Abstract
Publisher's Note: There is a Blood Commentary on this article in this issue.
TO THE EDITOR:
The World Health Organization recently launched an initiative to revise its recommended hemoglobin (Hb) thresholds for the diagnosis and assessment of anemia.1,2 The physiological Hb range varies, depending on age, sex, ethnicity, genetic background, possible pregnancy, smoking habits, socioeconomic and nutritional status (including iron availability), and residential altitude.3 There are little data about whether modest increases in altitude below 2000 meters above sea level (masl) impact Hb concentration.4,5 This knowledge helps to determine a person’s health status and diagnose anemia.6 Here, we analyze the association between Hb and residential altitude in healthy young Swiss men living between 200 and 2000 masl.
We used data collected between 2010 and 2012 from young Swiss male conscripts (covering >90% of Swiss male birth cohorts as a result of mandatory conscription).7 The total cohort consisted of 110 810 men (18-21 years); of those, 71 798 (64.8%) voluntarily consented to blood testing. Medical personnel took samples at the 6 conscription centers, and they were shipped to a single certified laboratory. All blood samples were analyzed using the same hemoglobinometer. See supplemental Material for additional information on the participants and methods (available on the Blood Web site).
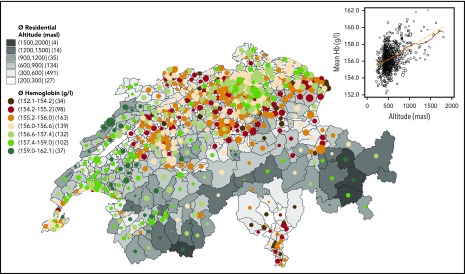
Blood test participants resided at an average altitude of 543.5 masl (range, 205-1989), with 91.1% living between 300 and 900 masl (see descriptive statistics in supplemental Material). Average Hb was 156.28 g/L (95% confidence interval, 156.22-156.34; standard deviation, 8.66; range, 67-217). Anemia (Hb < 130 g/L) affected 178 (0.25%) of the young men, with decreasing prevalence when residential altitude increased. Only 1 (0.002%) young man had excessive erythrocytosis, defined as Hb ≥ 210 g/L. Overall, the Hb distribution shifted to the right on the x-axis with increasing altitude (supplemental Figure 1), which is also reflected by decreasing percentages of lower Hb (<140 g/L) and increasing percentages of higher Hb (≥175 g/L) when residential altitude increased (supplemental Table 1). Mapping unadjusted average Hb against average residential altitude in all 705 MedStat regions (the official geographical regions of Switzerland) revealed that regions of higher residential altitude were associated with higher mean Hb concentrations (Figure 1). In other words, the Hb values mirrored Switzerland’s topography (“hemoglobinography”).
Figure 1.
Hemoglobinography: Hb concentrations mirror the topography of Switzerland. Map of 705 MedStat regions that represents the official geographical regions, allowing an anonymous indication of a place of residence for each person hospitalized in Switzerland. The map shows the conscripts’ average residential altitude, as well as average Hb level (circle size proportional to sample size, Nmin = 12, Nn < 30 = 12, Nmax = 335, Nmean = 102). The numbers in parentheses following the altitudes in the legend key indicate the number of MedStat regions in that particular category. The scatterplot (inset) shows the same MedStat data (altitude vs Hb). The solid red line indicates a smoothed local polynomial trend (bandwidth = 100 masl), the yellow dashed line is a linear fit, and the size of the circle is proportional to sample size.
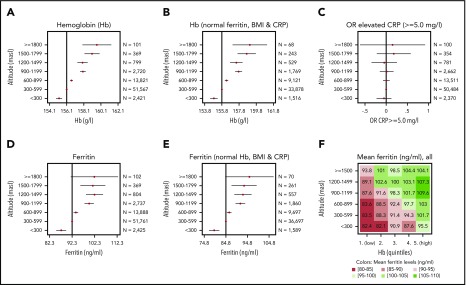
Mean Hb increased by a maximum of 2.84%, from 155.20 g/L for conscripts living at <300 masl to 159.61 g/L for those living at >1800 masl. Hb concentrations increased significantly and stepwise with every gain in altitude starting from 300 masl (Figure 2A). To exclude the possible impact of nonphysiological parameters, we restricted the data to conscripts with normal ferritin, C-reactive protein (CRP), and body mass index (BMI) values (Figure 2B). Hb still increased significantly with altitude gain; however, the coefficients of the regressions (supplemental Table 2) showed that the increase in Hb from 1 altitude step of 300 m to the next is not always the same (range, 0.6-1.3 g/L). There was no further increase between the altitude levels of 900 and 1199 masl and between 1200 and 1499 masl. If the association between Hb and altitude is considered continuously (supplemental Figure 1B), the linear and the smoothed modeling (via fine local polynomials with a bandwidth of 100 masl) differ modestly.
Figure 2.
Regression results of blood parameters at increasing altitute. Coefficient plots showing the linear regression results for residential altitude as explanatory variable against Hb (A), Hb for conscripts with normal ferritin (15-200 ng/mL), CRP (<5.0 mg/L), and BMI (18.5-24.9 kg/m2) only (B), ferritin (D), and ferritin for conscripts with normal Hb (<175 g/L), CRP (<5.0 mg/L), and BMI (18.5-24.9 kg/m2) only (E). (C) Odds ratios (OR) for elevated CRP (≥5.0 mg/L) were calculated using logistic regression. The vertical lines indicate the reference levels (constant, intercept, α) of the regressions, and the point estimates with 95% confidence intervals indicate the coefficients (β). Detailed results for regression (A, B, D, and E) can be found in supplemental Table 3. The robustness of these associations was checked in a sensitivity analysis by stratifying for age, occupational background, and recruitment centers (supplemental Figure 2). (F) Matrix plot of average ferritin levels (all blood test participants) by altitude and quintiles of Hb (first quintile is the lowest 20% of Hb values, fifth quintile is the highest 20% of Hb values).
Ferritin increased significantly with altitude (Figure 2D), whereas the odds ratios for elevated CRP did not (Figure 2C). This is important because systemic inflammation has a profound impact on hematopoiesis, gastrointestinal iron absorption, and ferritin plasma levels.8,9 To assess whether increased ferritin serum levels, a marker for body iron stores, directly depend on a concomitant increase in Hb concentrations, we analyzed the interrelationship among altitude, Hb, ferritin, CRP, and BMI. Analysis of conscripts with normal Hb concentrations (<175 g/L), normal CRP (<5.0 mg/L), and normal BMI (18.5-24.9 kg/m2) revealed that ferritin levels still increased significantly with altitude (Figure 2E). We further plotted average ferritin levels within a matrix of altitude vs Hb quintiles (Figure 2F). Conscripts with high Hb, and who lived at higher altitudes, had the highest mean ferritin levels. Within the 5 Hb quintile levels, mean ferritin increased with residential altitude. This suggests that, in addition to the adaptation of Hb concentrations to altitude, there are mechanisms that increase iron stores depending on altitude.
The most important observation is the significant increase in Hb concentration with every 300-meter increase in residential altitude. Remarkably, Mabel Purefoy FitzGerald speculated >110 years ago that small altitude changes affect Hb serum, but the number of measurements (including her own blood!) was too low to reach statistical significance.10 In contrast to World Health Organization and Centers for Disease Control and Prevention studies on Hb adjustment,11 we found a significant change in Hb concentrations below 1000 masl (supplemental Table 4). Although we observed that Hb increases from one altitude level of 300 masl to the next, another recent study models an almost linear increase of 3 g/L for every 500-meter increase in altitude between 1 and 2000 masl.5 The differences may be due to the narrower study population in our case (only young men in Switzerland), decreasing sample size at altitudes ≥1500 masl, and the fine-grained modeling.
Because Hb concentrations are primarily regulated by the cellular oxygen sensor mechanism involving the prolyl-hydroxylase-2–hypoxia-inducible factor-2 (HIF-2)–erythropoietin (Epo) axis,12 our observation implies that the oxygen sensor is very precise, enabling detection of even subtle changes in oxygen at low to moderate altitude. We hypothesize that the oxygen-sensing mechanism did not originally evolve to increase red blood cell production by increasing Epo synthesis. The observations that Epo and its receptor are expressed in mouse and human brains in an oxygen-dependent manner,13,14 Epo exerts neuroprotective effects,15,16 and cerebral Epo improves exercise performance in mice17 might point toward Epo’s function in enhancing cognitive and physical performance in conditions of oxygen deprivation, as it occurs at sea level upon (menstrual or accidental) blood loss. We hypothesize that, in hypoxic conditions, this precise oxygen sensor was originally thought to enhance Epo synthesis for neuroprotection and -regeneration and was later reused to increase erythropoiesis.
This study provides the first evidence that blood ferritin levels increase with altitude (by a maximum of 16.83% for conscripts living at <300 masl to those living at >1800 masl) in an Hb-independent manner. We expected that increased erythropoiesis leading to elevated Hb concentrations requires more iron that is supplied by dietary absorption and mobilization from iron stores.18 Consequently, we hypothesized that ferritin concentrations would directly correlate with elevated Hb. But this was not the case. We explain this phenomenon by the presence of the oxygen-sensing pathway in duodenal enterocytes, which acts independently of Hb concentrations. Accordingly, HIF-2 activity increases with low oxygenation in duodenal enterocytes to activate dietary iron absorption and iron release to the circulation. It does so by activating transcription of dimetal transporter-1, an iron transporter at the apical side of the enterocyte, as well as of ferroportin, an iron exporter releasing iron into the circulation at the basolateral side.12 Thus, enterocytes sense oxygen partial pressure and supply more iron when oxygen levels drop. Although Hb concentrations are not directly involved in this process, a direct cross talk occurs between the expression of the iron hormone hepcidin in the liver and HIF-2α expressed in duodenal cells. Hepcidin-mediated ferroportin degradation in enterocytes controls HIF-2α levels in a cell-autonomous manner, thereby determining the availability of iron required for the oxygen sensor prolyl-hydroxylase-2 to function.19 Our finding suggests that, in addition to the adaptation of Hb concentrations, certain mechanisms increase iron stores, depending upon altitude exposure.
In summary, even very modest increases in residential altitude significantly increase blood Hb and ferritin values in young men. Thus, altitude should be considered when defining the Hb threshold for anemia, even for populations living at <1500 masl. Because there appear to be different mechanisms of adaptation to high altitude in different populations,3 future analysis of Hb concentrations (and other physiological parameters) in populations living at low to moderate altitude, as well as studies on underlying mechanisms of adaption, is required to provide adequate thresholds.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Andreas Stettbacher, Surgeon General of the Swiss Armed Forces, for providing individual conscription data, as well as Katarina Matthes, Sabine Güsewell, Franz Frey, Tiziano Angelelli, Adrian Trapp, and Rock G. Arten for helpful comments. The authors also thank the Swiss Federal Office of Statistics and the Swiss National Cohort (SNC) for providing the altitude information for the source populations of the area units.
This work was supported by the Swiss Federal Office of Public Health (K.S.), the Mäxi Foundation (F.R.), the Swiss National Science Foundation (M.G.), and the German Research Foundation (Deutsche Forschungsgemeinschaft, SFB1036 and SFB1118) (M.U.M.).
Footnotes
For altitude-related matters, please contact Max Gassmann (maxg@access.uzh.ch).
Authorship
Contribution: K.S., F.R., and M.H. had the initial idea for the project; K.S. and F.R. obtained data from the Swiss Armed Forces; K.S., M.G., M.Z., R.P., and M.U.M. designed the data presentation; M.Z. and R.P. prepared the SNC altitude data; K.S. analyzed data; K.S. and M.G. wrote the first draft of the manuscript; N.N.G. provided valuable research assistance; and all authors conceived the presentation of the data and contributed to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kaspar Staub, Institute of Evolutionary Medicine, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland; e-mail: kaspar.staub@iem.uzh.ch.
REFERENCES
- 1.Pasricha S-R, Colman K, Centeno-Tablante E, Garcia-Casal M-N, Peña-Rosas J-P. Revisiting WHO haemoglobin thresholds to define anaemia in clinical medicine and public health. Lancet Haematol. 2018;5(2):e60-e62. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf. Accessed 16 February 2020.
- 3.Gassmann M, Mairbäurl H, Livshits L, et al. The increase in hemoglobin concentration with altitude varies among human populations. Ann. N. Y. Acad. Sci. 2019;1450(1):204-220. [DOI] [PubMed] [Google Scholar]
- 4.García-Erce JA, Lorente-Aznar T, Rivilla-Marugán L. Influence of gender, age and residence altitude on haemoglobin levels and the prevalence of anemia. Med. Clin. (Barc). 2019;153(11):424-429. [DOI] [PubMed] [Google Scholar]
- 5.Sharma AJ, Addo OY, Mei Z, Suchdev PS. Reexamination of hemoglobin adjustments to define anemia: altitude and smoking. Ann N Y Acad Sci. 2019;1450(1):190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107(5):1747-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruggisser M, Burki D, Haeusler M, Rühli FJ, Staub K. Multivariable analysis of total cholesterol levels in male Swiss Armed Forces conscripts 2006-2012 (N = 174,872). BMC Cardiovasc Disord. 2016;16(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetze O, Schmitt J, Spliethoff K, et al. Adaptation of iron transport and metabolism to acute high-altitude hypoxia in mountaineers. Hepatology. 2013;58(6):2153-2162. [DOI] [PubMed] [Google Scholar]
- 9.Fruehauf H, Vavricka SR, Lutz TA, et al. Evaluation of acute mountain sickness by unsedated transnasal esophagogastroduodenoscopy at high altitude [published online ahead of print 25 November 2019]. Clin Gastroenterol Hepatol. [DOI] [PubMed] [Google Scholar]
- 10.Purefoy FitzGerald M. Further observations on the changes in the breathing and the blood at various high altitudes. Proc R Soc Lond, B. 1914;88(602):248-258. [Google Scholar]
- 11.Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Haemoglobin adjustments to define anaemia. Trop Med Int Health. 2008;13(10):1267-1271. [DOI] [PubMed] [Google Scholar]
- 12.Gassmann M, Muckenthaler MU. Adaptation of iron requirement to hypoxic conditions at high altitude. J Appl Physiol (1985). 2015;119(12):1432-1440. [DOI] [PubMed] [Google Scholar]
- 13.Marti HH, Wenger RH, Rivas LA, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8(4):666-676. [DOI] [PubMed] [Google Scholar]
- 14.Digicaylioglu M, Bichet S, Marti HH, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92(9):3717-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrenreich H, Kästner A, Weissenborn K, et al. Circulating damage marker profiles support a neuroprotective effect of erythropoietin in ischemic stroke patients. Mol Med. 2011;17(11-12):1306-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm C, Wenzel A, Groszer M, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8(7):718-724. [DOI] [PubMed] [Google Scholar]
- 17.Schuler B, Vogel J, Grenacher B, Jacobs RA, Arras M, Gassmann M. Acute and chronic elevation of erythropoietin in the brain improves exercise performance in mice without inducing erythropoiesis. FASEB J. 2012;26(9):3884-3890. [DOI] [PubMed] [Google Scholar]
- 18.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz AJ, Das NK, Ramakrishnan SK, et al. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J Clin Invest. 2019;129(1):336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.