Abstract
Gastroenteritis remains a serious health condition among children under 5 years especially in Africa. We conducted a systematic review and meta-analysis to investigate the aetiologic pathogens of gastroenteritis in the region. We did a systematic search for articles with original data on the aetiology of gastroenteritis and acute diarrhoea among children younger than 5 years. Pooled results were extracted and analysed in STATA version 12.0 using random-effects for statistical test for homogeneity following the guidelines provided in the Cochrane Collaboration and Preferred reporting items for systematic reviews and meta-analyses. Overall, viruses accounted for 50.2% of the cases followed by bacteria with 31.6% of the cases. Parasites accounted for 12.1% of the case. Rotavirus was the most common cause of acute diarrhoea in all regions resulting in 29.2% of the cases followed by E. coli (15.6%) of diarrhoeal cases and Adenovirus (10.8%). The most prevalent parasite detected was Giardia lamblia (7.3%). Acute diarrhoea remains rampant with Rotavirus still being the major pathogen responsible for the disease in children less than 5 years old despite the introduction of vaccine. It is recommended that the vaccine should be promoted much more widely in the region.
Key words: Diarrhoea in children, diarrhoea, diarrhoeal enteric pathogens, gastroenteritis
Introduction
Mortality due to childhood diarrhoea has decreased over the recent decades due to improved detection, preventive and proper treatment; nonetheless, acute gastroenteritis is still a major concern especially in low-income countries including sub-Saharan Africa [1]. Diarrhoea is the eighth leading cause of mortality accounting for about 1.6 million deaths in 2016 among children of all ages and the fifth leading cause of death in children less than 5 years old [2]. Globally, acute gastroenteritis accounts for 10% of hospitalizations and 19% of deaths in children under 5 years [3]. It is said to be predominantly caused by viruses; with Rotavirus accounting for about 20% of fatal diarrhoea globally, though they are also associated with bacteria and some protozoans [3–5].
Clinical investigation of diarrhoeal aetiology can be expensive and time consuming and the results seldom directly affect patient treatment [6]. However, it is important to determine aetiology because it is a key determinant in diarrhoeal disease prevention and treatment. For example, Rotavirus which is the single leading aetiologic pathogen for gastroenteritis now has an effective vaccine [7, 8].
This systematic review accessed the aetiology of gastroenteritis in sub-Saharan Africa using multiple studies across different sub-regions in order to have a clearer picture of the true etiologic pathogens to serve as a reliable source of information in tackling the burden of gastroenteritis.
Materials and methods
This systematic review followed the guidelines provided in the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA checklist is included in Supporting Information.
Search strategy for identification of studies
A systematic search of three electronic databases (Pubmed, Google Scholar and Wiley Online Library) was conducted using a range of search strings (Etiology of gastroenteritis in Africa, Aetiology of Diarrhoea, Gastroenteritis in children under 5 years, Diarrhoea Disease in children, Acute Diarrhoea in Africa, Diarrhoea in Africa, Gastrointestinal Diseases children, Causes of Diarrhoea in children) and was limited to studies published from 2007 to 2019 in the English language.
Inclusion and exclusion criteria
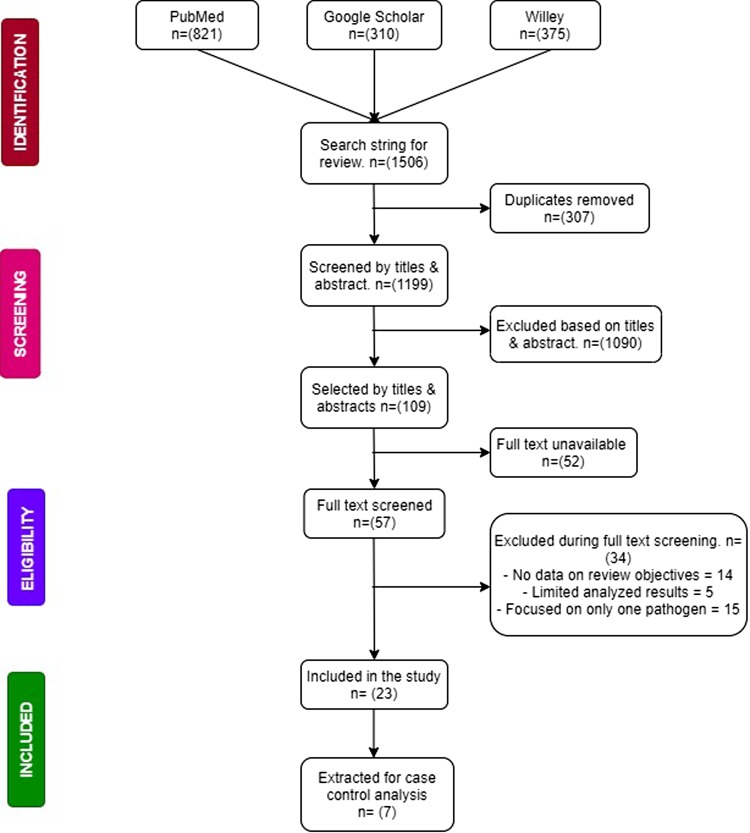
Articles had to report original data on the aetiology of viruses, bacteria and parasites associated with gastrointestinal diseases with a particular emphasis on diarrhoea before they could be included in this study. Articles were excluded if they focused on only one pathogen (being it a virus, bacteria or parasite) or focused on participants above 5 years. Included articles were also restricted to those conducted in Sub-Saharan Africa according to the United Nation's demarcations and reported clinically tested results of faecal samples to arrive at results. Articles were again excluded if quantitative data was not available or accessible. The process of data search and inclusion is summarised in Figure 1.
Fig. 1.
Flow chart of study selection and criteria.
Data selection
Data was extracted from full-text articles and were reviewed by three independent researchers after their titles and abstracts had been screened for relevance. During the screening process, an additional researcher was on standby to resolve any discrepancies that may arise with study selection. Relevant data such as the following were collected: characteristics of the study (study period, setting, design, country and sub region in which the study was conducted), etiological agents (viruses, bacteria and parasites), study population (age of participants, case definition, sample size) and analysed data (frequencies, percentages, odds ratios, confidence intervals).
Risk of bias assessment
The study adapted a customised checklist used by Oordt-Speets et al., which is based on the Critical Appraisal Skills Program (CASP) and on criteria relevant to the designs of studies included in the systematic review to assess study quality/risk of bias in individual studies. The checklist included eight questions that could be answered ‘yes’, ‘no’ or ‘cannot tell/not applicable’. Each question was given a weight of 10 or 15 points based on relevance. Each study was given an overall quality assessment score based on answers to the eight questions; 100 points were scored if all eight responses were positive. Overall study quality was categorised as ‘high’ (scores ≥80 points), ‘moderate’ (scores >50 to <80 points) or ‘low’ (scores ⩽50 points).
Data was categorised into four sub geographical regions according to the United Nation's demarcation of Sub Saharan Africa: West African Region, East African Region, Middle African Region and South African Region. Publication bias also assessed.
Statistical analysis
Analysis was performed when data on age and region were available in studies that reported frequencies of two or more pathogens. Meta-analysis was performed using proportions of the pathogens that were identified among studies to be associated with gastroenteritis among children under 5 years old including: viruses (Rotavirus, Norovirus, Astrovirus and Adenovirus), bacteria (E. coli, Salmonella species, Shigella species and Campylobacter species) and parasites (Giardia species, Entamoeba histolytica and Trichomonas intestinalis). Separate analysis was also done for pathogens with the highest frequencies stratified by case control studies. Pooled proportions were calculated using the DerSimonian and Laird method of the random effects model [9] with results depicted on the forest plot. Heterogeneity was accessed by the Cochran's Q test and quantified by the Higgins I2 test. The resultant heterogeneity was considered as low (I2 of 0–30%), moderate (30–60%), substantial (60–90%) or high (90–100%). P-Values were obtained by comparing the statistic with the χ2 distribution with k-1 degrees of freedom, with 0.10 considered the cut-off for statistically significant heterogeneity. Publication bias was also assessed using Egger's test. All statistical analyses were performed in STATA version 12.0.
Results and discussion
Study characteristics
Out of the 1251 results pooled from unique searches, 23 [10–26] of them met the criteria for the selection of studies. Of the 23 studies that were included, ~69.6% (16/23) were cross-sectional studies while 30.4% (7/17) [12, 19–22, 24, 27] were case control studies but only five [12, 19, 20, 22, 24] reported the necessary data to be added to the case-control analysis. The majority of the included studies were from the East and West African Regions (10/23 and 9/23 respectively). Only two studies came from both the South and Middle African Regions. The majority of the studies received a moderate quality assessment score (11/17). Six studies received a ‘low’ assessment score because of inadequate case definitions and the lack of adjustment for possible confounding factors, in addition to the lack of representativeness of some of the study populations. A detailed description of the study characteristics is presented in Table 1. A total of 5481 cases of gastroenteritis were recorded in all the 23 studies reviewed. Included studies identified participants from hospitals. Only four studies specified outpatients as their source participants. Viruses accounted for 50.2% of the cases whereas bacteria and parasites accounted for 37.8% and 12.1% of the cases respectively. Table 2 shows the types of pathogens and the number of infections they caused.
Table 1.
Study characteristics
| Author (first author) Year | Study design | Country, Region in Africa | Sample size | Confirmed cases | Outcome pathogen | Quality assessment score |
|---|---|---|---|---|---|---|
| Aminu [10] | Cross sectional | Nigeria, West Africa | 134 | 21 | Rotavirus, Astrovirus | 60 (Moderate) |
| Sambe-Ba [11] | Cross sectional | Senegal, West Africa | 223 | 80 | Rotavirus, Adenovirus, E. coli, Salmonella, Shigella, Giardia, T. intestinalis | 60 (Moderate) |
| Nitiema et al. [26] | Cross sectional | Burkina Faso, West Africa | 309 | 215 | Rotavirus, E. coli, Salmonella, Shigella, Gardia. E. histolytica, T. intestinalis | 45 (Low) |
| Ouédraogo et al. [12] | Case control | Burkina Faso, West Africa | 263 | 225 | Rotavirus, Norovirus, Astrovirus, Adenovirus | 60 (Moderate) |
| Bonkoungou et al. [27] | Case control | Burkina Faso, West Africa | 283 | 211 | Rotavirus, Adenovirus, E. coli, Salmonella, Shigella, Campylobacter | 85 (High) |
| Feleke et al. [34] | Cross sectional | Ethiopia, East Africa | 112 | 36 | Rotavirus, Salmonella, Shigella, Gardia | 100 (High) |
| Sang et al. [35] | Cross sectional | Kenya, East Africa | 651 | 111 | E. coli, Salmonella, Shigella. | 50 (Low) |
| Mayindou et al. [36] | Cross sectional | Congo, East Africa | 655 | 303 | Rotavirus, Adenovirus, Campylobacter | 50 (Low) |
| Mulatu et al. [37] | Cross sectional | Ethiopia, East Africa | 158 | 35 | Salmonella, Shigella, Campylobacter | 45 (Low) |
| Moyo et al. [38] | Cross sectional | Tanzania, East Africa | 270 | 94 | Rotavirus, Norovirus, Astrovirus, Adenovirus | 90 (High) |
| Swierczewski et al. [19] | Case control | Kenya, East Africa | 432 | 232 | Rotavirus, E. coli, Salmonella, Shigella, Campylobacter, Giardia, E. histolytica. | 75 (Moderate) |
| Randremanana et al. [20] | Case control | Madagascar, East Africa | 2692 | 1160 | Rotavirus, Astrovirus, Adenovirus, E. coli, Salmonella, Shigella, Campylobacter, Giardia, E. histolytica, T. intestinalis | 90 (High) |
| Njuguna et al. [21] | Case control | Kenya, East Africa | 284 | 116 | E. coli, Salmonella, Giardia, E. histolytica, T. intestinalis | 75 (Moderate) |
| Randremanana et al. [22] | Case control | Madagascar, East Africa | 199 | 108 | Rotavirus, Astrovirus, Adenovirus, E. coli, Shigella | 75 (Moderate) |
| Amukoshi et al. [25] | Cross sectional | Namibia, South Africa | 1392 | 108 | E. coli, Salmonella, Shigella, Giardia, E. histolytica | 65 (Moderate) |
| Breurec et al. [24] | Case control | Central African Republic, Middle Africa | 428 | 343 | Rotavirus, Norovirus, Astrovirus, Adenovirus, E. coli, Salmonella, Shigella, Campylobacter, Giardia, E. histolytica | 75 (Moderate) |
| Lekana-Douki [23] | Cross sectional | Gabon, Middle Africa | 317 | 241 | Rotavirus, Norovirus, Astrovirus, Adenovirus | 60 (Moderate) |
| Kafayat (2019) | Cross sectional | West Africa | 175 | 73 | Rotavirus, Astrovirus, Norovirus, Adenovirus | 60 (Moderate) |
| Japhet et al. [14] | Cross sectional | West Africa | 103 | 59 | Rotavirus, Norovirus, Astrovirus | 50 (Low) |
| Adeh [17] | Cross sectional | West Africa | 240 | 173 | Salmonella, E. coli, Shigella | 75 (Moderate) |
| Ledwaba [15] | Cross sectional | South Africa | 237 | 62 | E. coli, Shigella, Adenovirus, Norovirus, Rotavirus | 50 (Low) |
| Andersson et al. [16] | Cross sectional | East Africa | 994 | 857 | Adenovirus, Astrovirus, Norovirus, Rotavirus, Campylobacter, Salmonella, Shigella | 90 (High) |
| Tagbo [18] | Cross sectional | West Africa | 50 | 12 | Rotavirus, Adenovirus | 90 (High) |
Table 2.
Distribution of pathogens and the total number of detections in diarrhoeal cases
| Pathogen | Total number of cases | Percentage (%) |
|---|---|---|
| Viruses | ||
| Rotavirus | 1599 | 29.2 |
| Norovirus | 361 | 6.6 |
| Astrovirus | 197 | 3.6 |
| Adenovirus | 592 | 10.8 |
| Total | 2749 | 50.2 |
| Bacteria | ||
| E. coli | 854 | 15.6 |
| Salmonella species | 248 | 4.5 |
| Shigella | 526 | 9.6 |
| Campylobacter | 442 | 8.1 |
| Total | 2070 | 37.8 |
| Parasites | ||
| G. lamblia | 400 | 7.3 |
| E. histolytica | 100 | 1.8 |
| T. intestinalis | 162 | 3.0 |
| Total | 662 | 12.1 |
| Total | 5481 | 100 |
Aetiology of gastroenteritis
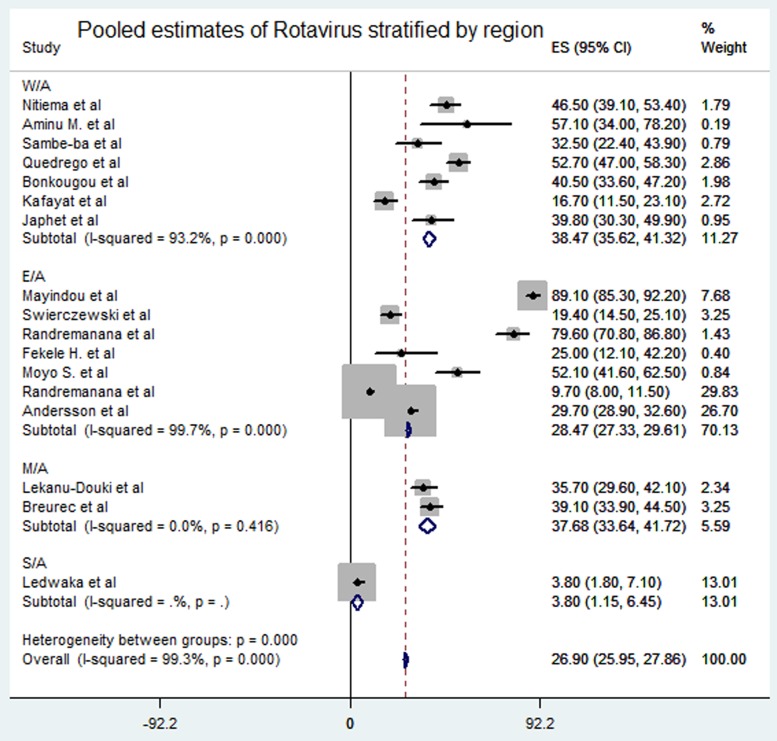
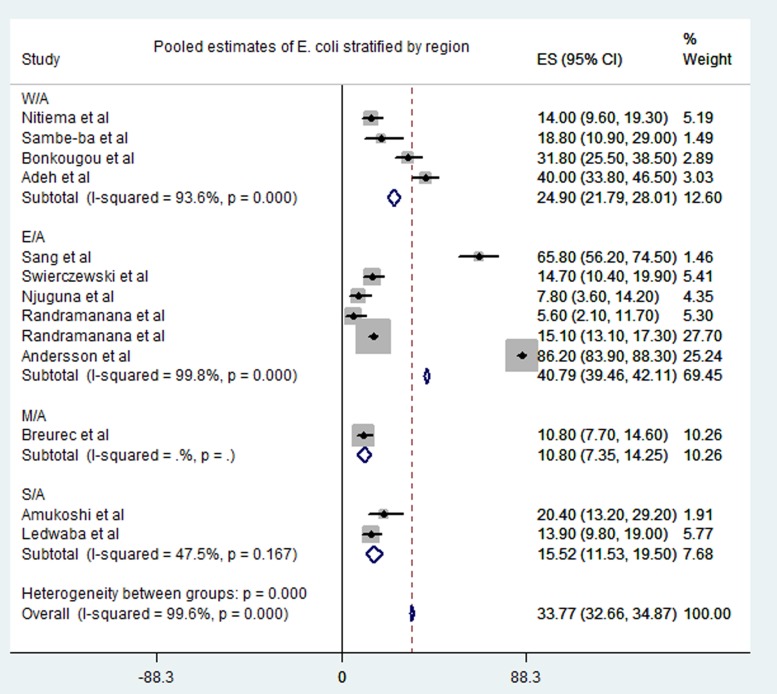
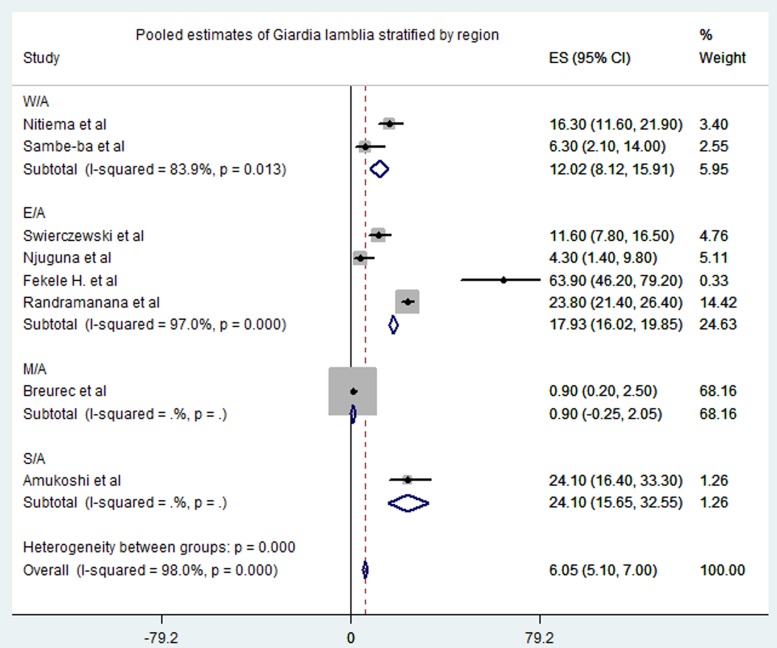
Meta-analysis of the 23 studies showed that the most prevalent virus associated with gastroenteritis across all the geographical regions was Rotavirus (pooled estimates from 3.8% to 38.5%) with an overall estimate of 26.% (95% CI 25.9–27.9) followed by Norovirus (pooled estimates between 5.1% and 29.5%) with an overall estimate of 8.4% (95% CI 7.5–9.2). Rotavirus detection was highest in the West African region and lowest in South Africa. Adenovirus and Astrovirus were the least detected viruses with an overall pooled estimate of 1.3% and 4.8% respectively. The most prevalent bacteria were E. coli (pooled estimates between 10.8% and 40.8%) with an overall estimate of 33.8% (95% CI 32.7–34.9). E. coli detection was highest in the East African region and lowest in middle Africa. The most prevalent parasite was T. intestinalis with an overall estimate of 12.6% (95% CI 11.4–13.7) pooled from only the west and east African regions. Generally, the Middle and South African regions contributed limited articles to this study, resulting in small detection rates of all pathogens in both sub-regions. A visual show of all the pathogens associated with gastroenteritis in all the regions is provided in Table 3. Viruses were predominant in gastrointestinal illness in sub Saharan Africa with the frequency of 1878 cases out of a total of 3712 cases. A distribution of cases and the type of pathogen can be seen in Table 2 above. Rotavirus was detected most frequently in all the regions with 1599 cases out of the 2749 cases associated with enteric viruses. The frequency of Rotavirus was highest in East Africa (899/1599), followed by West Africa (471/1599). Forest plots from the meta-analysis of the most prevalent virus, bacteria and parasite stratified by the region are included in Figures 2–4. A separate meta-analysis for diarrhoeal pathogens in the West, Middle and East African Regions stratified by case control studies (Table 4) also resulted in Rotavirus having the highest odds ratio compared to the detection of other enteric pathogens in diarrhoea cases (OR 4.585), followed by Giardia (OR 1.448) and E. coli (OR 1.734).
Table 3.
Overview of pathogens that caused gastrointestinal diseases from all studies stratified by regions
| Region | Rotavirus | Norovirus | Astrovirus | Adenovirus | E. coli | Salmonella | Shigella | Campylobacter | Giardia lamblia | E. histolytica | T. intestinalis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| West African Region (n) | 8 | 3 | 4 | 5 | 4 | 4 | 4 | 1 | 2 | 1 | 2 |
| No. of infections | 471 | 77 | 56 | 123 | 208 | 63 | 57 | 6 | 40 | 4 | 24 |
| Pooled estimate (%) | 38.5 | 5.4 | 9.9 | 9.1 | 24.9 | 6.7 | 7.6 | 0.3 | 12 | 2 | 7.1 |
| 95% CI | 35.6, 41.3 | 3.8, 6.9 | 7.8, 12 | 7.1, 11.2 | 21.8, 28.0 | 4.8, 8.6 | 5.6, 9.6 | −0.5, 1.1 | 8.1, 15.9 | 0.7, 3.3 | 3.9, 10.2 |
|
I2, % (P-value) |
93.2 (P < 0.001) |
29.5 (P = 0.24) |
73.7 (P = 0.01) |
93.8 (P < 0.001) |
93.6 (P < 0.001) |
79.1 (P = 0.002) |
0.0 (P = 0.7) |
NA | 83.9 (P = 0.01) |
NA | 74.3 (P = 0.048) |
| East African Region (n) | 7 | 2 | 4 | 5 | 6 | 7 | 8 | 5 | 4 | 3 | 2 |
| No. of infections | 899 | 166 | 81 | 382 | 683 | 136 | 387 | 431 | 331 | 86 | 138 |
| Pooled estimate (%) | 28.5 | 10.4 | 3.3 | 1.1 | 40.8 | 4.1 | 6.9 | 12.3 | 17.9 | 5 | 20.5 |
| 95% CI | 27.33, 29.6 | 8.9,11.9 | 2.6, 4.07 | 0.8, 1.4 | 39.5, 42.1 | 3.3, 4.9 | 6, 7.9 | 11, 13.6 | 16, 19.8 | 3.8, 6.2 | 18.7, 22.1 |
|
I2, % (P-value) |
99.7 (P < 0.001) |
90 (P = 0.002) |
0.0 (P = 0.4) |
98.7 (P < 0.001) |
99.8 (P < 0.001) |
92 (P ⩽ 0.001) |
97.7 (P < 0.001) |
97.6 (P < 0.001) |
97 (P < 0.001) |
83.8 (P < 0.001) |
99.3 (P < 0.001) |
| Middle African Region (n) | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| No. of infections | 220 | 106 | 53 | 81 | 37 | 13 | 61 | 5 | 3 | 5 | 0 |
| Pooled estimate (%) | 37.8 | 9.6 | 10.8 | 1.7 | 10.8 | 5.6 | 5.6 | 7 | 0.9 | 8 | |
| 95% CI | 33.6, 41.7 | 8.2, 11 | 8.7, 12.8 | 0.6, 2.8 | 7.35, 14.2 | 2.9, 8.3 | 3.7, 7.5 | 4.9, 9.1 | −0.3 2.1 | 5.5, 10.4 | NA |
|
I2, % (P-value) |
0.0 (P = 0.4) |
94.2 (P < 0.001) |
72.6 (P = 0.06) |
0.0 (P < 0.9) |
NA | NA | NA | NA | NA | NA | NA |
| South African Region (n) | 1 | 1 | 0 | 1 | 2 | 1 | 2 | 0 | 1 | 1 | 0 |
| No. of infections | 9 | 12 | 0 | 6 | 22 | 36 | 19 | 0 | 26 | 5 | 0 |
| Pooled estimate (%) | 3.8 | 5.1 | NA | 2.5 | 15.5 | 6.8 | 6.7 | NA | 24.1 | 4.3 | |
| 95% CI | 1.2, 6.5 | 2.0, 8.1 | NA | −24.1, 29.1 | 11.5, 19.5 | 4, 9.6 | 1.3, 4 | NA | 15.7, 32.5 | 2.5, 6 | NA |
|
I2, % (P-value) |
NA | NA | NA | NA | 47.5 (P = 0.2) |
NA | 97.4 (P < 0.001) |
NA | NA | NA | |
| Overall pooled estimate (%) | 26.9 | 8.4 | 4.8 | 1.3 | 33.8 | 4.7 | 5.7 | 3.9 | 6.1 | 4.2 | 12.6 |
| 95% CI | 25.9, 27.9 | 7.5, 9.2 | 4.1, 5.8 | 1.0, 1.6 | 32.7, 34.9 | 4.0, 5.3 | 5.1, 6.4 | 3.3, 4.5 | 5.1, 7 | 3.5, 5 | 11.4, 13.7 |
|
I2, % (P-value) |
99.3 (P < 0.001) | 88.1 (P < 0.001) | 89.8 (P < 0.001) | 97.5 (P < 0.001) | 99.6 (P < 0.001) | 87.8 (P < 0.001) | 96.2 (P < 0.001) | 98.6 (P < 0.001) | 98 (P < 0.001) | 85.1 (P < 0.001) | 98 (P < 0.001) |
Fig. 2.
Pooled estimates of Rotavirus stratified by region.
Fig. 3.
Pooled estimates of E. coli stratified by region.
Fig. 4.
Pooled estimates of Giardia lamblia stratified by region.
Table 4.
Meta-analysis of odds ratios of case control studies
| Rotavirus | Adenovirus | E. coli | Shigella | Gardia | |
|---|---|---|---|---|---|
| No. of studies | 5 | 4 | 3 | 5 | 3 |
| OR | 4.585 | 1.849 | 1.448 | 3.201 | 1.734 |
| 95% CI | 3.151, 6.67 | 1.219, 2.803 | 0.981, 2.1 | 1.993, 5.141 | 1.354, 2.22 |
| I2, % (P-value) | 88.8 (P < 0.001) | 0.00 (P = 0.433) | 71.4 (P = 0.15) | 44.6 (P = 0.12) | 0.00 (P = 0.42) |
Publication bias and source of heterogeneity
Egger's test of publication bias was not significant (P = 0.085 at 95% CI); supporting the hypothesis that there was no publication bias. Large heterogeneity was found among the included studies. Heterogeneity could partly be attributed to large variation in sampling. Some studies investigated large samples while others investigated small samples. Again, the number of pathogens reported by the different studies also varied greatly. The average number of pathogens reported by included studies was ~5 out of the 11 pathogens examined in this study. Lastly, heterogeneity could also be attributed to the diagnostic methods used to detect the diarrhoeal pathogens since different methods may have different sensitivities, specificities, positive predictive and negative predictive values
Discussion
This study investigated the aetiology of enteric pathogens associated with gastroenteritis in children under 5 years. To the best of our knowledge, this is the first systematic review and meta-analysis covering the frequency of enteric pathogens associated with gastroenteritis in children less than 5 years in sub-Saharan Africa. This systematic review indicated that viruses are the most common pathogens associated with gastroenteritis in children less than 5 years old; accounting for about 50.2% of the cases analysed (Table 2). This confirms the existing knowledge that viruses are accountable for over 70% of all gastroenteritis in infants [4, 5]. The Global Enteric Multicenter Study (GEMS) identified Rotavirus, Cryptosporidium, Shigella and certain strains of E. coli (particularly ST-only or LT/STST-ETEC strains) as the most predominant enteric pathogens associated with diarrhoeal diseases with Rotavirus having the highest attributable fraction in all their sites [28]. Even though the GEMS study included only four sites in Africa, their outcome pertaining to the most common pathogens associated with diarrhoeal diseases is comparable to this study except Cryptosporidium, which is not included in this analysis because it is not often reported in the region; an assertion the GEMS agrees on. In this review, Rotavirus accounted for 32.7% of cases which confirms that Rotavirus is the most common cause of severe gastroenteritis in children worldwide, accounting for 30% to 72% in all hospitalisations and 4% to 24% in acute gastroenteritis at the community level [4, 29]. Even though some studies [22, 30] assert that the introduction of Rotavirus vaccine has changed this trend, we found that Rotavirus is still the leading cause of acute diarrhoea in sub-Saharan Africa except in south Africa which had a relatively low estimate (3.8%). In fact, Randremanana et al., revealed that despite the introduction of Rotavirus vaccine in the year prior to their study, Rotavirus was still the leading cause of gastroenteritis in Madagascar [22]. This assertion is however not a measure of the impact of Rotavirus vaccine in the region as we did not include a pre- and post-analysis of Rotavirus infections in this study.
A relatively limited decline in Rotavirus cases in an area may be due to multiple factors including: reduced vaccine effectiveness (vaccine efficacy reported to be around 60% in low-income countries and up to about 90% high-income countries), failure to vaccinate resulting in low vaccine coverage, breastfeeding, malnutrition, co-administration of OPV and the kind of Rotavirus strain causing the infections [23, 31]. Our meta-analysis showed that the odds of Rotavirus being associated with gastroenteritis was more than four times in cases (OR 4.58 at 95% CI) as compared to other known etiologic pathogens like Shigella (OR 3.201), E. coli (OR 1.849) and Adenoviruses (OR 1.448) (Table 4). Adenovirus, Astrovirus and Norovirus have also been identified as important etiologic factors of gastroenteritis [30, 29].
Even though viruses are the most common causes of gastroenteritis, bacteria and parasites are also a serious concern especially in low-income countries in sub-Saharan Africa [5]. It has been noted that Salmonella, Campylobacter and Shigella species are most common bacteria in gastroenteritis [4]. This contradicts our results as the meta-analysis of this study revealed that E. coli cases (overall pooled estimate 33.8%) was the most prevalent bacteria associated with gastroenteritis in the regions of sub-Saharan Africa.
Parasitic infections causing gastroenteritis are common; for example, giardia is detected in about 5% to 30% of infectious gastroenteritis in low-income countries and is thought to be associated with poverty [32, 33]. This is consistent with this review which revealed that about 7.2% (Table 2) of the cases in our study were by Giardia lamblia. A study in Ghana indicated an overall incidence of Giardia lamblia to be 144 per 1000 persons with the parasite commonly detected (89.5%) in the population under study [33]. Among the three parasites included in this review, Giardia lamblia was most detected in cases (400/662). T. intestinalis followed with 162 cases out of the 662 parasites detected. These parasites are more frequent in low-income countries with poor sanitation, indicating that parasitic infections can be prevented by improving sanitation [4].
The major limitation we encountered with this review is that fewer studies from the Middle and South African Regions met the study criteria. Some studies lacked the necessary data for analysis. Again, most of the included studies did not report on all of the pathogens under review. This limits the ability to access the true impact of those pathogens not reported.
Conclusion
This systematic review and meta-analysis showed notable variations in the proportions of etiological pathogens associated with gastroenteritis in different regions across sub-Saharan Africa. Our study has also identified various enteric pathogens associated with gastroenteritis. We recommend that future aetiology studies focus on all known enteric pathogens associated with diarrhoea and not limited to only one pathogen. This will help know the true aetiology of diarrhoeal disease and allow design of prevention and treatment strategies. It is further recommended that Rotavirus vaccination should be intensified across sub-Saharan Africa.
Acknowledgments
Conflict of interest
None.
Author contributions
All authors made substantial contribution to the study. TBO designed and conceived the study. HY supervised and edited the manuscript. CAB and EKDK conducted electronic search, extracted data and analysed it. TA and DG wrote the initial manuscript. TBO and GO revised manuscript. HY, TBO and GO critically analysed the manuscript. All authors gave final approval for manuscript to be submitted for publication.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical standards
This study is a systematic review and meta-analysis using secondary data already published in 17 articles, all of which have been duly cited.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268820000618.
click here to view supplementary material
References
- 1.Vasco G et al. (2014) Identifying etiological agents causing diarrhea in low income Ecuadorian communities. American Journal of Tropical. Medicine and Hygiene 91, 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.C. GBD 2016 Diarrhoeal Disease Collaborators et al. (2018) Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infectious Disease 18, 1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adadey SM and Quaye O (2017) The burden of gastroenteritis in the post-Rotavirus vaccine era in Ghana: a hospital diagnoses-based study. International Journal of Medical. Research and Health Sciences 6, 45–49. [Google Scholar]
- 4.Chow CM, Leung AK and Hon KL (2010) Acute gastroenteritis: from guidelines to real life. Clinical and Experimental Gastroenterology 3, 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb A and Starr M (2005) Acute gastroenteritis in children. Australian Family Physician 34, 227–231. [PubMed] [Google Scholar]
- 6.Fauci AS and Morens DM (2012) The perpetual challenge of infectious diseases. New England Journal of Medicine, 366:454-461 DOI: 10.1056/NEJMra1108296 [DOI] [PubMed] [Google Scholar]
- 7.Goldman RD (2012) Effectiveness of Rotavirus vaccine in preventing severe acute gastroenteritis in children. Canadian Family Physician 58, 270–271. [PMC free article] [PubMed] [Google Scholar]
- 8.Wittenberg DF (2012) Management guidelines for acute infective diarrhoea/gastroenteritis in infants. South African Medical Journal 102, 104–107. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R and Laird N (2015) Meta-analysis in clinical trials revisited. Contemprory Clinical Trials 45, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aminu M et al. (2008) Epidemiology of Rotavirus and Astrovirus infections in children in Northwestern Nigeria. Annals of African Medicine 7, 168. [DOI] [PubMed] [Google Scholar]
- 11.Sambe-Ba B et al. (2013) Community-acquired diarrhea among children and adults in urban settings in Senegal: clinical, epidemiological and microbiological aspects. BMC Infectious Disease 13, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouédraogo N et al. (2016) Prevalence and genetic diversity of enteric viruses in children with diarrhea in Ouagadougou, Burkina Faso. PLoS ONE 11, e0153652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arowolo KO et al. (2019) Epidemiology of enteric viruses in children with gastroenteritis in Ogun State, Nigeria. Journal of Medical Virology 91, 1022–1029. [DOI] [PubMed] [Google Scholar]
- 14.Japhet MO et al. (2019) Viral gastroenteritis among children of 0–5 years in Nigeria: characterization of the first Nigerian aichivirus, recombinant Noroviruses and detection of a zoonotic Astrovirus. Journal of Clinical Virology 111, 4–11. [DOI] [PubMed] [Google Scholar]
- 15.Ledwaba SE et al. (2018) Enteric pathogen co-infections in the paediatric population from rural communities in the Vhembe District, South Africa. SAJCH South African Journal of Child Health 12, 170–174. [Google Scholar]
- 16.Andersson M et al. (2018) Coinfection with enteric pathogens in east African children with acute gastroenteritis – associations and interpretations. American Journal of Tropical Medicine and Hygiene 98, 1566–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeh I, Maikaje DB and Inabo HI (2019) Prevalence of bacterial gastroenteritis in children. Science World Journal 14, 86–91. [Google Scholar]
- 18.Tagbo BN et al. (2019) Adenovirus and Rotavirus associated diarrhoea in under 5 children from Enugu rural communities, South East Nigeria. World Journal of Vaccines 09, 71–83. [Google Scholar]
- 19.Swierczewski BE et al. (2013) Surveillance for enteric pathogens in a case-control study of acute diarrhea in western Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 107, 83–90. [DOI] [PubMed] [Google Scholar]
- 20.Randremanana R et al. (2012) Case-control study of the etiology of infant diarrheal disease in 14 districts in Madagascar. PLoS ONE 7, e44533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Njuguna C et al. (2016) Enteric pathogens and factors associated with acute bloody diarrhoea, Kenya. BMC Infectious Disease 16, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randremanana RV et al. (2016) Etiologies, risk factors and impact of severe diarrhea in the under-fives in Moramanga and Antananarivo, Madagascar. PLoS ONE 11, e0158862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lekana-Douki SE et al. (2015) Molecular epidemiology of enteric viruses and genotyping of Rotavirus A, Adenovirus and Astrovirus among children under 5 years old in Gabon. International Journal of Infectious Disease 34, 90–95. [DOI] [PubMed] [Google Scholar]
- 24.Breurec S et al. (2016) Etiology and epidemiology of diarrhea in hospitalized children from low income country: a matched case-control study in Central African Republic. PLoS Negleted Tropical Disease 10, e0004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amukoshi M. et al. (2017) Etiological agents isolated from stool samples of children under the age of five years in Windhoek, Namibia. Edorium Journal of Microbiology 3, 1–9. [Google Scholar]
- 26.Nitiema LW et al. (2011) Burden of Rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. International Journal of Infectious Diseases 15, e646–e652. [DOI] [PubMed] [Google Scholar]
- 27.Bonkoungou IJO et al. (2013) Bacterial and viral etiology of childhood diarrhea in Ouagadougou, Burkina Faso. BMC Pediatrics 13, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotloff KL et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet (London, England) 382, 209–222. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelmi I, Roman E and Sánchez-Fauquier A (2003) Viruses causing gastroenteritis. Clinical Microbiology and Infections 9, 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desselberger U and Goodfellow I (2014) Noroviruses: a global cause of acute gastroenteritis. Lancet Infectious Disease 14, 664–665. [DOI] [PubMed] [Google Scholar]
- 31.Burnett E et al. (2016) Rotavirus vaccines: current global impact and future perspectives. Future Virology 11, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minetti C et al. (2016) Giardiasis. BMJ 355, i5369. [DOI] [PubMed] [Google Scholar]
- 33.Nkrumah B and Nguah S (2011) Giardia lamblia: a major parasitic cause of childhood diarrhoea in patients attending a district hospital in Ghana. Parasite & Vectors 4, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feleke H et al. (2018) Enteric pathogens and associated risk factors among under-five children with and without diarrhea in Wegera District. Northwestern Ethiopia. Pan African Medical Journal 29, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sang WK, Oundo V and Schnabel D (2012) Prevalence and antibiotic resistance of bacterial pathogens isolated from childhood diarrhoea in four provinces of Kenya, pp. 572–5786. [DOI] [PubMed] [Google Scholar]
- 36.Mayindou G et al. (2016) Molecular epidemiology and surveillance of circulating rotavirus and adenovirus in Congolese children with gastroenteritis. Journal of Medical Virology 88(4), 596–604. [DOI] [PubMed] [Google Scholar]
- 37.Mulatu G, Beyene G and Zeynudin A (2014) Prevalence of Shigella, Salmonella and Campylobacter species and their susceptibility patters among under five children with diarrhea in Hawassa town, south Ethiopia. Ethiopian Journal of Health Sciences 4(2), 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moyo SJ, Gro N and Kirsti V (2007) Prevalence of enteropathogenic viruses and molecular characterization of group A rotavirus among children with diarrhea in Dar es Salaam Tanzania. BMC Public Health 1(7), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268820000618.
click here to view supplementary material