Summary
Background
Early menopause is linked to an increased risk of cardiovascular disease mortality; however, the association between early menopause and incidence and timing of cardiovascular disease is unclear. We aimed to assess the associations between age at natural menopause and incidence and timing of cardiovascular disease.
Methods
We harmonised and pooled individual-level data from 15 observational studies done across five countries and regions (Australia, Scandinavia, the USA, Japan, and the UK) between 1946 and 2013. Women who had reported their menopause status, age at natural menopause (if postmenopausal), and cardiovascular disease status (including coronary heart disease and stroke) were included. We excluded women who had hysterectomy or oophorectomy and women who did not report their age at menopause. The primary endpoint of this study was the occurrence of first non-fatal cardiovascular disease, defined as a composite outcome of incident coronary heart disease (including heart attack and angina) or stroke (including ischaemic stroke or haemorrhagic stroke). We used Cox proportional hazards models to estimate multivariate hazard ratios (HRs) and 95% CIs for the associations between age at menopause and incident cardiovascular disease event. We also adjusted the model to account for smoking status, menopausal hormone therapy status, body-mass index, and education levels. Age at natural menopause was categorised as premenopausal or perimenopausal, younger than 40 years (premature menopause), 40–44 years (early menopause), 45–49 years (relatively early), 50–51 years (reference category), 52–54 years (relatively late), and 55 years or older (late menopause).
Findings
Overall, 301 438 women were included in our analysis. Of these 301 438 women, 12 962 (4·3%) had a first non-fatal cardiovascular disease event after menopause, of whom 9369 (3·1%) had coronary heart disease and 4338 (1·4%) had strokes. Compared with women who had menopause at age 50–51 years, the risk of cardiovascular disease was higher in women who had premature menopause (age <40 years; HR 1·55, 95% CI 1·38–1·73; p<0·0001), early menopause (age 40–44 years; 1·30, 1·22–1·39; p<0·0001), and relatively early menopause (age 45–49 years; 1·12, 1·07–1·18; p<0·0001), with a significantly reduced risk of cardiovascular disease following menopause after age 51 years (p<0·0001 for trend). The associations persisted in never smokers, and were strongest before age 60 years for women with premature menopause (HR 1·88, 1·62–2·20; p<0·0001) and early menopause (1·40, 1·27–1·54; p<0·0001), but were attenuated at age 60–69 years, with no significant association observed at age 70 years and older.
Interpretation
Compared with women who had menopause at age 50–51 years, women with premature and early menopause had a substantially increased risk of a non-fatal cardiovascular disease event before the age of 60 years, but not after age 70 years. Women with earlier menopause need close monitoring in clinical practice, and age at menopause might also be considered as an important factor in risk stratification of cardiovascular disease for women.
Funding
Australian National Health and Medical Research Council.
Introduction
Natural menopause, defined as absence of menstruation over a period of 12 months,1 typically occurs between the ages of 49 and 52 years, but varies among different ethnicities,2 with a median age of 51·4 years in high-income countries.3, 4 Menopause between age 40 and 45 years, usually defined as early menopause, occurs in around 5% of women.5, 6 Premature menopause before 40 years (also known as primary ovarian insufficiency if periods stop spontaneously)6 affects about 1% of women.7
Although early menopause has been linked to an increased risk of cardiovascular disease mortality and all-cause mortality,8 few studies have examined the associations between a broad range of ages at menopause and risk of incident cardiovascular disease. For example, in a 2016 review and meta-analysis,8 the risk of incident cardiovascular disease was compared between women with early menopause and women with menopause at 45 years or older, which included both relatively early menopause (at 45–49 years) and late menopause (at ≥55 years). Women with early menopause (<45 years) had a 50% increased risk (relative risk 1·50, 95% CI 1·28–1·76) of coronary heart disease, whereas the association between age at menopause and incident stroke was inconsistent.8 Women now live around one-third of their life span after menopause,9 and this period of time might be even longer for women with earlier menopause. In this context, it is useful to investigate the association between early or premature menopause and the incidence and timing of cardiovascular disease.
Research in context.
Evidence before this study
We searched PubMed from database inception until March 1, 2019, for observational studies published in English, using the search terms “menopause”, “final menstrual period”, “cardiovascular disease”, “coronary heart disease”, “myocardial infarction”, “angina”, and “stroke”. We screened papers by title and abstract to identify full-text reports that were relevant to the study aims. We also screened citation lists from these full-text reports to identify other relevant research. The papers cited in this report were selected to be representative of the existing evidence base, and are not an exhaustive list of relevant research. Our search yielded more than 90 studies. We found previous evidence showing that early menopause is linked to an increased risk of cardiovascular disease mortality, but the associations between a broad range of ages at natural menopause (including premature menopause [<40 years]) and incidence and timing of first non-fatal cardiovascular disease event are unclear.
Added value of this study
To our knowledge, this is the largest study of individual-level data to investigate the associations between a broad range of ages at menopause (including premature menopause [<40 years]) and incident cardiovascular disease, adjusted for smoking status, body-mass index, and hormone therapy status. This is also the first study to assess the association between age at menopause and timing of onset of first cardiovascular disease event.
Implications of all the available evidence
Our results show that compared with women who had menopause at age 50–51 years, women with premature and early menopause had a substantially increased risk of first non-fatal cardiovascular disease event before the age of 60 years, but not after age 70 years. Early or premature menopause might be considered an important factor in risk stratification of cardiovascular disease for women. Preventive measures should consider this association as part of active management of cardiovascular disease risk factors for women.
We aimed to assess the associations between a broad range of ages at natural menopause and incident cardiovascular disease, coronary heart disease, and stroke. We hypothesised that earlier menopause would lead to higher risk and earlier onset of cardiovascular disease than menopause at average age or later menopause.
Methods
Study design and participants
The International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events (InterLACE) is a consortium of pooled individual-level data on reproductive health and chronic diseases for 537 153 women who participated in 25 observational studies across ten countries between 1946 and 2013. Most studies were of prospective longitudinal design and collected survey data on key reproductive, socio-demographic, lifestyle factors, and disease outcomes. After the studies had joined the InterLACE consortium, a harmonisation process was developed to combine individual-level data. A detailed description of the InterLACE consortium and data harmonisation has been published previously.10, 11, 12 Data for the present study were provided from 15 observational studies done in five countries and regions (Australia, Scandinavia, the USA, Japan, and the UK; appendix pp 1, 2) between 1946 and 2013.
We included women who had reported their natural menopause status (premenopause, perimenopause, and postmenopause), age at natural menopause (if postmenopausal), and cardiovascular disease status (including coronary heart disease and stroke). Women who had hysterectomy or oophorectomy and women who reported use of menopausal hormone therapy or oral contraceptives but did not report their age at menopause were excluded.
Women who had missing data on key covariates, including age at last follow-up, ethnicity, education level, body-mass index (BMI), smoking status, hypertension status at baseline, or postmenopausal hormone therapy status were also excluded (appendix pp 1, 2).
Ethical approval was obtained from the Institutional Review Board or Human Research Ethics Committee at each participating institution, and all participants provided written informed consent.
Exposure and outcome variables
We defined age at natural menopause as 12 consecutive months or more of amenorrhea that did not result from interventions (such as bilateral oophorectomy, hysterectomy, chemotherapy, or radiotherapy). Menopause status was assessed at last follow-up or at the first occurrence of coronary heart disease or stroke. Women who had a cardiovascular disease event before menopause or who were premenopausal (ie, women who had a period in the past 3 months with no change in regularity) or perimenopausal (ie, women who had a period in the past 12 months with a change in regularity) at the end of follow-up were categorised as premenopausal or perimenopausal. Thus, age at menopause was categorised as premenopause or perimenopause, younger than 40 years (premature menopause), 40–44 years (early menopause), 45–49 years (relatively early menopause), 50–51 years (reference category), 52–54 years (relatively late menopause), and 55 years or older (late menopause).
The primary endpoint of this study was the occurrence of first non-fatal cardiovascular disease, defined as a composite outcome of incident coronary heart disease (including heart attack and angina) or stroke (including ischaemic stroke or haemorrhagic stroke). Physician-diagnosed cardiovascular disease was self-reported or ascertained from hospital medical records. When ascertained from hospital records, incident coronary heart disease was defined according to International Classification of Diseases, 10th Revision (ICD-10) codes I21, I22, I23, I24, and I25, or ICD, 9th Revision (ICD-9) codes 410, 411, 412, and 413. Incident stroke was defined by ICD-10 codes I60, I61, I63, and I64, or ICD-9 codes 430, 431, 432, 433, and 434.
Covariates
We included the following factors in the analyses as covariates according to evidence from previous studies:2, 13, 14 age at last follow-up, ethnicity, years of education, BMI, smoking status, hypertension status, type 2 diabetes, parity, age at menarche, and pre-menopausal use of oral contraceptives, which were reported at baseline or at a mid-age survey for birth cohort studies. Information about menopausal hormone therapy collected after menopause was used to define women as users or non-users. We combined ethnicity into six categories: white European, white Australian or New Zealander, white American or Canadian, Asian, Black, and other. Years of education was categorised as 10 years or less, 11–12 years, and more than 12 years. BMI was categorised as less than 18·5 kg/m2, 18·5 to 24·9 kg/m2, 25 to 29·9 kg/m2, and 30 kg/m2 or higher. Smoking status was categorised as current, former, or never smoker. Hypertension or diabetes status was dichotomised as present or absent on the basis of self-report at baseline. Parity was categorised as 0, 1, 2, and 3 or more livebirths. Age at menarche was divided into five categories: 11 years or younger, 12, 13, 14, and 15 years or older. Premenopausal oral contraceptive use was classified as ever use or non-use.
Statistical analysis
Baseline characteristics were presented as means and SDs for continuous variables and as percentages for categorical variables. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% CIs. The proportional hazards assumption was checked using log cumulative hazard plots and seemed to be reasonable. Study level variability was included in the model as a random effect. Because women who had early menopause might have a longer follow-up time than those with later menopause and the entry age for each study in the InterLACE consortium varied, we used the minimum age at natural menopause (32 years) as a fixed baseline age for women in all studies to avoid left-truncation bias. For women who had a cardiovascular disease event, follow-up time was calculated as their age at first cardiovascular disease event minus 32 years; for women without a cardiovascular disease event, follow-up time was defined as their age at last follow-up minus 32 years. For postmenopausal women, the time between age 32 years and menopause was classified as the unexposed period to account for immortal time bias. Menopause at age 50–51 years was used as the reference category.
We first analysed all incident cardiovascular disease, followed by separate analyses for incident coronary heart disease and stroke. To examine whether women with earlier menopause had higher risk of incident cardiovascular disease at a younger age than those with later menopause, we stratified analyses by age at follow-up (<60 years, 60–69 years, and ≥70 years). HRs and 95% CIs were adjusted for age at last follow-up, ethnicity, education level, BMI, smoking status, hypertension status, menopausal hormone therapy use after menopause, and oral contraceptive use in the premenopausal group. Since age at menarche and parity were also potential confounders, the models were additionally adjusted for these covariates using a subset of the available studies.
To quantify dose-response relationships, we used restricted cubic spline models to examine the associations between age at menopause and incident cardiovascular disease; age at menopause was considered a continuous variable to estimate the effect of each year of decrease in age at natural menopause. We analysed the combined effects of age at menopause and menopausal hormone therapy use. In a subset of studies that collected time of initiation of menopausal hormone therapy and duration of menopausal hormone therapy at women's last follow-up, we further examined the effects of age at menopause with different duration of menopausal hormone therapy (<10 and ≥10 years) and different time of initiation of menopausal hormone therapy. We analysed the combined effects of age at menopause, smoking status, and BMI levels on the risk of incident cardiovascular disease. We also examined the role of socioeconomic status by analysing the combined effect of age at menopause and education level.
We did eight sensitivity analyses to test the robustness of our findings. We first analysed data from a subset of studies in which cardiovascular disease events were ascertained from hospital data, rather than only self-report. Second, because the UK Biobank contributed more than 50% of the total cardiovascular disease events, we did an analysis excluding this study. Third, we only included cardiovascular disease events which occurred at least 3 years after menopause. Fourth, we also analysed the associations between age at menopause and specific types of coronary heart disease (heart attack or angina) and subtype of stroke (ischaemic stroke or haemorrhagic stroke). Fifth, we compared women's characteristics in the complete dataset and dataset with missing values, and did 10 times multiple imputation to impute missing covariates. Sixth, we further adjusted for type 2 diabetes in a subset of studies. Seventh, we assessed heterogeneity between countries and regions (Australia, Scandinavia, the USA, Japan, and the UK) using country-specific random-effects meta-analysis, and I2 and p values were used to assess heterogeneity. Eighth, we further adjusted for family history of cardiovascular disease in studies with relevant information.
We used SAS (version 9.4) in all statistical analyses. The PHREG procedure was used to fit the Cox proportional hazards regression models. A two-sided p value of 0·05 or less was considered to indicate statistical significance.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Results
Our analysis included 301 438 women from 15 studies.12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 The mean (SD) age at last follow-up was 57·0 years (10·3) and more than half of the women were born after 1950. 192 525 (64·0%) of 301 438 were postmenopausal at the end of follow-up (table 1; appendix p 1). The mean age at menopause was 50·2 years (SD 4·4), and the median age was 50·0 years (IQR 48·0–53·0). 3424 (1·2%) of 301 438 women had premature menopause and 14 038 (4·7%) had early menopause. The mean time from menopause to incident cardiovascular disease was 13·5 years (SD 9·0), with a median time of 12·0 years (6·0–19·6). 12 962 (4·3%) of 301 438 women had non-fatal cardiovascular disease events after menopause, including 9369 (3·1%) coronary heart disease events and 4338 (1·4%) strokes. Compared with women who did not have a cardiovascular disease event, those who had a cardiovascular disease event were more likely to be less educated, obese, and current smokers with a history of hypertension (table 1).
Table 1.
Baseline characteristics by age at natural menopause and incident cardiovascular disease events
|
Age at natural menopause |
Incident cardiovascular disease |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Premenopausal or perimenopausal (n=108 913) | <40 years (n=3424) | 40–44 years (n=14 038) | 45–49 years (n=48 061) | 50–51 years (n=47 391) | 52–54 years (n=50 996) | ≥55 years (n=28 662) | No (n=287 231) | Yes (n=14 254) | |
| Ethnicity | |||||||||
| White Australian or New Zealander | 351 (0·3%) | 300 (8·8%) | 1084 (7·7%) | 3451 (7·2%) | 3552 (7·5%) | 3655 (7·2%) | 2258 (7·9%) | 12 974 (4·5%) | 1677 (11·8%) |
| White European | 65 402 (60·1%) | 2905 (84·8%) | 12 070 (86·0%) | 40 410 (84·1%) | 40 178 (84·8%) | 43 924 (86·1%) | 25 073 (87·5%) | 218 482 (76·1%) | 11 480 (80·5%) |
| White American or Canadian | 476 (0·4%) | 7 (0·2%) | 47 (0·3%%) | 382 (0·8%) | 404 (0·9%) | 497 (1·0%) | 177 (0·6%) | 1893 (0·7%) | 97 (0·7%) |
| Asian | 39 729 (36·5%) | 131 (3·8%) | 510 (3·6%) | 2669 (5·6%) | 2376 (5·0%) | 2073 (4·1%) | 711 (2·5%) | 47 626 (16·6%) | 573 (4·0%) |
| Black | 1844 (1·7%) | 53 (1·6%) | 212 (1·5%) | 740 (1·5%) | 535 (1·1%) | 522 (1·0%) | 293 (1·0%) | 3920 (1·4%) | 279 (2·0%) |
| Other | 1111 (1·0%) | 28 (0·8%) | 115 (0·8%) | 409 (0·9%) | 346 (0·7%) | 325 (0·6%) | 150 (0·5%) | 2336 (0·8%) | 148 (1·0%) |
| Body-mass index | |||||||||
| Underweight (<18·5 kg/m2) | 6332 (5·8%) | 108 (3·2%) | 326 (2·3%) | 1135 (2·4%) | 963 (2·0%) | 836 (1·6%) | 374 (1·3%) | 9694 (3·4%) | 380 (2·7%) |
| Normal (18·5–24·9 kg/m2) | 65 785 (60·4%) | 1392 (40·7%) | 5959 (42·5%) | 22 259 (46·3%) | 22 000 (46·4%) | 22 741 (44·6%) | 11 004 (38·4%) | 146 008 (50·8%) | 5132 (36·0%) |
| Overweight (25·0–29·9 kg/m2) | 24 082 (22·1%) | 1113 (32·5%) | 4873 (34·7%) | 15 835 (33·0%) | 15 969 (33·7%) | 17 848 (35·0%) | 10 936 (38·2%) | 85 840 (29·9%) | 4816 (33·8%) |
| Obese (≥30 kg/m2) | 12 714 (11·7%) | 811 (23·7%) | 2880 (20·5%) | 8832 (18·4%) | 8459 (17·9%) | 9571 (18·8%) | 6348 (22·2%) | 45 689 (15·9%) | 3926 (27·5%) |
| Smoking status | |||||||||
| Never | 69 794 (64·1%) | 1709 (49·9%) | 7172 (51·1%) | 25 829 (53·7%) | 27 472 (58·0%) | 30 492 (59·8%) | 17 372 (60·6%) | 173 013 (60·2%) | 6827 (47·9%) |
| Past smoker | 24 003 (22·0%) | 1080 (31·5%) | 4565 (32·5%) | 15 049 (31·3%) | 14 809 (31·3%) | 15 980 (31·3%) | 9382 (32·7%) | 79 928 (27·8%) | 4940 (34·7%) |
| Current smoker | 15 116 (13·9%) | 635 (18·6%) | 2301 (16·4%) | 7183 (15·0%) | 5110 (10·8%) | 4524 (8·9%) | 1908 (6·7%) | 34 290 (11·9%) | 2487 (17·5%) |
| Education level (years) | |||||||||
| ≤10 | 24 475 (22·5%) | 1926 (56·3%) | 7367 (52·5%) | 22 204 (46·2%) | 21 860 (46·1%) | 22 896 (44·9%) | 14 195 (49·5%) | 107 353 (37·4%) | 7570 (53·1%) |
| 11–12 | 13 367 (12·3%) | 410 (12·0%) | 1546 (11·0%) | 5483 (11·4%) | 5575 (11·8%) | 6293 (12·3%) | 3297 (11·5%) | 34 446 (12·0%) | 1525 (10·7%) |
| >12 | 71 071 (65·3%) | 1088 (31·8%) | 5125 (36·5%) | 20 374 (42·4%) | 19 956 (42·1%) | 21 807 (42·8%) | 11 170 (39·0%) | 145 432 (50·6%) | 5159 (36·2%) |
| Age at last follow-up (years) | |||||||||
| <60 | 108 913 (100%) | 1724 (50·4%) | 6329 (45·1%) | 24 334 (50·6%) | 21 373 (45·1%) | 21 785 (42·7%) | 6654 (23·2%) | 187 134 (65·2%) | 3951 (27·7%) |
| 60–64 | .. | 794 (23·2%) | 3573 (25·5%) | 11 009 (22·9%) | 12 942 (27·3%) | 15 570 (30·5%) | 12 176 (42·5%) | 52 808 (18·4%) | 3281 (23·0%) |
| 65–69 | .. | 612 (17·9%) | 2877 (20·5%) | 8750 (18·2%) | 9214 (19·4%) | 9744 (19·1%) | 7792 (27·2%) | 35 282 (12·3v) | 3709 (26·0%) |
| ≥70 | .. | 294 (8·6%) | 1259 (9·0%) | 3968 (8·3%) | 3862 (8·2%) | 3897 (7·6%) | 2040 (7·1%) | 12 007 (4·2%) | 3313 (23·3%) |
| Hypertension | |||||||||
| No | 99 482 (91·3%) | 2581 (75·4%) | 10 494 (74·8%) | 37 069 (77·1%) | 35 968 (75·9%) | 38 137 (74·8%) | 20 165 (70·4%) | 236 457 (82·3%) | 7439 (52·2%) |
| Yes | 9431 (8·7%) | 843 (24·6) | 3544 (25·3%) | 10 992 (22·9%) | 11 423 (24·1%) | 12 859 (25·2%) | 8497 (29·7%) | 50 774 (17·7%) | 6815 (47·8%) |
| Postmenopausal menopausal hormone therapy | |||||||||
| No | 105 459 (96·8%) | 1478 (43·2%) | 7202 (51·3%) | 29 284 (60·9%) | 30 232 (63·8%) | 32 597 (63·9%) | 16 194 (56·5%) | 213 791 (74·4%) | 8655 (60·7%) |
| Yes | 3454 (3·25) | 1946 (56·8%) | 6836 (48·7%) | 18 777 (39·1%) | 17 159 (36·2%) | 18 399 (36·1%) | 12 468 (43·5%) | 73 440 (25·6%) | 5599 (39·3%) |
| Number of children | |||||||||
| 0 | 27 897 (26·1%) | 756 (22·5%) | 2668 (19·2%) | 8879 (18·7%) | 7795 (16·6%) | 7337 (14·5%) | 3574 (12·6%) | 56 880 (20·1%) | 2026 (14·5%) |
| 1 | 15 038 (14·1%) | 517 (15·4%) | 1992 (14·4%) | 6365 (13·4%) | 5752 (12·3%) | 5892 (11·7%) | 3136 (11·0%) | 36 853 (13·0%) | 1839 (13·1%) |
| 2 | 40 623 (38·0%) | 1238 (36·9%) | 5513 (39·8%) | 19 768 (41·7%) | 20 249 (43·2%) | 22 594 (44·7%) | 13 086 (46·0%) | 117 824 (41·6%) | 5247 (37·4%) |
| ≥3 | 23 410 (21·9%) | 849 (25·3%) | 3695 (26·6%) | 12 439 (26·21%) | 13 107 (27·9%) | 14 733 (29·1%) | 8663 (30·4%) | 71 988 (25·4%) | 4908 (35·0%) |
| Age at menarche (years) | |||||||||
| ≤11 | 20 331 (19·1%) | 764 (23·1%) | 2818 (20·8%) | 8296 (17·9%) | 7837 (17·1%) | 8577 (17·4%) | 5328 (19·2%) | 51 338 (18·4%) | 2613 (19·4%) |
| 12 | 25 699 (24·1%) | 577 (17·4%) | 2537 (18·7%) | 8992 (19·4%) | 8898 (19·5%) | 9753 (19·7%) | 5139 (18·5%) | 59 143 (21·2%) | 2452 (18·2%) |
| 13 | 27 900 (26·1%) | 742 (22·4%) | 3138 (23·1%) | 11 777 (25·4%) | 11 809 (25·8%) | 12 838 (26·0%) | 6698 (24·1%) | 71 753 (25·7%) | 3149 (23·4%) |
| 14 | 19 945 (18·7%) | 612 (18·5%) | 2700 (19·9%) | 9430 (20·3%) | 9495 (20·8%) | 10 350 (21·0%) | 5693 (20·5%) | 55 501 (19·9%) | 2724 (20·2%) |
| ≥15 | 12 871 (12·1%) | 613 (18·5%) | 2368 (17·5%) | 7914 (17·0%) | 7694 (16·8%) | 7919 (16·0%) | 4968 (17·9%) | 41 816 (15·0%) | 2531 (18·8%) |
Data are n (%).
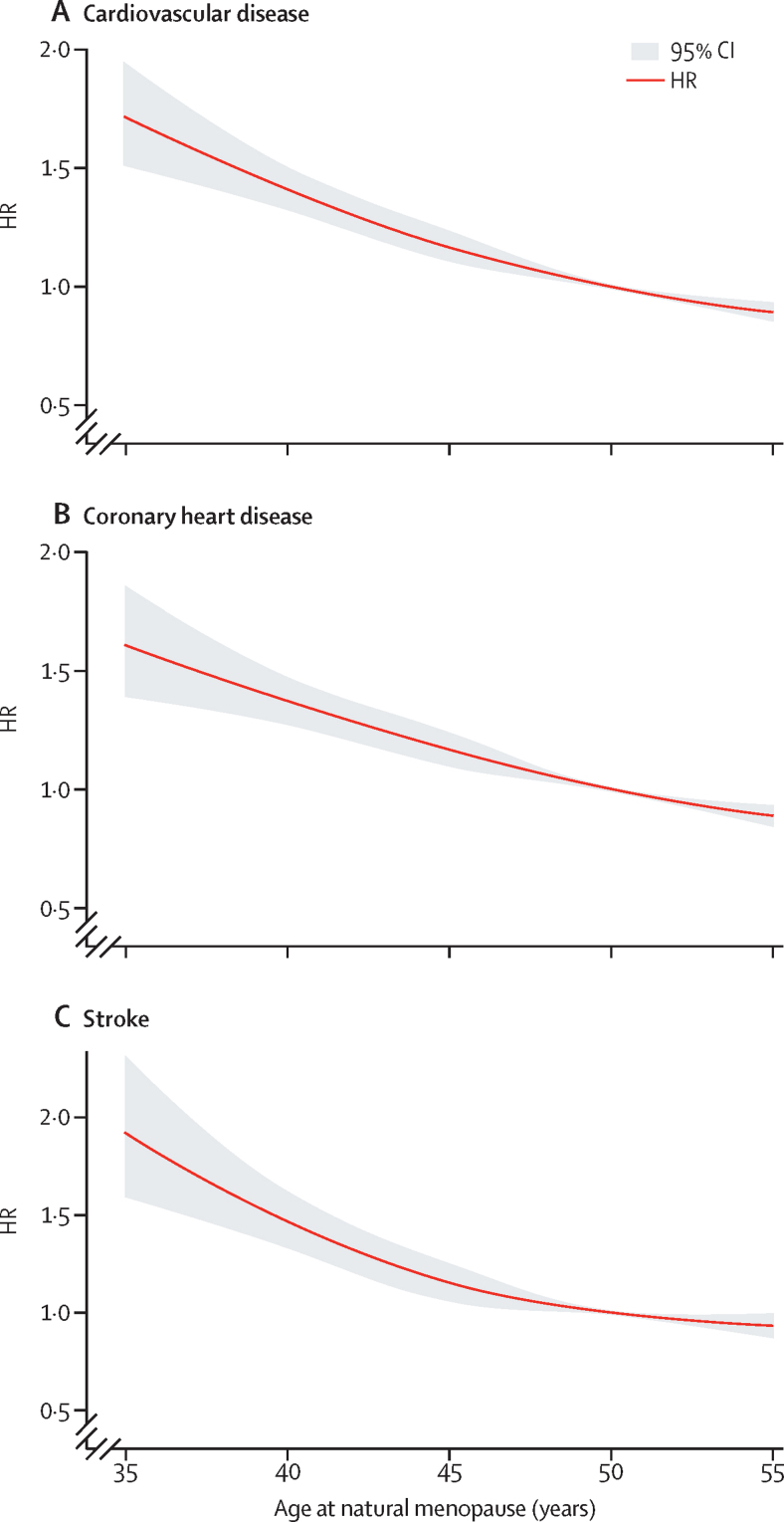
Compared with women who had menopause at 50–51 years, risk of cardiovascular disease was higher among women with premature (HR 1·55, 95% CI 1·38–1·73; p<0·0001), early (1·30, 1·22–1·39; p<0·0001), or relatively early menopause (1·12, 1·07–1·18; p<0·0001). By contrast, risk of cardiovascular disease was lower in women with late menopause (0·88, 0·83–0·93; p<0·0001) and women who were premenopausal or perimenopausal (0·52, 0·48–0·56; p<0·0001) at last follow-up (p<0·0001 for trend; table 2). Similar associations were observed when incident coronary heart disease and stroke were analysed separately. The estimates remained largely similar when the model was adjusted for age at menarche and parity (based on data from 12 studies,12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 data not shown). The cubic spline models showed an approximately linear relationship between age at menopause and risk of incident cardiovascular disease, coronary heart disease, and stroke (figure 1). When we analysed age at menopause as a continuous variable, each year of decrease in age was associated with a 3% increased risk of incident cardiovascular disease (1·03, 1·02–1·04; p<0·0001).
Table 2.
Associations between age at natural menopause and first non-fatal cardiovascular disease, coronary heart disease, and stroke events
| Women (n) | Cardiovascular disease events (n) | Person-years (n) | Events per 1000 person-years (n) | Adjusted HR (95% CI)* | ||
|---|---|---|---|---|---|---|
| Cardiovascular disease | ||||||
| Age at natural menopause (years) | ||||||
| <40 | 3424 | 346 | 84 961·6 | 4·1 | 1·55 (1·38–1·73) | |
| 40–44 | 14 038 | 1216 | 372 142·8 | 3·3 | 1·30 (1·22–1·39) | |
| 45–49 | 48 061 | 3316 | 1 269 834·9 | 2·6 | 1·12 (1·07–1·18) | |
| 50–51 | 47 391 | 3016 | 1 303 876·1 | 2·3 | 1 (ref) | |
| 52–54 | 50 996 | 3146 | 1 429 883·3 | 2·2 | 0·96 (0·91–1·01) | |
| ≥55 | 28 662 | 1922 | 852 776·4 | 2·3 | 0·88 (0·83–0·93) | |
| Premenopause or perimenopause | 108 913 | 3253 | 2 842 537·1 | 1·1 | 0·52 (0·48–0·56) | |
| Trend p value† | .. | .. | .. | .. | <0·0001 | |
| Coronary heart disease | ||||||
| Age at natural menopause (years) | ||||||
| <40 | 3419 | 253 | 85 414·1 | 3·0 | 1·52 (1·34–1·74) | |
| 40–44 | 14 022 | 881 | 373 417·4 | 2·4 | 1·30 (1·20–1·41) | |
| 45–49 | 48 016 | 2418 | 1 272 920·1 | 1·9 | 1·13 (1·06–1·20) | |
| 50–51 | 47 362 | 2180 | 1 307 497·1 | 1·7 | 1 (ref) | |
| 52–54 | 50 944 | 2260 | 1 432 324·2 | 1·6 | 0·96 (0·90–1·02) | |
| ≥55 | 28 628 | 1377 | 854 420·9 | 1·6 | 0·90 (0·85–0·97) | |
| Premenopause or perimenopause | 108 902 | 2229 | 2 710 689·7 | 0·8 | 0·59 (0·53–0·65) | |
| Trend p value† | .. | .. | .. | .. | <0·0001 | |
| Stroke | ||||||
| Age at natural menopause (years) | ||||||
| <40 | 3412 | 126 | 86 474·8 | 1·5 | 1·72 (1·43–2·07) | |
| 40–44 | 13 993 | 416 | 376 099·8 | 1·1 | 1·32 (1·18–1·48) | |
| 45–49 | 47 892 | 1078 | 1 279 465·0 | 0·8 | 1·09 (1·00–1·18) | |
| 50–51 | 47 301 | 1018 | 1 31 4206·0 | 0·8 | 1 (ref) | |
| 52–54 | 50 868 | 1048 | 1 439 717·0 | 0·7 | 0·94 (0·86–1·02) | |
| ≥55 | 28 644 | 652 | 860 938·3 | 0·8 | 0·89 (0·81–0·99) | |
| Premenopause or perimenopause | 108 805 | 1120 | 2 065 658·4 | 0·5 | 0·46 (0·40–0·52) | |
| Trend p value† | .. | .. | .. | .. | <0·0001 | |
HR=hazard ratio.
Cox proportional hazards models were used to estimate HRs and 95% CIs. All HRs were adjusted for age at last follow-up, ethnicity, education level, body-mass index, smoking status, hypertension status, and postmenopausal menopausal hormone therapy status, or oral contraception use in the premenopause or perimenopause group.
χ2 values of >50, with 6 degrees of freedom.
Figure 1.
Association between age at natural menopause and first cardiovascular events
Association between age at menopause and incident cardiovascular disease (A), coronary heart disease (B), and stroke (C). Restricted cubic spline models were used to visualise the shape. HR=hazard ratio.
Compared with women who had menopause at 50–51 years, women with premature menopause (1·88, 1·62–2·20; p<0·0001), early menopause (1·40, 1·27–1·54; p<0·0001), or relatively early menopause (1·17, 1·09–1·25; p=0·0002) had the highest excess risk of first non-fatal cardiovascular disease event before the age of 60 years, with only modest associations at age 60–69 years, and no significant associations at older ages (≥70 years; table 3).
Table 3.
Associations between age at natural menopause and age at first cardiovascular disease, coronary heat disease, and stroke events*
|
Aged <60 years at first event |
Aged 60–69 years at first event |
Aged ≥70 years at first event |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular disease events (n) | Events per 1000 person-years (n) | Adjusted HR (95% CI) | Cardiovascular disease events (n) | Events per 1000 person-years (n) | Adjusted HR (95% CI) | Cardiovascular disease events (n) | Events per 1000 person-years (n) | Adjusted HR (95% CI) | ||
| Cardiovascular disease | ||||||||||
| Age at natural menopause (years) | ||||||||||
| <40 | 193 | 2·5 | 1·88 (1·62–2·20) | 99 | 2·0 | 1·35 (1·10–1·66) | 54 | 3·9 | 1·14 (0·86–1·52) | |
| 40–44 | 597 | 1·8 | 1·40 (1·27–1·54) | 413 | 1·8 | 1·30 (1·16–1·46) | 206 | 3·2 | 1·09 (0·93–1·27) | |
| 45–49 | 1634 | 1·4 | 1·17 (1·09–1·25) | 1035 | 1·4 | 1·08 (0·99–1·17) | 647 | 3·2 | 1·09 (0·97–1·22) | |
| 50–51 | 1472 | 1·3 | 1 (ref) | 986 | 1·2 | 1 (ref) | 558 | 2·7 | 1 (ref) | |
| 52–54 | 1523 | 1·2 | 0·91 (0·85–0·98) | 1065 | 1·2 | 0·99 (0·91–1·08) | 558 | 2·8 | 1·02 (0·91–1·15) | |
| ≥55 | 875 | 1·2 | 0·82 (0·75–0·89) | 729 | 1·1 | 0·91 (0·83–1·00) | 318 | 2·8 | 1·07 (0·93–1·23) | |
| Trend p value | .. | .. | <0·0001 | .. | .. | <0·0001 | .. | .. | 0·0342 | |
| Coronary heart disease | ||||||||||
| Age at natural menopause (years) | ||||||||||
| <40 | 139 | 1·8 | 1·85 (1·48–2·10) | 79 | 1·5 | 1·44 (1·14–1·81) | 35 | 2·5 | 1·03 (0·73– ·46) | |
| 40–44 | 423 | 1·3 | 1·38 (1·23–1·54) | 307 | 1·3 | 1·33 (1·16–1·52) | 151 | 2·3 | 1·06 (0·88–1·27) | |
| 45–49 | 1195 | 1·1 | 1·19 (1·10–1·30) | 768 | 1·0 | 1·11 (1·00–1·22) | 455 | 2·2 | 1·02 (0·89–1·17) | |
| 50–51 | 1052 | 0·9 | 1 (ref) | 713 | 0·9 | 1 (ref) | 415 | 2·0 | 1 (ref) | |
| 52–54 | 1111 | 0·9 | 0·93 (0·86–1·01) | 763 | 0·9 | 0·99 (0·89–1·10) | 386 | 1·9 | 0·96 (0·84–1·10) | |
| ≥55 | 653 | 0·9 | 0·86 (0·78–0·95) | 522 | 0·8 | 0·92 (0·82–1·03) | 202 | 1·7 | 0·93 (0·79–1·11) | |
| Trend p value | .. | .. | <0·0001 | .. | .. | <0·0001 | .. | .. | 0·0046 | |
| Stroke | ||||||||||
| Age at natural menopause (years) | ||||||||||
| <40 | 62 | 0·8 | 1·93 (1·48–2·52) | 32 | 0·6 | 1·32 (0·91–1·89) | 32 | 2·0 | 1·73 (1·19–2·52) | |
| 40–44 | 199 | 0·6 | 1·44 (1·22–1·70) | 133 | 0·5 | 1·23 (1·01–1·50) | 84 | 1·2 | 1·17 (0·91–1·51) | |
| 45–49 | 509 | 0·4 | 1·11 (0·98–1·26) | 318 | 0·4 | 0·99 (0·85– 1·15) | 251 | 1·2 | 1·15 (0·95–1·38) | |
| 50–51 | 484 | 0·4 | 1 (ref) | 332 | 0·4 | 1 (ref) | 202 | 0·9 | 1 (ref) | |
| 52–54 | 467 | 0·4 | 0·85 (0·75–0·96) | 358 | 0·4 | 0·98 (0·85–1·14) | 223 | 1·0 | 1·10 (0·91–1·33) | |
| ≥55 | 249 | 0·3 | 0·71 (0·60–0·82) | 251 | 0·4 | 0·90 (0·76–1·06) | 152 | 1·2 | 1·38 (1·12–1·71) | |
| Trend p value | .. | .. | <0·0001 | .. | .. | 0·0051 | .. | .. | 0·3826 | |
HR=hazard ratio.
Cox proportional hazards models were used to estimate HRs and 95% CIs. All HRs were adjusted for age at last follow-up, ethnicity, education level, body-mass index, smoking status, hypertension status, and postmenopausal menopausal hormone therapy status.
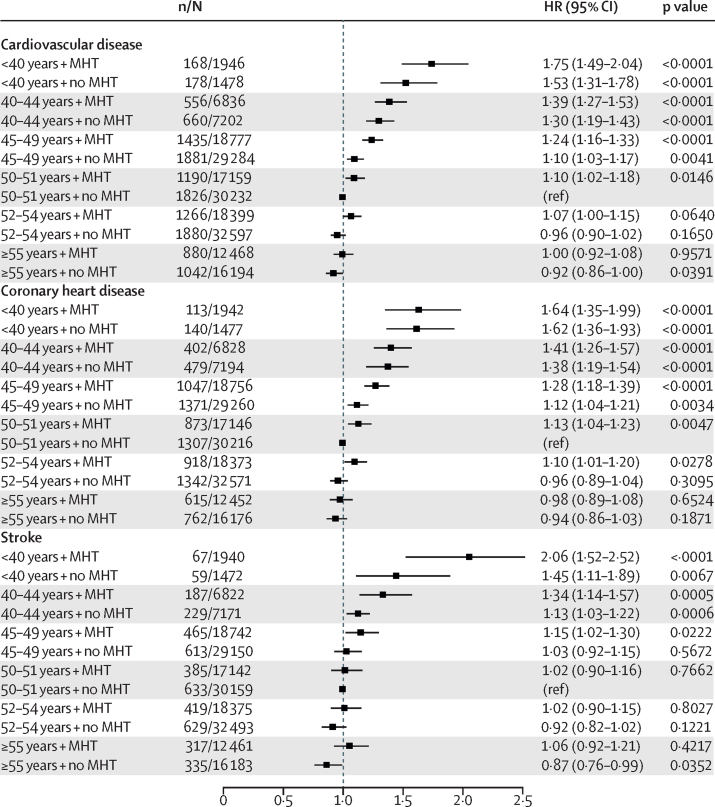
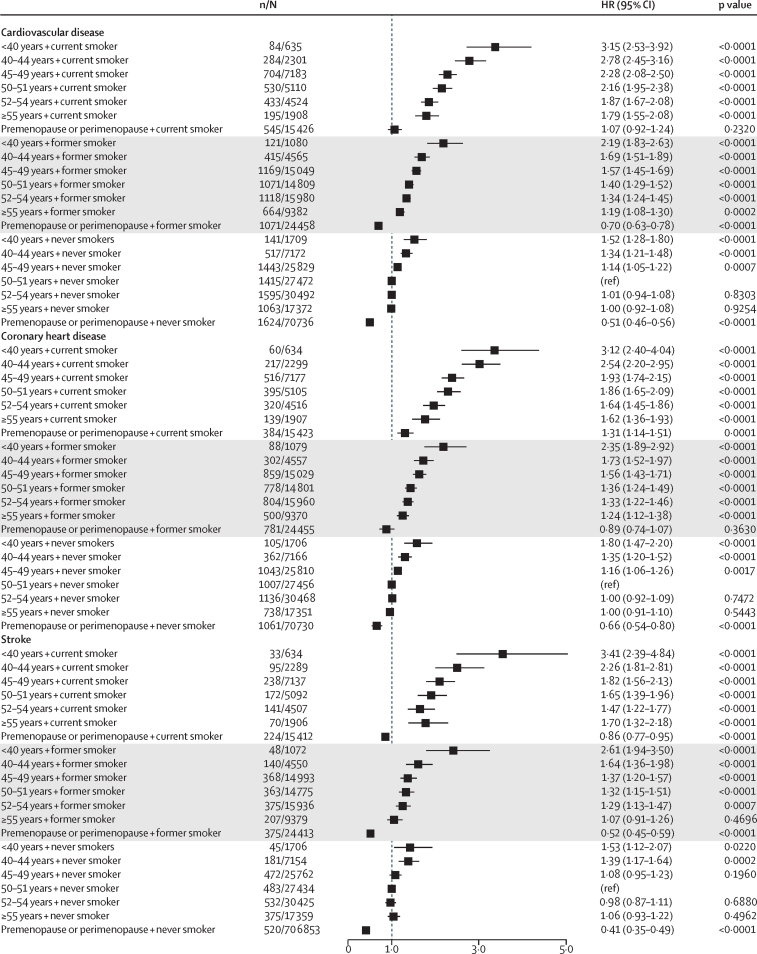
When we combined age at menopause and post-menopausal menopausal hormone therapy status, women who had premature menopause and used menopausal hormone therapy had a similar risk of coronary heart disease compared with women who did not use menopausal hormone therapy (HR 1·64, 1·35–1·99; p<0·0001 vs 1·62, 1·36–1·93; p<0·0001); however, women who had premature menopause and used menopausal hormone therapy had higher risk of stroke (2·06, 1·52–2·52; p<0·0001) than women who did not report the use of menopausal hormone therapy (1·45, 1·11–1·89; p=0·0067; figure 2; appendix p 3). Further analyses of seven studies12, 15, 16, 21, 22, 23, 24 that collected time of initiation of menopausal hormone therapy and duration of menopausal hormone therapy at women's last follow-up suggested that women with premature or early menopause who used menopausal hormone therapy for longer than 10 years had the lowest risk of cardiovascular disease when compared with women with early or premature menopause who did not use menopausal hormone therapy and women with early or premature menopause who used menopausal hormone therapy for less than 10 years. Additionally, among these women, those who had initiated menopausal hormone therapy 1 year after menopause had the lowest risk of cardiovascular disease when compared with women who did not use menopausal hormone therapy and women who initiated menopausal hormone therapy more than 1 year after menopause (appendix pp 4, 5). Women who initiated menopausal hormone therapy after age 60 years had higher HRs than those who started menopausal hormone therapy before age 60 years (appendix pp 4, 5). Analyses stratified by smoking status showed that the effects of premature or early menopause on incident cardiovascular disease were stronger among former and current smokers than never smokers (figure 3; appendix pp 6, 7). However, compared with the overall HRs (table 2), HRs among never smokers were only slightly attenuated (p<0·0001) showing decreasing risk of cardiovascular disease with increasing age at natural menopause (figure 3; appendix pp 6, 7). Underweight women and women with obesity with earlier menopause had a higher risk of incident cardiovascular disease than did those with normal BMIs (appendix p 8). Furthermore, poorly educated women with earlier menopause had a higher risk of incident cardiovascular disease than those who were more highly educated (appendix p 9).
Figure 2.
Combined effect of age at menopause and menopausal hormone therapy on risk of incident cardiovascular disease, coronary heart disease, and stroke events
Cox proportional hazards models were used to estimate HRs and 95% CIs. HRs were adjusted for age at last follow-up, ethnicity, education level, body-mass index, smoking status, and hypertension status. n=number of events. N=number of participants. HR=hazard ratio. MHT=menopausal hormone therapy.
Figure 3.
Combined effect of age at menopause and smoking status on risk of incident cardiovascular disease, coronary heart disease, and stroke
Cox proportional hazards models were used to estimate HRs and 95% CIs. All HRs were adjusted for age at last follow-up, ethnicity, education level, body-mass index, hypertension status, and postmenopausal menopausal hormone therapy status or oral contraception use in the premenopause or perimenopause group. n=number of events. N=number of participants. HR=hazard ratio.
When only cardiovascular disease cases ascertained by hospital record were analysed (three studies12, 18, 19), results were similar to the main analysis (appendix p 10). After excluding the UK Biobank study,12 associations between earlier menopause and risk of cardiovascular disease also remained largely unchanged (appendix p 11). When only cardiovascular disease events that occurred at least 3 years after menopause were included, we found the associations were strengthened (appendix p 12). Furthermore, the associations remained unchanged when age at menopause was stratified by subtype of coronary heart disease (data from MCCS,17 DNC,18 WLH,19 NSHD,23 ELSA,25 UKWCS,26 WHITEHALL II,27 SWAN,21 JNHS,22 SABRE,28 and UK Biobank12 studies) and stroke (data from WLH19 and UK Biobank12 studies; appendix p 13). Overall, the characteristics of women with complete datasets and missing datasets were comparable. The adjusted HRs (95% CIs) were similar for complete case analyses and multiple imputation-based analyses and after adjustment for type 2 diabetes (data from 12 studies [ALSWH,15 HOW,16 MCCS,17 DNC,18 SWAN,21 NSHD,23 NCDS,24 ELSA,25 UKWCS,26 WHITEHALL II,27 SABRE,28 and UK Biobank12 studies; appendix pp 14–16). Country-specific random-effects meta-analyses revealed no significant heterogeneity between countries (data not shown). When only studies with available data on family history of cardiovascular disease were included in the model (HOW,16 DNC,18 UKWCS,26 WHITEHALL II,27 JNHS,22 and UK Biobank12 studies), the results remained unchanged (data not shown).
Discussion
The risk of non-fatal cardiovascular disease events was more than 1·5 times higher among women with premature menopause (<40 years) and 1·3 times higher among women with early menopause (40–44 years) than women who had natural menopause at 50–51 years. Additionally, the risk of experiencing a cardiovascular disease before the age of 60 years was around two times higher among women with premature menopause and 1·4 times higher among women with early menopause than women in the reference group.
Although short reproductive duration29 and surgical menopause have been shown to increase the risk of coronary heart disease,30 data on the association between earlier natural menopause and incident cardiovascular disease have been inconsistent.31 In one study, early menopause was associated with an approximately two times higher risk of a coronary heart disease event, but after adjusting for family history of coronary heart disease, the association was not statistically significant (HR 1·80, 95% CI 0·99–3·29); this study included both women with early natural and surgical menopause.32 In the Nurses' Health Study,33 the relative risk of 1·04 (95% CI 1·01–1·07) of coronary heart disease for each year of decrease in age at natural menopause was only observed for current smokers. Also, in the DNC study,34 a significant association was found between premature menopause and risk of coronary heart disease (HR 2·2, 1·0–4·9), but the association was not statistically significant when women were stratified by menopausal hormone therapy use.
We found that both premature and early menopause were associated with a higher risk of first coronary heart disease event when menopause at 50–51 years was used as the reference group, which is consistent with the results from two previous studies.29, 35 An approximately linear relationship was observed, with a 3% increased risk of incident coronary heart disease per 1 year decrease in age at menopause, which is consistent with the results of a previous study.36 The association remained unchanged when family history of cardiovascular disease was included in the statistical model and was not materially affected by smoking or menopausal hormone therapy status. In the Women's Health Initiative study, women who began menopausal hormone therapy before age 60 years had a lower risk of coronary heart disease than those who began therapy after age 60 years, while no association was observed for stroke.37 However, the Women's Health Initiative studies included older women (mean age 63·2 years) and the results might be generalisable to women with premature or early menopause.37
Existing literature on the association between age at natural menopause and risk of stroke is inconclusive.8, 38 In three cohort studies, premature and early menopause were not significantly associated with risk of ischaemic or haemorrhagic stroke.33, 39, 40 However, two studies33, 40 only included women who never used menopausal hormone therapy, and one40 had only a small number of stroke events. In the Framingham cohort study, women with natural menopause before age 42 years had twice the risk of ischaemic stroke compared with women with natural menopause later than 42 years; this association persisted in never smokers and non-menopausal hormone therapy users.41 Consistent with two recent studies,29, 35 we found premature and early menopause were similarly associated with both ischaemic and haemorrhagic stroke. We also found that menopausal hormone therapy users had a higher risk of incident stroke than non-users. A recent Cochrane review also found a 24% increased risk of stroke following use of menopausal hormone therapy prescribed for primary or secondary prevention of cardiovascular disease.42
One of our main findings was that the excess risk of cardiovascular disease following premature or early menopause was largely confined to events occurring at ages younger than 70 years and was highest before the age of 60 years. Few studies have examined in detail the association between age at menopause and timing of onset of cardiovascular disease events. In one study, women with earlier menopause had higher risk of cardiovascular mortality at age 65 years than those with later menopause.43 However, this study did not include non-fatal cardiovascular disease. Our findings might help to identify women at high risk of cardiovascular disease at an early age. Additionally, the J-shaped curve observed for risk of stroke after age 70 years might arise because more than 50% of women in this age group were overweight or obese, and a J-shaped curve has been found between overweight BMI and age at natural menopause.14
Several mechanisms have been proposed to explain the association between earlier menopause and increased risk of postmenopausal cardiovascular disease. Endogenous oestrogens have protective effects on the cardiovascular system.44 Oestrogen increases vasodilatation45 and inhibits the response of blood vessels to injury and the development of atherosclerosis.44 Early loss of oestrogen might impair vascular function and increase the expression of inflammatory cytokines at younger ages, which might further damage the vascular function.46 Circulating androgen and sex hormone-binding globulin concentrations are also associated with risk of cardiovascular disease.47 High concentrations of androgen and low concentrations of sex hormone-binding globulin are associated with both risk of postmenopausal cardiovascular disease events48 and adverse cardiovascular disease risk factor profiles. Additionally, genetic or environmental factors need to be considered. An adverse cardiovascular disease risk factor profile in premenopausal women might be associated with both earlier menopause and occurrence of cardiovascular disease.49 Also, the changes in cardiovascular disease risk factors (eg, lipid concentrations), which coincide with the oestrogen reduction during the menopausal transition, might contribute to risk of cardiovascular disease, although no consistent conclusions have been reported in the literature.50, 51
The main strength of our study was the large sample of pooled individual-level data, which was obtained from 15 studies across different geographical regions and populations, representing many different ethnic groups. The participant-level data in InterLACE enabled us to harmonise variables using common definitions, coding, and cutoff points, which is not usually possible with meta-analyses of published results.
Our study also has several limitations. First, about 40% of postmenopausal cardiovascular disease events were self-reported but consistent findings were observed in analyses confined to cardiovascular disease events ascertained through medical records. Second, smoking is a well-known shared risk factor for early menopause and cardiovascular disease13 and BMI is another modifiable variable associated with both age at menopause and cardiovascular disease.14 Additionally, postmenopausal menopausal hormone therapy status might mediate the association between age at menopause and cardiovascular disease.42 We used variables reported at middle age (41–53 years) and postmenopausal menopausal hormone therapy status at a specific timepoint as covariates rather than treating them as time-varying covariates, which could have caused some bias. However, of the studies included in InterLACE that included women who reported smoking status and BMI levels both before and after menopause (ie, UK Biobank,12 NSHD,23 NCDS,24 SWAN,21 and SABRE28), the concordance rate was approximately 85%. Additionally, around 80% of women used menopausal hormone therapy for more than 6 years.52 Thus, we assume the bias caused by not using time-varying covariates is small. Third, information about post-menopausal lipid concentrations was not available, which might mediate the association between age at natural menopause and cardiovascular disease events. However, no evidence of changes in lipid concentrations between premenopause and perimenopause was found in the NSHD study.51 Fourth, little information was available about types and doses of menopausal hormone therapy. Nevertheless, one study has found the association among different types of menopausal hormone therapy (oestrogen alone or oestrogen with progestin) and incident coronary heart disease or stroke was similar.53 Fifth, menopause status was based on self-report, which might induce recall bias. However, previous studies have shown that the validity and reproducibility of self-reported age at menopause was good.54 Also, validated questionnaires and standard questions were used in each study; thus we assume the heterogeneity of menopause status among studies is limited. Sixth, as the outcome of this study was non-fatal cardiovascular disease events, the exclusion on fatal cardiovascular disease events might bias our results. However, since only 7·2% of first cardiovascular disease events are fatal,55 and earlier menopause has been associated with higher cardiovascular disease mortality,8 the inclusion of fatal events in the analyses would only strengthen the association between earlier age at menopause and incident cardiovascular disease. Finally, more than 80% of the women included in our study were white, which could limit the generalisability of the findings to other ethnicities.
Compared with women with average age at menopause, women with premature and early menopause had higher risk of a non-fatal cardiovascular disease event with an almost linear dose-response relationship for each year of decrease in age at menopause. This excess risk was highest before the age of 60 years, but was greatly diminished by the age of 70 years. A systematic review showed that the prevalence of premature (3·7%) and early menopause (12·2%) in women is considerable.56 WHO estimates that 1·2 billion women worldwide will be perimenopausal or postmenopausal by the year 2030, with 47 million women becoming menopausal each year.57 Studies have shown that in the past 30 years, cardiovascular disease mortality rates have decreased sharply whereas the decrease in incidence has been less steep.58, 59 Our findings could have important clinical and public health implications. First, strategies to reduce the risk of early menopause, such as the avoidance of cigarette smoking and maintaining a normal BMI, provide primary prevention measures for cardiovascular disease. Second, identification of women with early menopause offers a window of opportunity to implement active management of other cardiovascular disease risk factors in these women to improve overall cardiovascular health in postmenopausal years. These women might also need close monitoring in clinical practice. Third, early or premature menopause might also be considered as an important factor in risk stratification of cardiovascular disease. Further research is needed to assess the added value of including the timing of menopause as a predictor in existing cardiovascular disease models for women and to provide physicians with a more accurate prediction model for women.
Acknowledgments
Acknowledgments
The InterLACE consortium is funded by the Australian National Health and Medical Research Council project (grant APP1027196). GDM is supported by Australian National Health and Medical Research Council Principal Research Fellowship (APP1121844). The data on which this research is based were drawn from 15 observational studies included in the InterLACE consortium. The findings and views reported in this Article are not necessarily those of the original studies or their respective funding agencies. All studies would like to thank the participants for volunteering their time to be involved in the respective studies.
Contributors
DZ did the literature review, statistical analyses, and drafted the manuscript. H-FC and NP harmonised the data and contributed to the interpretation of the results. AJD contributed to the statistical analyses and interpretation of the results. GGG, FB, EJB, DK, RH, NEA, EBG, CAD, KAM, JEC, DCG, PD, DEB, LLS, DA, KH, JSL, HM, TT, MKS, H-OA, and EW provided study data. GDM conceived the study design and contributed to interpretation of the results. All authors critically revised the manuscript.
Declaration of interests
We declare no competing interests. Where authors are identified as personnel of the International Agency for Research on Cancer or WHO, the authors alone are responsible for the views expressed in this Article, and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer or WHO.
Supplementary Material
References
- 1.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 2.Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol. 2014;43:1542–1562. doi: 10.1093/ije/dyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold EB, Crawford SL, Avis NE. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold EB, Bromberger J, Crawford S. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 5.Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol. 2003;64:87–92. [PubMed] [Google Scholar]
- 6.Shifren JL, Gass ML, NAMS Recommendations for Clinical Care of Midlife Women Working Group The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21:1038–1062. doi: 10.1097/GME.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 7.Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod. 2003;18:199–206. doi: 10.1093/humrep/deg005. [DOI] [PubMed] [Google Scholar]
- 8.Muka T, Oliver-Williams C, Kunutsor S. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 9.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra GD, Anderson D, Schoenaker DA. InterLACE: a new International Collaboration for a Life Course Approach to Women's Reproductive Health and Chronic Disease Events. Maturitas. 2013;74:235–240. doi: 10.1016/j.maturitas.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Mishra GD, Chung HF, Pandeya N. The InterLACE study: design, data harmonization and characteristics across 20 studies on women's health. Maturitas. 2016;92:176–185. doi: 10.1016/j.maturitas.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudlow C, Gallacher J, Allen N. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu D, Chung HF, Pandeya N. Relationships between intensity, duration, cumulative dose, and timing of smoking with age at menopause: a pooled analysis of individual data from 17 observational studies. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu D, Chung HF, Pandeya N. Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. Eur J Epidemiol. 2018;33:699–710. doi: 10.1007/s10654-018-0367-y. [DOI] [PubMed] [Google Scholar]
- 15.Lee C, Dobson AJ, Brown WJ. Cohort Profile: the Australian Longitudinal Study on Women's Health. Int J Epidemiol. 2005;34:987–991. doi: 10.1093/ije/dyi098. [DOI] [PubMed] [Google Scholar]
- 16.Seib C, Whiteside E, Humphreys J. A longitudinal study of the impact of chronic psychological stress on health-related quality of life and clinical biomarkers: protocol for the Australian Healthy Aging of Women Study. BMC Public Health. 2014;14:9. doi: 10.1186/1471-2458-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milne RL, Fletcher AS, MacInnis RJ. Cohort profile: the Melbourne Collaborative Cohort Study (Health 2020) Int J Epidemiol. 2017;46:1757–1778. doi: 10.1093/ije/dyx085. [DOI] [PubMed] [Google Scholar]
- 18.Hundrup YA, Simonsen MK, Jorgensen T, Obel EB. Cohort profile: the Danish nurse cohort. Int J Epidemiol. 2012;41:1241–1247. doi: 10.1093/ije/dyr042. [DOI] [PubMed] [Google Scholar]
- 19.Roswall N, Sandin S, Adami HO, Weiderpass E. Cohort profile: the Swedish Women's Lifestyle and Health cohort. Int J Epidemiol. 2017;46:e8. doi: 10.1093/ije/dyv089. [DOI] [PubMed] [Google Scholar]
- 20.Sievert LL, Morrison LA, Reza AM, Brown DE, Kalua E, Tefft HA. Age-related differences in health complaints: the Hilo women's health study. Women Health. 2007;45:31–51. doi: 10.1300/J013v45n03_03. [DOI] [PubMed] [Google Scholar]
- 21.Sowers MFR, Crawford SL, Sternfeld B. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RAKJ, Marcus R, editors. Menopause: biology and pathobiology. 1st edn. Academic Press; New York: 2000. pp. 175–188. [Google Scholar]
- 22.Hayashi K, Mizunuma H, Fujita T. Design of the Japan Nurses' Health Study: a prospective occupational cohort study of women's health in Japan. Ind Health. 2007;45:679–686. doi: 10.2486/indhealth.45.679. [DOI] [PubMed] [Google Scholar]
- 23.Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: the 1946 National Birth Cohort (MRC National Survey of Health and Development) Int J Epidemiol. 2006;35:49–54. doi: 10.1093/ije/dyi201. [DOI] [PubMed] [Google Scholar]
- 24.Power C, Elliott J. Cohort profile: 1958 british birth cohort (National Child Development Study) Int J Epidemiol. 2006;35:34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- 25.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42:1640–1648. doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cade JE, Burley VJ, Alwan NA. Cohort profile: the UK Women's Cohort Study (UKWCS) Int J Epidemiol. 2017;46:e11. doi: 10.1093/ije/dyv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 28.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N, SABRE Study Group Southall And Brent REvisited: cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol. 2012;41:33–42. doi: 10.1093/ije/dyq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ley SH, Li Y, Tobias DK. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32:745–750. doi: 10.1093/eurheartj/ehq477. [DOI] [PubMed] [Google Scholar]
- 31.Tunstall-Pedoe H. Myth and paradox of coronary risk and the menopause. Lancet. 1998;351:1425–1427. doi: 10.1016/S0140-6736(97)11321-6. [DOI] [PubMed] [Google Scholar]
- 32.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu FB, Grodstein F, Hennekens CH. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 34.Løkkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53:226–233. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Shen L, Song L, Liu B. Effects of early age at natural menopause on coronary heart disease and stroke in Chinese women. Int J Cardiol. 2017;241:6–11. doi: 10.1016/j.ijcard.2017.03.127. [DOI] [PubMed] [Google Scholar]
- 36.Dam V, van der Schouw YT, Onland-Moret NC. Association of menopausal characteristics and risk of coronary heart disease: a pan-European case-cohort analysis. Int J Epidemiol. 2019 doi: 10.1093/ije/dyz016. published online Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossouw JE, Anderson GL, Prentice RL. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 38.Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol. 2012;11:82–91. doi: 10.1016/S1474-4422(11)70269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17:506–510. doi: 10.1097/gme.0b013e3181c7dd41. [DOI] [PubMed] [Google Scholar]
- 40.Choi SH, Lee S-M, Kim Y, Choi N-K, Cho YJ, Park B-J. Natural menopause and risk of stroke in elderly women. J Korean Med Sci. 2005;20:1053–1058. doi: 10.3346/jkms.2005.20.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40:1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boardman HM, Hartley L, Eisinga A. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;3 doi: 10.1002/14651858.CD002229.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 44.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 45.Mazzuca MQ, Mata KM, Li W, Rangan SS, Khalil RA. Estrogen receptor subtypes mediate distinct microvascular dilation and reduction in [Ca2+]I in mesenteric microvessels of female rat. J Pharmacol Exp Ther. 2015;352:291–304. doi: 10.1124/jpet.114.219865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135:54–70. doi: 10.1016/j.pharmthera.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton-Tyrrell K, Wildman RP, Matthews KA. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 48.Rexrode KM, Manson JE, Lee IM. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 49.Zhu D, Chung HF, Pandeya N. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. Eur J Epidemiol. 2019;34:235–246. doi: 10.1007/s10654-019-00490-w. [DOI] [PubMed] [Google Scholar]
- 50.Matthews KA, Crawford SL, Chae CU. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuh D, Langenberg C, Hardy R. Cardiovascular risk at age 53 years in relation to the menopause transition and use of hormone replacement therapy: a prospective British birth cohort study. BJOG. 2005;112:476–485. doi: 10.1111/j.1471-0528.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 52.Karim R, Dell RM, Greene DF, Mack WJ, Gallagher JC, Hodis HN. Hip fracture in postmenopausal women after cessation of hormone therapy: results from a prospective study in a large health management organization. Menopause. 2011;18:1172–1177. doi: 10.1097/gme.0b013e31821b01c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168:861–866. doi: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas. 1997;27:117–123. doi: 10.1016/s0378-5122(97)01122-5. [DOI] [PubMed] [Google Scholar]
- 55.Jorstad HT, Colkesen EB, Boekholdt SM. Estimated 10-year cardiovascular mortality seriously underestimates overall cardiovascular risk. Heart. 2016;102:63–68. doi: 10.1136/heartjnl-2015-307668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22:403–411. doi: 10.1080/13697137.2019.1574738. [DOI] [PubMed] [Google Scholar]
- 57.Schneider HPG, Birkhauser M. Quality of life in climacteric women. Climacteric. 2017;20:187–194. doi: 10.1080/13697137.2017.1279599. [DOI] [PubMed] [Google Scholar]
- 58.GBD 2016 Neurology Collaborators Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moran AE, Forouzanfar MH, Roth GA. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.