YX, Zhao DY, Wang G, Jiang QK, Guo MR, Kan QM, et al. A novel oral prodrug-targeting transporter MCT 1: 5-fluorouracil-dicarboxylate monoester conjugates. 2019; 10.1016/j.ajps. 2019.04.001. [DOI] [PMC free article] [PubMed]
- 125.Pollack M.H., Matthews J., Scott E.L. Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry. 1998;155:992–993. doi: 10.1176/ajp.155.7.992. [DOI] [PubMed] [Google Scholar]
- 126.Garcia-Borreguero D., Larrosa O., de la L., lave Y. Treatment of restless legs syndrome with gabapentin: a doubleblind, cross-over study. Neurology. 2002;59:1573–1579. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 127.Guttuso T., Jr., Kurlan R., McDermott M.P. Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337–345. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 128.Wu L., Liu M., Shan W. Bioinspired butyrate-functionalized nanovehicles for targeted oral delivery of biomacromolecular drugs. J Control Release. 2017;262:273–283. doi: 10.1016/j.jconrel.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 129.Paradis R., Noel C., Page M. Use of pluronic micelles to overcome multidrug resistance. Int J Oncol. 1994;5(6):1305–1308. doi: 10.3892/ijo.5.6.1305. [DOI] [PubMed] [Google Scholar]
- 130.Kabanov A.V., Batrakova E.V., Alakhov V.Y. Pluronic (R) block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82(2–3):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 131.Batrakova E.V., Zhang Y., Li Y. Effects of pluronic P85 on GLUT1 and MCT1 transporters in the blood-brain barrier. Pharm Res. 2004;21(11):1993–2000. doi: 10.1023/b:pham.0000048189.79606.6e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.