Abstract
We present the first case of a Japanese patient with familial hypobetalipoproteinemia (FHBL) caused by a protein-truncating variant in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene. A 34-year-old woman was referred to our hospital due to her low low-density lipoprotein (LDL)-cholesterolemia (34 mg/dL). She did not have any secondary causes of hypobetalipoproteinemia. Her father and her younger sister also exhibited low LDL cholesterol levels. We identified a protein-truncating variant in the PCSK9 gene (c.1090_1091del/p.Pro364ArgfsTer62) among them. None of them exhibited atherosclerotic cardiovascular diseases nor any other complications associated with low LDL cholesterol, including fatty liver, neurocognitive disorders, or cerebral hemorrhaging.
Keywords: FHBL, PCSK9, LDL cholesterol
Introduction
Familial hypobetalipoproteinemia (FHBL) type 1 (OMIM 615558) has been described as a codominant disorder mainly caused by a protein-truncating variants (PTVs) in the apolipoprotein B (APOB) gene (1). Individuals with this condition typically exhibit low low-density lipoprotein (LDL) cholesterol associated with low APOB levels. We have shown that individuals with a single PTV in the APOB gene are quite cardioprotective (2), although some of them present with hepatic steatosis (3). In contrast, individuals with a double PTV in the APOB gene exhibit more severe phenotypes, resembling those of patients with abetalipoproteinemia (ABL) caused by microsomal triglyceride transfer protein (MTTP) gene mutations (4). In addition to a PTV in the APOB gene, a PTV in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene, which encodes a protein that targets the LDL receptor for lysosomal degradation, has also been shown to cause FHBL1. Of note, particular types of genetic mutations in APOB and PCSK9 can cause the opposite phenotype [familial hypercholesterolemia (FH)].
These observations motivated us to develop medications mimicking these mutations in order to reduce the LDL cholesterol levels (5-8). In addition, we can predict the “side-effects” of those medications through observations, such as Mendelian disorders (9). In that sense, the development of a fatty liver through APOB or MTTP inhibitors is predictable given that FHBL is caused by APOB mutations, and ABL is caused by MTTP mutations exhibiting fatty liver symptoms (10,11). However, few data are available regarding this issue in cases with a PTV in the PCSK9 gene, especially in the Asian population, because of the rarity of such mutations (12,13). We herein report a family with FHBL caused by a PTV in the PCSK9 gene, among whom we found no adverse effects associated with this mutation, thus providing supporting evidence for the safe use of PCSK9 inhibitors.
Case Report
Study subjects
A 34-year-old Japanese woman was referred to our lipid clinic due to her low level of LDL cholesterol without any apparent secondary causes. Her initial LDL cholesterol level was 34 mg/dL. Both of her parents showed no evidence of consanguineous marriage, and her grandmother, parents, and younger sister were also included in this study. The characteristics of the study subjects are listed in Table.
Table.
Characteristics of the Family.
| Subject (gender) | I.1 (female)* | II.1 (male) | II.2 (female) | III.1 (female) | III.2 (female) |
|---|---|---|---|---|---|
| Genotype | W/W | W/M | W/W | W/M | W/M |
| Age (years) | 87 | 62 | 60 | 35 | 29 |
| Total cholesterol (mg/dL) | 180 | 163 | 220 | 126 | 115 |
| Triglyceride (mg/dL) | 39 | 42 | 51 | 30 | 31 |
| HDL cholesterol (mg/dL) | 56 | 60 | 72 | 79 | 66 |
| LDL cholesterol (mg/dL) | 116 | 91 | 138 | 34 | 43 |
| ApoA-I (mg/dL) | 132 | 150 | 173 | 170 | 144 |
| ApoB (mg/dL) | 98 | 67 | 94 | 30 | 33 |
| ApoE (mg/dL) | 4.1 | 3.1 | 7.0 | 4.6 | 5.2 |
| ApoE phenotype | E3/E3 | E3/E3 | E3/E3 | E3/E3 | E3/E3 |
| Hetero-dimer PCSK9 (ng/mL) | 250 | 97 | 235 | 96 | 82 |
| Furin-cleaved PCSK9 (ng/mL) | 41 | 20 | 38 | 12 | 12 |
Genotype: M=PCSK 9 gene (c.1090_1091del, or p.Pro364ArgfsTer62)
Biochemical analyses
Fasting blood samples were drawn for assays without any lipid-modifying treatments, except for her grandmother, who had been under statin therapy. The serum concentrations of total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, and LDL cholesterol were determined enzymatically (Qualigent; Sekisui Medical, Tokyo, Japan). The ApoE phenotype was separated by isoelectric focusing and detected by Western blotting with ApoE polyclonal antibody (phenotyping ApoE IEF system; JOKOH, Tokyo, Japan). The serum PCSK9 concentrations were determined using an enzyme-linked immunosorbent assay (14).
Genetic analyses
We isolated the genomic DNA for each participant from peripheral white blood cells using a standard DNA extraction protocol. DNA was pooled, selected for size, ligated to sequencing adapters, and amplified to enrich for targets that were sequenced using the Kapa DNA Library Preparation. A custom NimbleGen in-solution DNA capture library (Roche NimbleGen, Madison, USA) was designed to capture all coding exons in 21 dyslipidemia-related Mendelian genes, including 4 associated with primary hypobetalipoproteinemia (ANGPTL3, APOB, MTTP, and PCSK9). The details have been described previously (15). Target-enriched products were sequenced using the Illumina MiSeq. The target coverage for each subject was ≥20-fold in ≥98% of all targeted exons.
Ethical considerations
Genetic analyses were approved by the Ethics Committee at Kanazawa University. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1975 Declaration of Helsinki, as revised in 2008. Informed consent for genetic analyses was obtained from all of the subjects for inclusion in the study.
Determination of causative variants
We defined a variant as causative if it met any of the following criteria: a) rare (minor allele frequency <1% among the East Asian population) protein-truncating variant (premature stop, insertions or deletions that shift frames, or canonical splice-sites); b) rare damaging missense variant, defined as those predicted as damaging by all five in silico software programs (SIFT, Polyphen2-HDIV, Polyphen2-HVAR, MutationTaster-2, and LRT); and c) ClinVar-registered pathogenic or likely pathogenic variants that cause primary hypobetalipoproteinemia.
Characteristics of study subjects
The initial LDL cholesterol level of the proband was 34 mg/dL. She did not exhibit any secondary causes for hypobetalipoproteinemia, including hyperthyroidism, bleeding, or any malignancies. She did not exhibit fatty liver on computed tomography or echo imaging. Our cascade screening revealed that her father and her younger sister also had hypobetalipoproteinemia, suggesting its dominant pattern of inheritance (Table). In addition to low levels of APOB-containing lipoprotein, these subjects' PCSK9 mass levels were markedly lower than those of other family members. None of the family members exhibited atherosclerotic cardiovascular disease (ASCVD) nor any other complications typically considered to be associated with low LDL cholesterol levels, including fatty liver, neurocognitive disorder, or cerebral hemorrhaging.
Genetic analyses
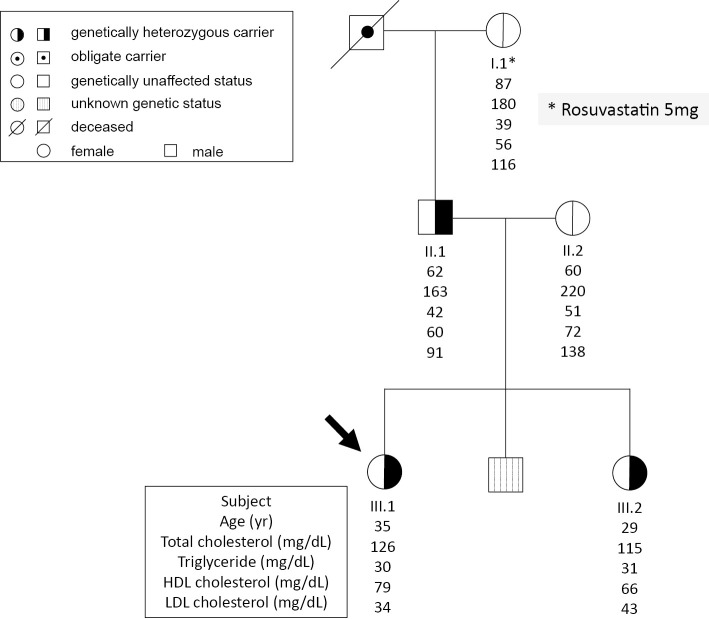
There were no apparent deleterious mutations in the APOB gene, which is the most frequent cause of this situation in the proband. However, one protein-truncating variant was found in her PCSK9 gene (c.1090_1091del/p.Pro364ArgfsTer62). This particular mutation was inherited from her father, whose LDL cholesterol level was relatively low compared with the average levels expected for his age (Fig. 1). In addition, this variant was inherited by her younger sister as well, whose LDL cholesterol was extremely low as well.
Figure 1.
Family tree. Arrow indicates the proband. Black color indicates a carrier of a PCSK9 mutation (c.1090_1091del, or p.Pro364ArgfsTer62). *under rosuvastatin 5 mg/daily.
Assessments of systemic atherosclerosis and other complications
We assessed systemic atherosclerosis in the proband and her father using carotid ultrasonography and brachial-ankle pulse wave velocity. Neither of them had any plaque in their carotid arteries, and their arterial stiffness was within the normal ranges for their ages.
Discussion
Several different types of mutations associated with a reduced LDL cholesterol level as well a reduced risk of ASCVD in the PCSK9 gene have been described (16,17); however, few reports have described “familial” hypobetalipoproteinemia caused by a PCSK9 genetic mutation where the segregation pattern was well-confirmed. We herein report the first Japanese case of FHBL caused by a PTV in the PCSK9 gene using comprehensive genetic analyses.
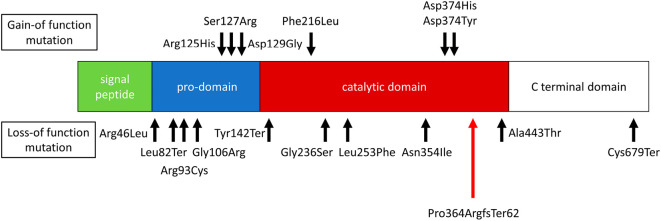
While the proband's father's LDL cholesterol was not very low, a survey by the National Institute of Health and Nutrition (18) indicated that the mean LDL cholesterol among men in their 60s is roughly 120 mg/dL, which was much higher than the father's value. In contrast, the average LDL cholesterol level among women in their 30s is 107 mg/dL. In addition to the absolute difference, a substantial proportion of men in their 60s are treated, while only a few women in their 30s are treated. Accordingly, the LDL cholesterol level of 91 mg/dL in the father without any treatment could be considered to be a low LDL cholesterol level. The novel mutation identified in this family has not been reported before and is believed to produce protein truncation in a catalytic domain of the PCSK9 protein (Fig. 2).
Figure 2.
Domain of the PCSK9 gene. PCSK9 encodes a 692-amino-acid protein composed of a signal peptide, a pro-domain, catalytic, and C-terminal domains. Black arrows indicate the mutations reported thus far. Red arrows indicate a mutation identified in this study.
Interestingly, both the hetero-dimer and furin-cleaved PCSK9 levels were decreased in the affected members. In that sense, another nonsense mutation (Cys670Ter) has been associated with an inability of PCSK9 to exit the endoplasmic reticulum, leading to a reduction in both hetero-dimer PCSK9 and furin-cleaved PCSK9 (19). However, another loss-of-function mutation (Ala443Thr) has been shown to increase furin cleavage, leading to elevated levels of furin-cleaved PCSK9 (19). Accordingly, we speculate that the current frameshift mutation is likely to be classified into the former in this context. In addition, other loss-of-function mutations in the PCSK9 gene, such as Tyr142Ter, Cys679Ter, and Arg46Leu, have been shown to be associated with reduced LDL cholesterol levels and a reduced risk for ASCVD (20). Those mutations have frequencies of approximately 3% and are associated with a reduction in the LDL cholesterol level of 13-35 mg/dL, depending on the ethnicity. It has also been shown that rarer genetic variations tend to have larger effect sizes, based on purifying selection. Accordingly, it is not surprising that the mutation identified in this study had a rather marked effect on reducing the LDL cholesterol level.
The frequency of this condition in the general population has been estimated to be 1 in 1,000-3,000. Among cases of FHBL, the APOB gene seems to be the major cause of this condition, and it is estimated that almost half of such cases may be caused by a PTV in the APOB gene. We previously showed that a rare PTV in the APOB gene was associated with a reduced LDL cholesterol level and protection against ASCVD (2). However, a PTV in the PCSK9 gene has also been shown to cause FHBL, although few data on this point exist except for studies in an African population where a particular PTV in the PCSK9 gene is relatively common.
We herein reported a rare family with FHBL caused by a PTV in the PCSK9 gene, where apparently “healthy” phenotypes were observed. The accumulation of data from such rare individuals may help us better understand the roles of PCSK9 in human.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by a scientific research grant from the Ministry of Education, Science, and Culture of Japan (No. 19K08575).
Acknowledgement
We thank Ms. Kazuko Honda and Sachio Yamamoto for their technical assistance.
References
- 1. Blanco-Vaca F, Martin-Campos JM, Beteta-Vicente Á, et al. Molecular analysis of APOB, SAR1B, ANGPTL3, and MTTP in patients with primary hypocholesterolemia in a clinical laboratory setting: evidence supporting polygenicity in mutation-negative patients. Atherosclerosis 283: 52-60, 2019. [DOI] [PubMed] [Google Scholar]
- 2. Peloso GM, Nomura A, Khera AV, et al. Rare protein-truncating variants in APOB, lower low-density lipoprotein cholesterol, and protection against coronary heart disease. Circ Genom Precis Med 12: e002376, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Filippo M, Moulin P, Roy P, et al. Homozygous MTTP and APOB mutations may lead to hepatic steatosis and fibrosis despite metabolic differences in congenital hypocholesterolemia. J Hepatol 61: 891-902, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Kawashiri MA, Tada H, Hashimoto M, et al. Extreme contrast of postprandial remnant-like particles formed in abetalipoproteinemia and homozygous familial hypobetalipoproteinemia. JIMD Rep 22: 85-94, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuchel M, Meagher EA, du Toit Theron H, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 381: 40-46, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 376: 1713-1722, 2017. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 379: 2097-2107, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Reeskamp LF, Kastelein JJP, Moriarty PM, et al. Safety and efficacy of mipomersen in patients with heterozygous familial hypercholesterolemia. Atherosclerosis 280: 109-117, 2019. [DOI] [PubMed] [Google Scholar]
- 9. Tada H, Kawashiri MA, Yamagishi M. Clinical perspectives of genetic analyses on dyslipidemia and coronary artery disease. J Atheroscler Thromb 24: 452-461, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang XP, Inazu A, Yagi K, Kajinami K, Koizumi J, Mabuchi H. Abetalipoproteinemia caused by maternal isodisomy of chromosome 4q containing an intron 9 splice acceptor mutation in the microsomal triglyceride transfer protein gene. Arterioscler Thromb Vasc Biol 19: 1950-1955, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Katsuda S, Kawashiri MA, Inazu A, et al. Apolipoprotein B gene mutations and fatty liver in Japanese hypobetalipoproteinemia. Clin Chim Acta 399: 64-68, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 79: 514-523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cariou B, Ouguerram K, Zaïr Y, et al. PCSK9 dominant negative mutant results in increased LDL catabolic rate and familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol 29: 2191-2197, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Hori M, Ishihara M, Yuasa Y, et al. Removal of plasma mature and furin-cleaved proprotein convertase subtilisin/kexin 9 by low-density lipoprotein-apheresis in familial hypercholesterolemia: development and application of a new assay for PCSK9. J Clin Endocrinol Metab 100: E41-E49, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Tada H, Kawashiri MA, Nomura A, et al. Oligogenic familial hypercholesterolemia, LDL cholesterol, and coronary artery disease. J Clin Lipidol 12: 1436-1444, 2018. [DOI] [PubMed] [Google Scholar]
- 16. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 354: 1264-1272, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Kathiresan S, Myocardial Infarction Genetics Consortium . A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med 358: 2299-2300, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Health Japan 21 (the second term) Analysis and Assessments Projects. National Institute of Health and Nutrition [Internet]. [cited 2019 Aug 25]. Available from: http://www.nibiohn.go.jp/eiken/kenkounippon21/ (in Japanese).
- 19. Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah NG. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J Biol Chem 281: 30561-30572, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Kent ST, Rosenson RS, Avery CL, et al. PCSK9 Loss-of-function variants, low-density lipoprotein cholesterol, and risk of coronary heart disease and stroke: data from 9 studies of blacks and whites. Circ Cardiovasc Genet 10: e001632, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]