Abstract
Purpose
We investigated the expression of Nrf2 in colorectal cancer and its correlation with clinicopathological characteristics as well as mechanisms and roles of Nrf2 expression including cell signaling pathway, survival, proliferation, and migration.
Methods
Nrf2 expression was measured in 12 and 30 different colorectal cancer (CRC) tissues by western blot (WB) and immunohistochemistry (IHC), respectively. SW480 cells were used for cell proliferation and cell migration tests. The correlation between the expression of Nrf2 and clinicopathologic parameters were evaluated using the chi-square or Fisher exact test. Data are expressed as the mean ± standard deviation for 3 independent experiments. P < 0.05 was considered statistically significant.
Results
Analysis of WB demonstrated that Nrf2 proteins were increased in CRC tissues, and decreased in normal tissues. IHC staining showed that the Nrf2 expression was elevated in CRC tissues, compared to matched normal tissues. When SW480 cells were suppressed with small interfering RNA of Nrf2, cell viability was inhibited, and cell apoptosis was increased. These results were found along with suppression of the phosphorylated form of extracellular signal-regulated kinase 1/2 and AKT.
Conclusion
This study suggests that overexpression of Nrf2 may be related to carcinogenesis and progression of CRC.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignant cancers. The survival rate of localized CRC patients from 2003 to 2009 was 90.5%, but in the case of patients with distant metastasis, it drops to 12.5% [1]. Currently, although, in addition to radical surgery, adjuvant therapy, such as chemotherapy and targeted therapy are used broadly, there is no effective treatment for CRC with lymph node metastasis, and most CRC patients also have liver metastases [2]. It is, therefore, necessary to uncover the factors related to cell metastasis, as well as cell survival in CRC.
Oxidative stress interferes with normal oxidation-reduction balance and thereby, generates reactive oxygen species (ROS). ROS damages cells and changes signaling pathways. ROS can cause pathological symptoms, including inflammation, neurodegenerative diseases, and cancer [3]. Cells have various protective processes against oxidative damage, such as antioxidant production and expression of enzymes against ROS.
Nuclear factor erythroid2-related factors (Nrfs) series play a crucial role in protecting cells from oxidative damages. It has been proven that the Nrf-2 (Nrf2) plays a critical role when cells react to stress, and is normally regulated by Kelch-like ECH-associated protein 1 (Keap1) known as powerful cytoplasmic suppressor of Nrf2 by making a complex composed of Keap1/Nrf2 compounds [4]. In normal environment, Nrf2 inside the cytoplasm stays transcriptionally inactive via combination with the suppressor Keap1; and that is quickly degraded by proteasomes, so only low levels of the protein are maintained. But when the cell is exposed to oxidative stress or chemopreventive compounds, Nrf2 moves to the nucleus to form a heterodimer with small Maf proteins, where it binds with ARE series of DNA to activate genetic transcription of antioxidants and phase II detoxification enzymes [5]. This proves the Nrf2-dependant protection against cancer, neurodegenerative diseases, cardiovascular diseases, inflammations, pulmonary fibrosis, acute pulmonary injury, and a number of other human diseases and pathological conditions, by emphasizing the important biological role of Nrf2 [6,7,8,9,10]. Therefore, Nrf2 has been considered to be a “good” transcriptional factor, essential for protecting against oxidative stress.
However, new data finding that Nrf2 can cause cancer, such as lung cancer, liver cancer, breast cancer, squamous cell carcinoma, and head and neck cancer was recently reported [11]. It was found that abnormal activation of Nrf2 pathways occurs frequently in cancer cells and tissues. Nrf2 not only prevented normal cells from turning into cancer cells, but it was also associated with protecting cancer cells from cell stress, promoting the survival of cancer cells, and the growth and progression of cancer [10]. Nrf2 is considered to be a tumor gene, since it was found in excess in several cancers [10,12]. Previous studies showed that Nrf2 signaling pathways were abnormally activated in CRC [13,14]. However, only few studies have been done on Nrf2 expression of CRC in vivo [12]. Therefore, we conducted research on the expression of Nrf2 proteins in CRC tissues and normal tissues in vivo as well as in vitro experiments to confirm roles of Nrf2 expression such as cell survival, proliferation, and migration, and its mechanisms in colon cancer cells.
METHODS
Patients and samples
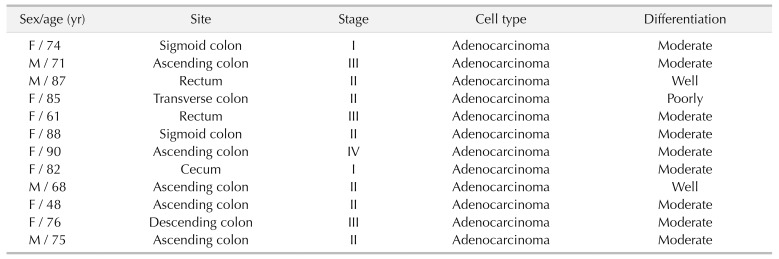
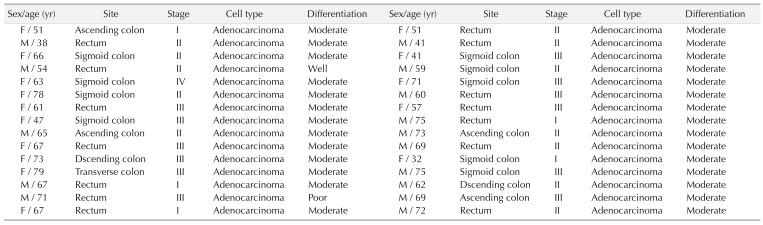
CRC tissues were obtained from 12 postoperative patients from July 2016 to June 2018, and were paired with normal tissue for western blot (Table 1). CRC and normal tissues were collected for immunohistochemical staining in 30 postoperative patients between December 2011 and August 2012 in the general surgery department of Soonchunhyang University Seoul Hospital (Table 2). Samples for western blot were acquired immediately after specimen delivery out and transferred with portable liquid nitrogen bottle and stored at −80℃ refrigerator. Also, we conducted protein extraction from those samples within 3 weeks and stored it at−80℃ refrigerator. Then, western blot was performed in protein extraction. Other samples for immunohistochemistry (IHC) were fixed in cold 10% neutral buffered formalin for 24 hours, then paraffin block. All of the CRC were diagnosed by conventional pathological examination. The Institutional Review Board of Soonchunhyang University Seoul Hospital reviewed and approved this research protocol related to the use of tissue samples (IRB No. SCHUH 2018-08-011). The human CRC cell line SW480 (ATCC CCL-228) was purchased from the American Type Culture Collection. The cell culture was performed in Dulbecco's Modifed Eagle's medium, containing 10% fetal calf serum, 100-U/mL penicillin, and 100-mg/mL streptomycin at 37℃, 5% CO2.
Table 1. Clinical characteristics of subjects on western blotting.
Table 2. Clinical characteristics of subjects on immunohistochemistry.
Western blot analysis
SW480 cells were used as a positive control for Nrf2 expression. Homogenization was done for tissue samples in radio immunoprecipitation assay buffer (1× phosphate-buffered saline [PBS], 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 10-µg/mL phenylmethanesulfonylfluoride, and a protease inhibitor cocktail tablet [Boehringer Mannheim, Germany]) for 30 minutes on ice followed by centrifugation at 10,000 ×g for 10 minutes at 4℃. Supernatants at −70℃ were used for western blotting. The protein concentration was measured by a bicinchoninic acid assay kit (Thermo Scientific Inc., Waltham, MA, USA), following the manufacturer's protocol. Proteins were separated by SDS-polyacrylamide gel electrophoresis, using NuPAGE 4%–12% bis-Tris gels (Invitrogen, Waltham, MA, USA), and transferred to ImmunoBlot polyvinylidene fluoride membranes (GE Healthcare Life Sciences, Amersham, Bucks, Germany). The membrane was blocked using casein blocking buffer (Sigma-Aldrich Corp., St. Louis, MI, USA). Then, the membrane was reacted with the primary antibody, rabbit anti-Nrf2 antibodies (Santa Cruz Biotechnology Inc., Dallas, TX, USA), diluted 1:500, at 4℃ for 16 hours. After 16 hours, the membrane was washed with washing buffer and PBS tween-20 buffer 3 times for 15 minutes, and reacted for 1 hour with anti-rabbit IgG (Santa Cruz Biotechnology Inc.)-horseradish peroxidase-linked species-specific whole antibody diluted 1:5,000. After reaction with antibody, the membrane was washed again 3 times for 15 minutes. Proteins on the membrane were detected by an enhanced chemiluminescence solution kit (Promega Corp., Madison, WI, USA). The membrane was stripped and reprobed with anti-beta-actin antibody (Sigma-Aldrich Corp.).
Immunohistochemical analysis
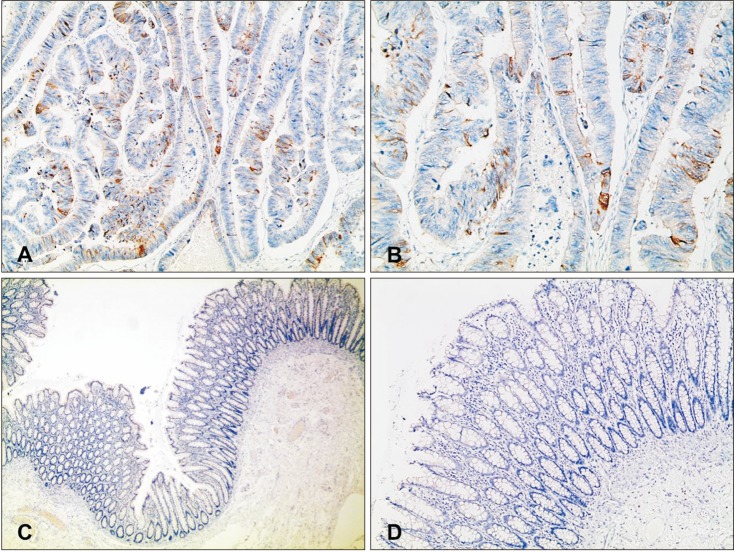
CRC tissues for routine diagnostic pathologic examinations were used for immunohistochemical staining of the anti-Nrf2 (1:100; Abcam plc, Cambridge, UK). Paraffin-embedded tissue sections after formalin fixation were immunohistochemically stained for anti-Nrf2 using a BenchMark XT automatic immunostaining device (Ventana Medical Systems Inc., Oro Valley, AZ, USA) with OptiView DAB IHC Detection Kit (Ventana Medical SystemsInc.), following the manufacturer's protocol. Four-micrometer-thick sections, obtained with a microtome, were transferred onto silanized charged slides and allowed to dry for 10 minutes at room temperature, followed by 20 minutes in an incubator at 65℃. Sections were processed by the heat-induced epitope retrieval method using cell conditioning 1 (CC1) buffer for 32 minutes and incubated for 16 minutes with antibodies in the autoimmunostainer. Antigen-antibody reactions were visualized using Ventana OptiView DAB IHC Detection Kit (Optiview HQ Linker 8 minutes, Optiview HRP Multimer 8 minutes, Optiview H2O2/DAB 8 minutes, Optiview Copper 4 minutes). Counterstaining was performed using Ventana Hematoxylin II for 12 minutes and Ventana Bluing reagent for 4 minutes. Finally, all slides were removed from the stainer, dehydrated, and cover slipped for microscopic examination. It was scored for a positive reaction of the antibody into 4 categories, as follows: 0 (0%), 1+ (1%–33%), 2+ (34%–66%), and 3+ (67%–100%). Nrf2 expression was defined as being expressed in the nucleus or cytoplasm, with the intensity ≥1 (Fig. 1).
Fig. 1. Representative immunohistochemistry staining for nuclear factor erythroid 2-related factor 2 protein expression in paraffin-embedded colorectal cancer tissue (A, B) and normal tissue (C, D). (A, B) Some of the tumor cells show positive cytoplasmic staining for Nrf2 (A: ×100, B: ×200). (C, D) Normal mucosa of colon shows negativity for Nrf2 (C: ×40, D: ×100).
Small interfering RNA transfection
RNA interference was done by siRNAs targeting Nrf2 (Oligo ID: HSS107130). Briefly, the cells were seeded in 96-well or 6-well plates and transfected at 40% confluency with siRNA duplex, using lipofectamine RNAiMAX (Invitrogen), following the manufacturer's protocol. Cells transfected with the Stealth RNAi negative control duplex (Invitrogen) were used as controls.
Apoptosis assay
Apoptotic cell distribution was analysed by MuseTM Annexin V & Dead Cell kit (Merck Millipore Co., Burlington, MS, USA). This kit includes a fluorescent phycoerythrin (PE) dye conjugated to Annexin V to detect phosphatidylserine on the external membrane of apoptotic cells, and 7-amino-actinomycin D as a dead cell marker. Trypsinized cells were harvested by centrifugation, washed with PBS, and resuspended in MuseTM Annexin V and Dead Cell reagent. Data were analyzed using a Muse Cell Analyzer (Merck Millipore Co.).
Cell viability assay
Cells (5 × 103 cells/well) were seeded in 96-well cell culture plates, followed by transfection with 10 nM of Nrf2-specific siRNA duplex or the Stealth RNAi negative control duplex at 40% confluency for 24, 48, or 72 hours, after which they were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; final concentration, 0.1 mg/mL) for 3 hours. Purple formazan crystals were dissolved in 200 µL of dimethyl sulfoxide and the absorbance at 560 nm was measured with a GloMax-Multi Microplate Multimode Reader (Promega Corp.). The results were assessed as a percentage, based on the ratio of the absorbance between the treated cells and the controls (100%).
Wound healing assay
Cells (1.5 × 105 cells/well) were seeded in 6-well plates, cultured overnight, and transfected with Nrf2-specific siRNA. At 24-hour posttransfection, cells had grown to near confluence and were wounded by dragging a 10-µL pipette tip through the monolayer. Cells were washed using prewarmed 1× PBS and allowed to migrate for an additional 48 hours. Cell migration images were taken soon after the wound was introduced (0 hour) and at a designated time point (48 hours after wounding), under microscopic examination. Migrating cells from the opposing wound edge were measured.
Statistical analysis
The correlation between the expression of Nrf2 and clinicopathologic parameters were evaluated using the chisquare or Fisher exact test. Data are expressed as the mean ± standard deviation for 3 independent experiments. P < 0.05 was considered statistically significant.
RESULTS
Nrf2 protein is overexpressed in malignant CRC tissues
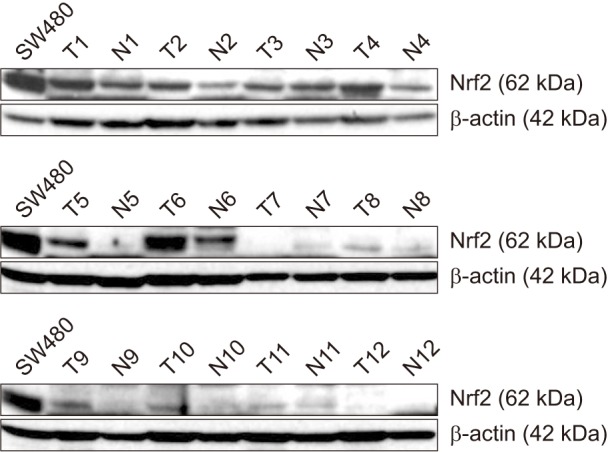
To determine Nrf2 protein expression in CRC and paired normal tissues, western blot analyses were performed. The Nrf2 expression was more increased in 10 of 12 CRC tissues than in normal tissues (Fig. 2). IHC analysis confirmed the results of western blotting; the expression of Nrf2 protein was upregulated in 15 of 30 CRC tissues. Representative IHC staining results for Nrf2 in CRC and normal colorectal tissues are shown (Fig. 1).
Fig. 2. Nrf2 protein was expressed on colorectal cancer and normal tissues. β-actin used as a loading control. The human colorectal cancer cell line SW480 served as a positive control for Nrf2 expression.
Association between the expression of Nrf2 and clinicopathological parameters
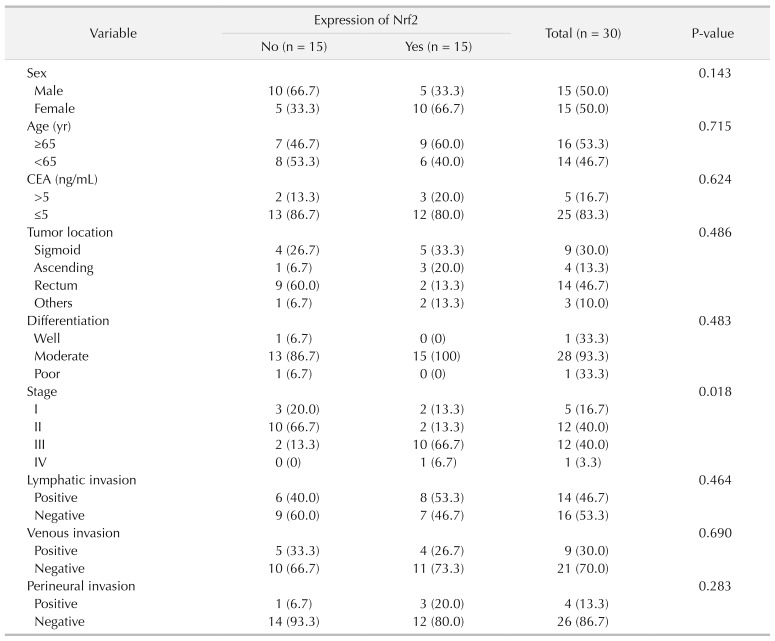
Nrf2 expression was not related to patents' sex (P = 0.143). Also, Nrf2 expression was not associated with age (P = 0.715), CEA (P = 0.624), lymphatic invasion (P = 0.464), venous invasion (P = 0.690), perineural invasion (P = 0.283), tumor location (P = 0.486), or differentiation (P = 0.483). However, pathological stage classification was significantly associated with Nrf2 expression (P = 0.018) (Table 3).
Table 3. The association between Nrf2 expression and clinicopathologic features.
Values are presented as number (%).
Knockdown of Nrf2 inhibits activation of ERK1/2, PI3K/AKT, and Bcl-2
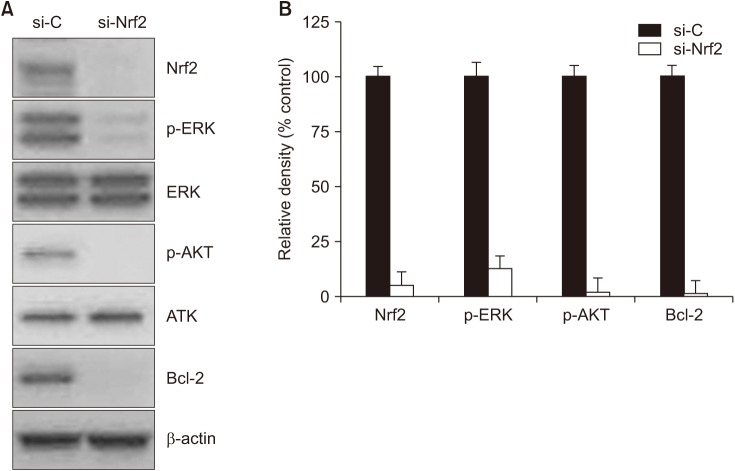
To find downstream molecules of Nrf2 signaling in SW480 cells, western blotting was performed following transfection of cells with Nrf2-specfic siRNA in SW480. Down-regulation of Nrf2 expression was observed 48 hours, following siRNA treatment (Fig. 3A). Densitometric analysis showed phosphorylated forms of ERK1/2 and AKT, which were normalized to the total ERK1/2 and AKT, respectively (Fig. 3B). Treatment of SW480 cells with siRNA inhibited the phosphorylation of ERK1/2, AKT, and Bcl-2. Collectively, these results indicated that Nrf2 may stimulate MEK/ERK1/2 and/or PI3K/AKT pathways, as well as Bcl-2 in SW480 cells.
Fig. 3. Effects of Nrf2 knockdown on phosphorylation of ERK and AKT. (A) Western blot analysis. After Nrf2-specific siRNA transfection in SW480, phosphorylation of ERK and AKT was downregulated. In addition, Bcl-2 expression was decreased. β-actin used as a loading control. (B) Densitometric analysis showed relative density of Nrf2, p-ERK, p-AKT, and Bcl-2. si-C, Stealth RNAi control; si-Nrf2, small interfering Nrf2.
Knockdown of Nrf2 induces growth inhibition and promotes apoptosis
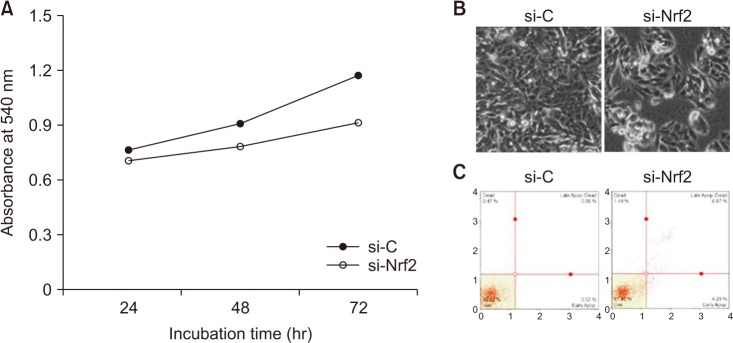
An increased rate of apoptosis was observed in SW480 cells after transfection with Nrf2-specific siRNA (Fig. 4). Based on the MTT assay, cell viability was decreased in a time-dependent manner, following knockdown of Nrf2 (Fig. 4A). At 48-hour posttransfection, the cell morphology changed, and some cells appeared to be detached (Fig. 4B). In the apoptosis assay, 3 different cell populations were detected after Annexin V-PE staining, including a live cell group (lower left panel), an early apoptotic cell group (lower right panel), and a late apoptotic cell group (top right panel). An increase in the apoptotic cell population was observed following transfection with siRNA (Fig. 4C). These results suggest that Nrf2 may promote the survival of SW480 cells.
Fig. 4. Confirmation of cell viability in SW480 after transfection with Nrf2-specific siRNA. (A) Effects of Nrf2 knockdown on proliferation of SW480 cells. (B) Phase-contrast images of cells treated with Nrf2-siRNA and control cells. (C) Annexin V-PE binding assay. 7-Amino-actinomycin D (7-AAD), phycoerythrin (Annexin V-PE). si-C, Stealth RNAi control; si-Nrf2, small interfering Nrf2.
Knockdown of Nrf2 inhibits migration of SW480 cells
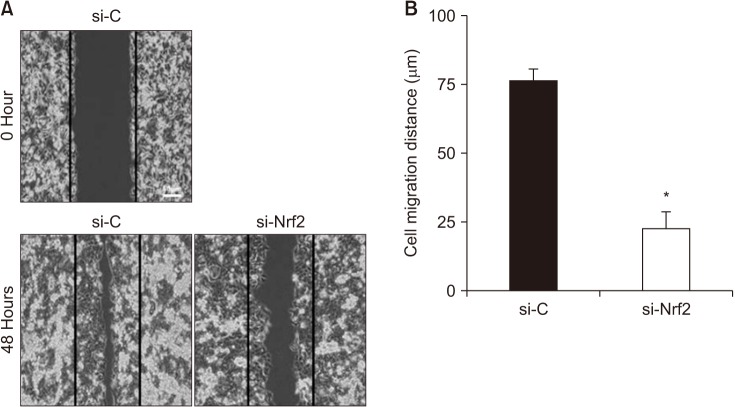
Cell migration and invasion are hallmarks for the development of metastasis. Therefore, we examined the silencing effect of Nrf2 on cell migration, using a scratch wound healing assay in SW480 cells. As shown in Fig. 5, silencing of Nrf2 significantly decreased the wound filling ability of SW480 cells. This result indicated that Nrf2 may be involved in cancer cell migration.
Fig. 5. Comparison of migration distances in SW480. si-C, Stealth RNAi control; si-Nrf2, small interfering Nrf2. *P < 0.05 compared to respective controls.
DISCUSSION
Nrf2 is in the cnc (“cap ‘n’ collar”) family of the basic region leucine zipper transaction factors. When reacting to oxidative stress, Nrf2 regulates the fate of cells through transcriptional adjustment of ARE genes, including the genes that code endogenous antioxidants, phase II detoxifying enzymes, and transporters. Therefore, Nrf2 is an important factor that adjusts the cell defence response of human diseases. Nrf2 plays the role of a core sensor for oxidative stress and is an important transcriptional regulating factor that removes oxidative damages, while protecting the cells from becoming cancer cells [15,16].
But, Nrf2 has dual roles to protect both normal and cancer cells against cell stress. It has been shown to increase normal and cancer cell survival, to play roles in both prevention and promotion of cancer [3,15,16]. Previous reports have demonstrated Nrf2's role in cancer promotion and increased expression of Nrf2 in many cancers including primary head and neck cancers [10], hepatocellular carcinoma [17], and stomach cancer [18].
Nrf2 transcriptional factor clearly has a very complex role in cells and it is evident that its expression is strongly influenced by external factors. Increased or decreased Nrf2 expression can also promote the generation and progression of CRC. It was reported that Nrf2 overexpression may increase the risk of CRC [19]. Its overexpression can occur for various reasons, including the mutation of the Keap1 gene or Nrf2 gene. Colon tissue inflammation and formation of tumor can be promoted when ROS induce overexpression of Nrf2 [20]. In another study, overexpression of Nrf2 in tissues exposed to inflammatory macrophages was reported and it was demonstrated that reduced apoptosis led to unregulated proliferation, while activity of proteasomal gene increased simultaneously. Therefore, increased Nrf2 expression may lead to greater risks of CRC [21]. In this case, promotion of colon inflammation, reduction of apoptosis, and unregulated cell proliferation can increase the risk of CRC.
Our research findings were consistent with the results of previous reports suggesting that overexpression of Nrf2 contributes to CRC formation [22,23]. Nrf2 expression was increased in CRC tissues, compared with normal tissues (Fig. 2). In addition, the immunohistochemistry demonstrated that Nrf2 expression was upregulated in the CRC tissue, but Nrf2 expression was definitely decreased in normal tissues (Fig. 1). However, although we investigated levels of Keap1 expression, it was not different between CRC tissues and normal tissues in western blot (data not shown), indicating that Nrf2 might be regulated by a Keap1-independent pathway. We also investigated the correlation between Nrf2 expression and clinicopathologic characteristics (Table 3). Although there was a limitation of small sample size, Nrf2 expression was related with pathologic stage (P = 0.018). These findings indicate that overexpression of Nrf2 may be related with formation and stage of CRC.
There are several reports stating that Nrf2 can control cell signal transduction through the MEK/ERK1/2 pathways and the PI3K/AKT pathways, regulating cell reactions such as cell survival, proliferation, and migration. Some studies conducted research on the roles of Nrf2, including its effect on ERK and PI3K signal transduction pathways [17,24,25]. Nrf2 promoted PI3K/AKT signal transduction pathways and this procedure was related to the proliferation of HCC [17]. Based on those previous researches, we conducted further experiments with knockdown of Nrf2 expression in SW480 cells to confirm signaling pathway of Nrf2. As shown in Fig. 3, we discovered that in SW480 cells transfected with Nrf2-siRNA, the protein levels of phosphorylated ERK1/2 and AKT were decreased. Considering that MEK/ERK1/2 and PI3K/AKT pathways are important in cell reactions that include cell survival, proliferation, and migration, Nrf2 may regulate cell progression together with cross-communication of MEK/ERK1/2 and PI3K/AKT signaling pathways.
Nrf2 increased expression of Bcl2, one of the anti-apoptotic proteins, leading to protection of cells from apoptosis [26]. From this finding, we hypothesized that down-regulation of Nrf2 in SW480 may decrease expression of Bcl-2, thereby activating apoptosis of cancer cells. In our study, we observed down-regulation of Bcl-2 and decreases in cell survival of SW480 cells transfected with Nrf2-specific siRNA (Figs. 3, 4). This result indicates that Nrf2 increases cancer cell survival by reducing cellular apoptosis.
Different types of cancers metastases possessing increase in Nrf2 have been reported [27]. It was found that E-cadherin prevented nucleic accumulation by binding with the C terminus of Nrf2, and that the overexpression of N-cadherin during epithelial-mesenchymal transition reduced Nrf2 inhibition, thereby increasing Nrf2 activation [28]. Furthermore, Nrf2 suppression due to shRNA in esophageal squamous cell carcinoma suppressed MMP-2 expression, and increased the E-cadherin mRNA levels, thus reducing invasion and migration of cancer cells [29]. According to previous reports showing association between Nrf2 and cancer cell migration, we further performed wound healing assay to figure out effects of Nrf2 expression related to migration of CRC. In our study, inhibition of Nrf2 by siRNA interfered with cell migration in SW480 cells (Fig. 5). These results indicate that Nrf2 expression may contribute to cancer cell migration.
In current study, we found increased expression of Nrf2 may be associated with CRC because cancer cells escaped from microenvironments and promoted cancer progression when CRC cells were faced with cell stress. Until now, the oncogenic roles of Nrf2 have been studied in CRC, but not well in vivo. The results of this study, together with that of previous studies, suggest that the proliferation and migration of SW480 cells are regulated by Nrf2. Also, our results showed that Nrf2 was overexpressed in CRC tissues and that Nrf2 siRNA-mediated silencing in SW480 cells could reduce not only cell proliferation and migration, but also phosphorylation of ERK and AKT. These results imply that Nrf2 plays a decisive role in the proliferation and progression of CRC tissues and cells. Furthermore, it was reported recently that upregulation of Nrf2 in CRC may be associated with poor prognosis across all stages of CRC [30]. Collectively, the overexpression of Nrf2 may play a critical role in CRC progression and Nrf2 may represent a new candidate for targeted treatment.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: SWC, MKC.
- Formal Analysis: YJL, WIK, SHL.
- Investigation: YJL, JHB, HSN, IHC.
- Methodology: YJL, JHB, SHL.
- Project Administration: YJL, WIK.
- Writing — Original Draft: YJL, WIK, JHB, IHC.
- Writing — Review & Editing: SWC, MKC, SHL, HSN.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78:237–248. doi: 10.1159/000315730. [DOI] [PubMed] [Google Scholar]
- 3.Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3:188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, et al. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 6.Kim HR, Kim S, Kim EJ, Park JH, Yang SH, Jeong ET, et al. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer. 2008;60:47–56. doi: 10.1016/j.lungcan.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Bogner PN, Ramnath N, Park Y, Yu J, Park YM. Elevated peroxiredoxin 1, but not NF-E2-related factor 2, is an independent prognostic factor for disease recurrence and reduced survival in stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:3875–3882. doi: 10.1158/1078-0432.CCR-06-2893. [DOI] [PubMed] [Google Scholar]
- 8.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 9.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 10.Stacy DR, Ely K, Massion PP, Yarbrough WG, Hallahan DE, Sekhar KR, et al. Increased expression of nuclear factor E2 p45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head Neck. 2006;28:813–818. doi: 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- 11.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arlt A, Bauer I, Schafmayer C, Tepel J, Muerkoster SS, Brosch M, et al. Increased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2) Oncogene. 2009;28:3983–3996. doi: 10.1038/onc.2009.264. [DOI] [PubMed] [Google Scholar]
- 13.Hu T, Yao Y, Yu S, Guo H, Han L, Wang W, et al. Clinicopathologic significance of CXCR4 and Nrf2 in colorectal cancer. J Biomed Res. 2013;27:283–290. doi: 10.7555/JBR.27.20130069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li CQ, Kim MY, Godoy LC, Thiantanawat A, Trudel LJ, Wogan GN. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc Natl Acad Sci U S A. 2009;106:14547–14551. doi: 10.1073/pnas.0907539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 16.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Zhang Y, Wei Y, Liu G, Liu Y, Gao Q, et al. Activation of AKT pathway by Nrf2/PDGFA feedback loop contributes to HCC progression. Oncotarget. 2016;7:65389–65402. doi: 10.18632/oncotarget.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HB, Zhou CJ, Song SZ, Chen P, Xu WH, Liu B, et al. Evaluation of Nrf2 and IGF-1 expression in benign, premalignant and malignant gastric lesions. Pathol Res Pract. 2011;207:169–173. doi: 10.1016/j.prp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghi MR, Jeddi F, Soozangar N, Somi MH, Samadi N. The role of Nrf2-Keap1 axis in colorectal cancer, progression, and chemoresistance. Tumour Biol. 2017;39:1010428317705510. doi: 10.1177/1010428317705510. [DOI] [PubMed] [Google Scholar]
- 20.Stachel I, Geismann C, Aden K, Deisinger F, Rosenstiel P, Schreiber S, et al. Modulation of nuclear factor E2-related factor-2 (Nrf2) activation by the stress response gene immediate early response-3 (IER3) in colonic epithelial cells: a novel mechanism of cellular adaption to inflammatory stress. J Biol Chem. 2014;289:1917–1929. doi: 10.1074/jbc.M113.490920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebens S, Bauer I, Geismann C, Grage-Griebenow E, Ehlers S, Kruse ML, et al. Inflammatory macrophages induce Nrf2 transcription factor-dependent proteasome activity in colonic NCM460 cells and thereby confer anti-apoptotic protection. J Biol Chem. 2011;286:40911–40921. doi: 10.1074/jbc.M111.274902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoo Y, Kijima A, Ishii Y, Takasu S, Tsuchiya T, Umemura T. Effects of Nrf2 silencing on oxidative stress-associated intestinal carcinogenesis in mice. Cancer Med. 2016;5:1228–1238. doi: 10.1002/cam4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KM, Kang K, Lee SB, Nho CW. Nuclear factor-E2 (Nrf2) is regulated through the differential activation of ERK1/2 and PKC α/βII by Gymnasterkoreayne B. Cancer Lett. 2013;330:225–232. doi: 10.1016/j.canlet.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X, Xu C, Pan Z, Keum YS, Kim JH, Shen G, et al. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol Carcinog. 2006;45:841–850. doi: 10.1002/mc.20234. [DOI] [PubMed] [Google Scholar]
- 26.Niture SK, Jaiswal AK. Nrf2 protein upregulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012;287:9873–9886. doi: 10.1074/jbc.M111.312694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Zhang M, Zhang L, Cai H, Zhou S, Zhang J, et al. Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. J Surg Res. 2010;164:e99–e105. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 28.Kim WD, Kim YW, Cho IJ, Lee CH, Kim SG. E-cadherin inhibits nuclear accumulation of Nrf2: implications for chemoresistance of cancer cells. J Cell Sci. 2012;125(Pt 5):1284–1295. doi: 10.1242/jcs.095422. [DOI] [PubMed] [Google Scholar]
- 29.Shen H, Yang Y, Xia S, Rao B, Zhang J, Wang J. Blockage of Nrf2 suppresses the migration and invasion of esophageal squamous cell carcinoma cells in hypoxic microenvironment. Dis Esophagus. 2014;27:685–692. doi: 10.1111/dote.12124. [DOI] [PubMed] [Google Scholar]
- 30.O'Cathail SM, Wu CH, Lewis A, Holmes C, Hawkins MA, Maughan T. A metagene of NRF2 expression is a prognostic biomarker in all stage colorectal cancer. bioRxiv. 2019 Jul 02; doi: 10.1101/690974. [DOI] [PubMed] [Google Scholar]