Abstract
Autoimmune encephalitis associated with autoantibodies to the gamma-aminobutyric acid B receptor (GABABR-AE) typically involves limbic symptoms with limbic abnormalities visible in brain magnetic resonance imaging (MRI). We herein report a case of a 48-year-old man with GABABR-AE whose initial presentation was limited to syncope without limbic symptoms or MRI abnormalities. Interestingly, serial MRI also revealed no abnormalities even after the appearance of limbic symptoms. Our findings suggest that GABABR-AE can initially mimic common syncope and that MRI findings may remain normal throughout the clinical course. Even if patients have normal MRI findings, GABABR-AE should be considered if limbic symptoms worsen.
Keywords: autoimmune encephalitis, gamma-aminobutyric acid B receptor (GABABR), magnetic resonance imaging (MRI), syncope
Introduction
Autoimmune encephalitis associated with autoantibodies to the gamma-aminobutyric acid B receptor (GABABR-AE) can be improved by early immunotherapy (1). Patients with GABABR-AE typically present with limbic symptoms, such as consciousness disturbance, psychiatric manifestations, and seizures, and brain magnetic resonance imaging (MRI) typically reveals limbic lesions similar to those observed in other forms of limbic encephalitis (1, 2).
We herein report a case of GABABR-AE involving an initial presentation of syncope without limbic symptoms. Serial MRI findings remained normal even after the patient's symptoms worsened. The subtle initial symptoms and lack of MRI abnormalities mimicking common syncope made an early diagnosis of GABABR-AE difficult.
Case Report
A 48-year-old man with no significant medical history of epilepsy, arrhythmia, or other major medical conditions developed syncope upon waking in February 2016. Incontinence occurred during this syncope episode, but convulsions, tongue-biting, auras, blackout, and postictal paresis did not. He fully regained consciousness within 1 minute. Three days later, he fainted again while sitting in his office performing work. Ten days after the first attack, he experienced a third syncope episode and was admitted to our hospital. He had never presented with convulsions, automatism, psychiatric symptoms, chest pain, palpitation, or autonomic failure before, and he had no history of suspected vasovagal or drug-induced syncope.
At admission, he had a fever (body temperature, 38℃), but his neurological and mental states were normal with no signs of meningeal irritation. Examinations with 12-lead and Holter electrocardiograms and echocardiography revealed no arrhythmias or valvular abnormalities that could cause syncope. The patient's orthostatic blood pressure did not decrease during the Schellong test. These results indicated that our patient's syncope was not cardiogenic, vasovagal, or related to orthostatic hypotension.
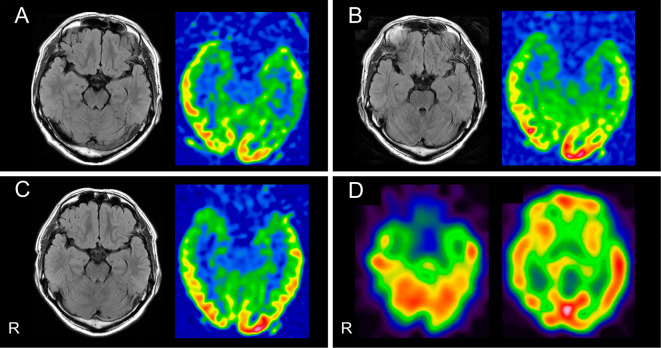
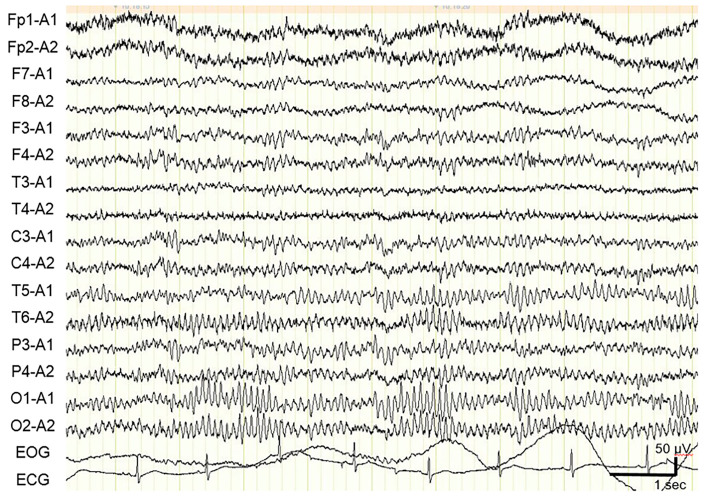
Routine laboratory tests revealed no abnormalities besides slight leukocytosis. Serological tests for systemic infection and autoimmune disease were negative. Testing of serum samples for rheumatoid factor, antinuclear antibodies, and antibodies to thyroid (thyroglobulin and thyroid peroxidase), and glutamic acid decarboxylase (GAD) returned negative results. A cerebrospinal fluid (CSF) analysis revealed a slightly elevated cell count of 27 /μL (all cells were monocytes) with normal protein (38 mg/dL) and glucose (72 mg/dL) levels. The CSF IgG index was also elevated (0.74; normal range, <0.65). Tests for oligoclonal bands returned negative results. The patient's CSF levels of myelin basic protein and adenosine deaminase were normal. The CSF was negative for herpes simplex virus 1 and varicella zoster virus DNA. Brain MRI including both fluid-attenuated inversion recovery (FLAIR) and arterial spin labeling (ASL) perfusion sequences revealed no limbic system abnormalities on day 12 (Fig. 1A). An electroencephalogram showed normal 10-Hz background activity with no epileptiform patterns on day 13 (Fig. 2). Computed tomography images of the chest, abdomen, and pelvis showed no evidence of cancer.
Figure 1.
Imaging findings in our patient and the characteristics of select cases of GABABR-AE. (A-C) FLAIR and ASL perfusion brain MRI sequences. Normal findings were obtained (A) at admission 12 days after syncope onset, at which point no neurological symptoms had been observed; (B) on day 14, at which point the patient had experienced repeated seizures; and (C) on day 38, after the patient’s limbic symptoms had markedly worsened. (D) Qualitative surface views of 123I-IMP perfusion SPECT images revealed no marked abnormalities on day 40. R: right, GABABR-AE: gamma-aminobutyric acid B receptor, FLAIR: fluid-attenuated inversion recovery, ASL: arterial spin labeling, 123I-IMP: 123I-N-isopropyl-p-iodoamphetamine
Figure 2.
An electroencephalogram from our patient showing normal 10-Hz background activity with no epileptiform patterns on day 13.
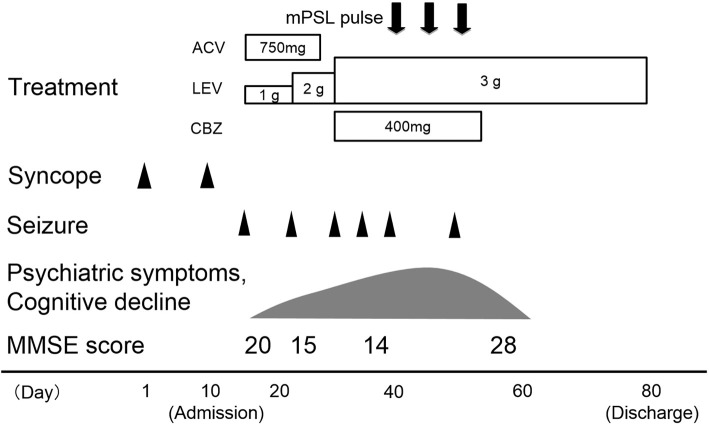
On day 14, he developed new-onset complex partial seizures with secondary generalization. Follow-up brain MRI showed no abnormalities on day 14 (Fig. 1B). Seizures occurred repeatedly even after appropriate antiepileptic drug administration. Psychiatric symptoms and progressive cognitive decline were observed. His Mini-Mental State Examination (MMSE) score was recorded as 20/30 on day 15. An electroencephalogram indicated generalized slowing (8 Hz) without focal findings or epileptiform activity on day 31. Despite the symptom progression and electroencephalographic abnormalities, brain MRI revealed no abnormalities on day 38 (Fig. 1C). Furthermore, perfusion single-photon emission computed tomography (SPECT) with 123I-N-isopropyl-p-iodoamphetamine (123I-IMP) showed no abnormalities on day 40 (Fig. 1D). Despite the normal MRI and SPECT findings, his clinical symptoms caused us to suspect that he had autoimmune encephalitis. He was treated with 3 rounds of methylprednisolone pulse therapy (1 g/day), which produced a good response (Fig. 3). His clinical symptoms and CSF findings improved following this treatment. His MMSE score was recorded as 28/30 on day 58. He was discharged on day 80 due to the resolution of his neurological symptoms and cognitive decline.
Figure 3.
The patient’s clinical course. ACV: acyclovir, CBZ: carbamazepine, LEV: levetiracetam, MMSE: mini-mental state examination, mPSL: methylprednisolone
Cell-based assays for serum autoantibodies associated with autoimmune encephalitis yielded positive results for anti-GABABR antibodies in his serum and CSF. Other autoantibodies to the N-methyl-D-aspartate receptor (NMDAR), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), leucine-rich glioma-inactivated protein 1 (LGI1), contactin-associated protein 2 (Caspr2), glycine receptor, and metabotropic glutamate receptor (mGluR) 1 and 5 were not detected. The patient was therefore ultimately diagnosed with GABABR-AE. In the more than three years since the symptom onset, he has not relapsed or needed post-immunotherapy treatments. No evidence of cancer has been observed during follow-up.
Discussion
In the present case of GABABR-AE, the patient's initial presentation was limited to syncope without limbic manifestations, and serial MRI revealed no abnormalities despite symptom progression. Further examinations that included cardiac and autonomic evaluations and an electroencephalogram initially failed to identify the cause of his syncope episodes, but a CSF analysis revealed a slightly elevated cell count. This clinical presentation mimicked common syncope or idiopathic epilepsy, which made a diagnosis difficult. The present case suggests that presentations involving etiologically uncharacterized repeated syncope episodes with CSF pleocytosis should be considered potentially indicative of early-stage GABABR-AE, even if no limbic symptoms or MRI abnormalities are observed.
In recent case series studies, approximately one third (27-44%) of patients with GABABR-AE had normal brain MRI findings, most of which were probably obtained in the disease's early phase (1-4). Interestingly, a few case studies concerning patients with GABABR-AE reported that normal MRI findings were observed in early-phase GABABR-AE but that MRI abnormalities were observed during symptom progression (5, 6). This suggests that the emergence of MRI abnormalities tends to accompany clinical progression in GABABR-AE. In contrast, our patient exhibited no abnormalities in serial MRI scans despite symptom progression and abnormal electroencephalogram and CSF findings. Some case studies have also reported that perfusion SPECT and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) may reveal abnormal perfusion or metabolic patterns in patients with GABABR-AE who have normal MRI findings (7-9). Although our patient did not undergo 18F-FDG PET, repeated ASL perfusion MRI and perfusion SPECT assessments revealed normal findings that mirrored those obtained with conventional MRI.
In addition to normal MRI findings, the normal electroencephalogram findings and absence of limbic symptoms at admission contributed to the diagnostic delay for our patient, and the repetitive syncope episodes were initially mistaken for common syncope. However, we eventually discovered that these attacks were epileptic seizures due to GABABR-AE. Recent case series studies have reported that limbic symptoms, including seizures, are present at the onset in ≥85% of patients with GABABR-AE and that only ≤17% of patients with GABABR-AE experience seizures without other limbic symptoms (1-4).
We reviewed three reported cases of GABABR-AE in which the patients initially presented with only syncope or seizures without non-seizure limbic manifestations and exhibited normal MRI findings (Table) (5-7). All three patients eventually developed repetitive refractory seizures with other limbic symptoms and recovered with immunotherapy. An interesting commonality between these three cases and our patient's case is that immunotherapy was initiated after non-seizure limbic symptoms emerged. In two patients, follow-up MRI scans revealed emergent abnormalities (5, 6), but the third patient, like ours, exhibited no abnormal findings in follow-up MRI scans (7). As mentioned earlier, some case reports showed abnormalities in PET or SPECT images (7-9), but our patient exhibited no abnormalities in ASL perfusion MRI or perfusion SPECT images. Although we cannot explain the lack of detectable MRI or SPECT abnormalities in our patient, his remarkable responsiveness to immunotherapy indicates that the neuronal effects of anti-GABABR antibodies are often latent and reversible. The pathophysiological mechanisms underlying between-patient differences in longitudinal imaging findings should be examined in future studies.
Table.
GABABR-AE Cases in which the Initial Presentation Involved Only Syncope Or Seizures and MRI Findings were Initially Normal.
| Reference | (5) | (6) | (7) | Present case | |
|---|---|---|---|---|---|
| Age / Sex | 23 / M | 57 / M | 62/ F | 48 / M | |
| Initial symptoms | Limbic symptoms | - | - | - | - |
| Only seizures | + | - | + | - | |
| Syncope | - | + | - | + | |
| Repeated seizures | + | + | + | + | |
| Immunotherapy response | + | + | + | + | |
| Last day MRI showed normal | day 2 | >day 10 | day 24 | day 38 | |
| First day MRI showed abnormal | day 15 | >day 70 | - | - | |
| EEG (initial) | Epileptiform activities | - | - | - | - |
| Slow waves | - | - | + | - | |
| CSF | Pleocytosis | + | - | + | + |
| Elevated protein | - | - | - | - | |
CSF: cerebrospinal fluid, EEG: electroencephalogram, F: female, GABABR-AE: gamma-aminobutyric acid B receptor, M: male, MRI: magnetic resonance imaging
In conclusion, the present case suggests that patients with GABABR-AE may initially present with only syncope and may exhibit normal MRI findings throughout their clinical courses. Even in patients with normal MRI findings, the possibility of GABABR-AE should be considered in the event of progressive limbic symptoms, including refractory seizures.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Professor Josep Dalmau (ICREA-IDIBAPS, Service of Neurology, Hospital Clínic, University of Barcelona, Barcelona, Spain, and the University of Pennsylvania, Philadelphia, PA, USA) for his help with screening the serum for autoimmune encephalitis-related autoantibodies with cell-based assays.
References
- 1. Lancaster E, Lai M, Peng X, et al. . Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 9: 67-76, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Höftberger R, Titulaer MJ, Sabater L, et al. . Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology 81: 1500-1506, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan HZ, Ren HT, Yang XZ, et al. . Limbic encephalitis associated with anti-γ-aminobutyric acid B receptor antibodies: a case series from China. Chin Med J (Engl) 128: 3023-3028, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X, Liu F, Li JM, et al. . Encephalitis with antibodies against the GABAB receptor: seizures as the most common presentation at admission. Neurol Res 39: 973-980, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Hainsworth JB, Shishido A, Theeler BJ, Carroll CG, Fasano RE. Treatment responsive GABAB-receptor limbic encephalitis presenting as new-onset super-refractory status epilepticus (NORSE) in a deployed U.S. soldier. Epileptic Disord 16: 486-493, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Hui AT, Lam YO, Chan CK, Cheung KY, Fung BH, Ng PW. A case of refractory seizure with cognitive impairment due to anti-GABA encephalitis. Hong Kong Med J 22: 509-511, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Ohta K, Seki M, Dalmau J, Shinohara Y. Perfusion IMP-SPECT shows reversible abnormalities in GABAB receptor antibody associated encephalitis with normal MRI. Brain Behav 1: 70-72, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su M, Xu D, Tian R. 18F-FDG PET/CT and MRI findings in a patient with anti-GABAB receptor encephalitis. Clin Nucl Med 40: 515-517, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Kim TJ, Lee ST, Shin JW, et al. . Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol 270: 45-50, 2014. [DOI] [PubMed] [Google Scholar]