Abstract
Ovarian cancer is a critically lethal gynecologic malignancy. More than 80% of patients with ovarian cancer have relapses within 5 years after initial treatment. However, recurrence from ovarian cancer more than 20 years later is extremely rare. We report a case of a 67-year-old female with mediastinal metastasis from ovarian cancer 29 years after initial gynecologic surgery and chemotherapy.
Keywords: Ovarian serous carcinoma, Mediastinal metastasis, Late recurrence
1. Introduction
Ovarian cancer is the 7th leading cancer diagnosis and 8th leading cause of cancer mortality among women in the worldwide [1]. Although intraperitoneal route of dissemination is the most common, ovarian cancer may also metastasize through lymphatic path and haematogenic spread. A large retrospective study demonstrated that 87% of patients with ovarian cancer had relapses within 5 years after initial treatment [2]. However, recurrence from ovarian cancer more than 20 years later is extremely rare. Limited literatures demonstrated that the late recurrence occurred at intraperitoneal, retroperitoneum, distant lymph nodes, or thoracic wall [[3], [4], [5]]. We present a case report of a 67-year-old female with mediastinal metastasis from ovarian cancer 29 years after initial gynecologic surgery and chemotherapy.
2. Case presentation
A 67-year-old female was referred to our hospital because of an abnormal shadow on a chest X-ray. The patient underwent radical hysterectomy, omentectomy and ovariectomy, and received adjuvant chemotherapy at age of 38 years. The extensive local resection was performed with a negative surgical margin, but adjuvant chemotherapy was done for stage III of ovarian serous carcinoma. Since no local and distant recurrence were observed, follow-up of outpatient clinic visit was terminated at 5 years after the initial treatment. Then the patient stayed asymptomatic.
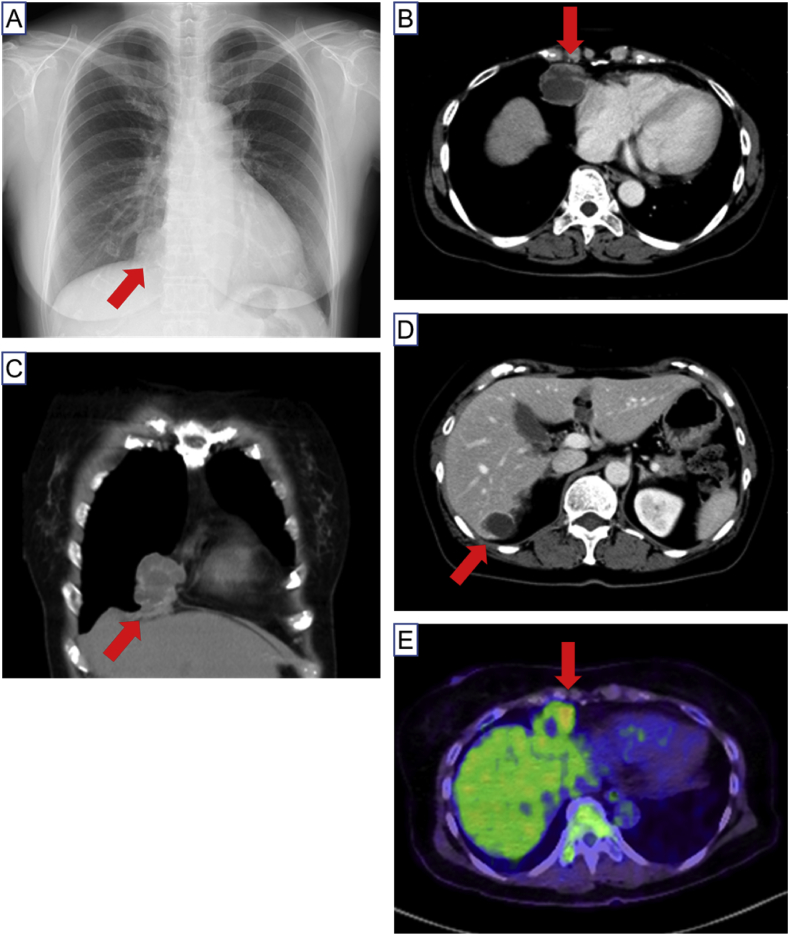
Physical examination of the patient was normal. Chest X-ray showed a sole nodular lesion adjacent to heart in right lower lung field, and contrast-enhanced computed tomography revealed a multiloculated cystic heterogeneous mass in anterior mediastinum and liver (Fig. 1A–D). A positron emission tomography revealed a higher standardized uptake value, ranging 2.6 to 4.2 of maximum value, of cystic wall, suggesting a possibility of malignancy in the lesion (Fig. 1E). The serum marker CA 125 was 33.9 U/mL within normal range. Since bronchoscopic study could not reach the mediastinal mass, the patient underwent resection of the mediastinal tumor by right video-assisted thoracic surgery (VATS).
Fig. 1.
A, Chest X-ray showed a sole nodular lesion (arrow) adjacent to heart in right lower lung fields. B and C, Chest contrast-enhanced computed tomography shows a multiloculated cystic heterogeneous mass (arrow) in anterior mediastinum. D, A multiloculated cystic heterogeneous mass is also shown in liver (arrow). E, A positron emission tomography revealed a higher standardized uptake value of the mediastinal mass (arrow).
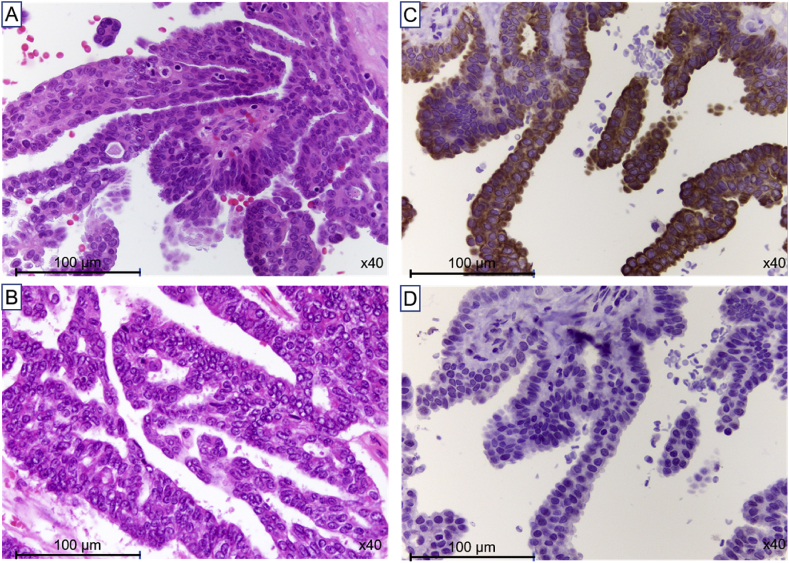
Pathological examination of the specimens from VATS revealed malignant cells lying in a cuboidal and columnar epithelial layer with focal papillary growth and psammoma body (Fig. 2A). Pathological findings of the specimens from ovary of the initial gynecologic surgery matched with those of specimens from VATS (Fig. 2B). On immunohistochemical staining of Cytokeratin (CK) 7 and CK 20, all specimens demonstrated positive immunoreactivity in the present case (Fig. 2C and D). Collectively, these pathological findings suggested that mediastinal metastasis arose from ovarian cancer at 29 years after initial gynecologic surgery and chemotherapy.
Fig. 2.
A, Specimen form video-associated thoracic surgery (VATS) shows papillary structures of atypically cuboidal and columnar epithelial cells and psammoma body. (Hematoxylin and eosin (HE) stain, x40). B, Pathological findings of specimens from ovary of the initial gynecologic surgery match with those of specimens from VATS (HE stain, x40). C and D, Specimens from VATS show positive immunoreactivity of both Cytokeratin (CK) 7 and CK 20 (x40).
Since the patient received chemotherapy with two courses of carboplatin + docetaxel + bevacizumab, subsequently with bevacizumab alone, and still stayed asymptomatic, no overt progressive disease was seen in follow-up CT one year later.
3. Discussion
Ovarian cancer is the most cause of death among women with gynecologic malignancy. Ovarian cancer is also characterized by a propensity for asymptomatic spread in peritoneum and lymphatic system. In fact, more than 75% of patients with ovarian cancer were diagnosed in third or fourth stage because early stage disease was usually asymptomatic and symptoms of late stage disease were nonspecific [6]. Distant metastases may occur at diagnosis of advanced ovarian cancer or may arise throughout the course of disease. A retrospective cohort study of 400 patients with ovarian cancer demonstrated that while 87% of the patients had relapses within 5 years after initial treatment, 8% relapsed at 5–10 years and 6% relapsed at more than 10 years after initial treatment [2].
Despite of the high rate of relapses, recurrence from ovarian cancer more than 20 years later is extremely rare. Limited literatures demonstrated that the late recurrence occurred at intraperitoneal, retroperitoneum, distant lymph nodes, or thoracic wall [[3], [4], [5]] (Table 1). Menczer J et al. reviewed characteristics of five cases of recurrence more than 20 years after initial treatment of ovarian cancer, and demonstrated as follows: younger age than 36 and mostly in stage III at diagnosis, time to recurrence ranged from 21 to 28 years, and histological type of serous carcinoma in those cases [5]. In the present case, characteristics of the patient were almost comparable with the suggestion of Menczer J et al. Although the late recurrence site varied, to our knowledge, the present case may be the first case report to show mediastinal metastasis from ovarian cancer more than 20 years after initial treatment.
Table 1.
Characteristics of recurrence from ovarian cancer more than 20 years later.
| Study | Age | Stage | Time to recurrence (years) | Histological type | Recurrence site |
|---|---|---|---|---|---|
| Zylberberg et al. [3] | 29 | IC | 21 | Serous | Intraperitoneal |
| 33 | IIIC | 21 | Serous | Axillary nodule | |
| 32 | IIIC | 26 | Serous | Para-aortic lymph node | |
| Testelmans et al. [4] | 36 | III | 23 | Serous | Thoracic wall |
| Menczer et al. [5] | 22 | IIIB | 28 | Serous | Retroperitoneal nodule |
| Present case | 38 | III | 29 | Serous | Anterior mediastinum |
Precise mechanism of late recurrence from ovarian cancer is uncertain. The incidence of recurrence is affected by several factors as follows: histological type, stage of progression and degree of tumor differentiation at diagnosis, undergoing of surgical scope, usage of adjuvant therapy, and sensitivity to platinum derivatives [7]. On the other hand, it is hypothesized that late recurrence of ovarian cancer may result from either regrowth of dormant tumor cells or from new cancer caused by field cancerization [5].
Nature of tumor dormancy is unknown. During tumor dormancy, cancer cells survived initial treatment may not be proliferative under an arrest in cell cycle resulting in prolonged G0 phase. The survived cancer cells may persist in patient's body for long time, and potentially initiate regrowth in the future. Late recurrence through tumor dormancy occurs most frequently in cutaneous malignant melanoma and renal cell carcinoma [8]. In preclinical and experimental models, as immunomodulation influenced cell cycle of cancer cells [9], tumor dormancy is, at least in part, considered to be regulated with equilibration between immune system and cancer cell proliferation [10].
Field cancerization is a replacement of normal cell population by cancer-primed cell population resulting from a biological process by carcinogenic alteration, and is now recognized to underlie development of many types of cancer [11]. Initial molecular changes of the biological process, such as genetic mutation and epigenetic change, may subsequently progress to premalignant foci of dysplasia and eventually to carcinoma in situ or cancer. The biological process may arise from exposure to an injurious environment, often over a lengthy period [12]. Buller RE et al. reported late recurrences of ovarian cancer through field cancerization. The authors demonstrated that 77% of tumor cells from the late recurrences had different genotypes of those from the original tumor of ovarian cancer, suggesting a possibility of carcinogenesis through field cancerization [13].
Curative treatment of late recurrence from ovarian cancer is unlikely. Secondary debulking may be indicated in selected patients with late recurrence from ovarian cancer. If the recurrent disease is located, a good performance is present, and response to first-line therapy occurred in the patients, secondary debulking surgery (i.e. cytoreduction) followed by combination chemotherapy is recommended [14]. A retrospective cohort study of 123 patients with recurrent ovarian cancer demonstrated that complete secondary cytoreduction was the strongest survival predictor of the whole course [15]. Thus, the patient may stay in curative course because she underwent secondary debulking surgery with a negative surgical margin in the present case.
4. Conclusion
We present here a case report of 67-year-old female with mediastinal metastasis from ovarian cancer 29 years after initial gynecologic surgery and chemotherapy. Although recurrence from ovarian cancer more than 20 years later is extremely rare, regular long-term monitoring may be, in some, necessary for the patients after gynecologic surgery and chemotherapy. Pulmonologist need differential diagnoses of late recurrence from ovarian cancer for abnormality on chest X-ray and computed tomography when seeing patients undergone gynecologic surgery and chemotherapy in the past.
Declaration of competing interest
The authors have no conflict of interest to declare. No fund supports the present case report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101003.
Contributor Information
Eri Inoue, Email: inoue.eri.rq@mail.hosp.go.jp.
Takuma Yotsumoto, Email: tyotsumoto-ths@umin.ac.jp.
Yuta Inoue, Email: inoue.yuta.ry@mail.hosp.go.jp.
Takeshi Fukami, Email: fukami.takeshi.ej@mail.hosp.go.jp.
Masashi Kitani, Email: kitani.masashi.ge@mail.hosp.go.jp.
Yuta Hirano, Email: hirano.yuta.gx@mail.hosp.go.jp.
Maki Nagase, Email: nagase.maki.sy@mail.hosp.go.jp.
Yoshiteru Morio, Email: morio.yoshiteru.pn@mail.hosp.go.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Coburn S.B., Bray F., Sherman M.E. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Canc. 2017;140:2451–2460. doi: 10.1002/ijc.30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahdev A., Hughes J.H., Barwick T. Computed tomography features of recurrent ovarian carcinoma according to time to relapse. Acta Radiol. 2007;48:1038–1044. doi: 10.1080/02841850701557255. [DOI] [PubMed] [Google Scholar]
- 3.Zylberberg B., Dormont D., Madelenat P. Relapse after more than 20 years of follow-up for epithelial ovarian carcinoma. Obstet. Gynecol. 2004;103:1082–1084. doi: 10.1097/01.AOG.0000114990.34357.b0. [DOI] [PubMed] [Google Scholar]
- 4.Testelmans D., Van Raemdonck D., Amant F. Late recurrent ovarian carcinoma metastatic to the thoracic wall. Acta Clin. Belg. 2010;65:354–356. doi: 10.1179/acb.2010.076. [DOI] [PubMed] [Google Scholar]
- 5.Menczer J., Schreiber L., Peled O. Very late recurrence (after more than 20 years) of epithelial ovarian carcinoma: case report and literature review. Arch. Gynecol. Obstet. 2015;291:1199–1203. doi: 10.1007/s00404-014-3597-6. [DOI] [PubMed] [Google Scholar]
- 6.Doubeni C.A., Doubeni A.R., Myers A.E. Diagnosis and management of ovarian cancer. Am. Fam. Physician. 2016;93:937–944. [PubMed] [Google Scholar]
- 7.Iżycka N., Lubin J., Markowska A. Late recurrence of ovarian cancer: a literature review and description of two cases. Eur. J. Gynaecol. Oncol. 2015;36:351–353. [PubMed] [Google Scholar]
- 8.Friberg S., Nyström A. Cancer metastases: early dissemination and late recurrences. Canc. Growth Metastasis. 2015;8:43–49. doi: 10.4137/CGM.S31244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y.Z., Chang H., Yu Y. Thymopentin (TP5), an immunomodulatory peptide, suppresses proliferation and induces differentiation in HL-60 cells. Biochim. Biophys. Acta. 2006;1763:1059–1066. doi: 10.1016/j.bbamcr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Al-Tameemi M., Chaplain M., d'Onofrio A. Evasion of tumours from the control of the immune system: consequences of brief encounters. Biol. Direct. 2012;7:31. doi: 10.1186/1745-6150-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtius K., Wright N.A., Graham T.A. An evolutionary perspective on field cancerization. Nat. Rev. Canc. 2018;18:19–32. doi: 10.1038/nrc.2017.102. [DOI] [PubMed] [Google Scholar]
- 12.Nonn L., Ananthanarayanan V., Gann P.H. Evidence for field cancerization of the prostate. Prostate. 2009;69:1470–1479. doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buller R.E., Skilling J.S., Sood A.K. Field cancerization: why late "recurrent" ovarian cancer is not recurrent. Am. J. Obstet. Gynecol. 1998;178:641–649. doi: 10.1016/s0002-9378(98)70473-9. [DOI] [PubMed] [Google Scholar]
- 14.Chi D.S., McCaughty K., Diaz J.P. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933–1939. doi: 10.1002/cncr.21845. [DOI] [PubMed] [Google Scholar]
- 15.Tian W.J., Jiang R., Cheng X. Surgery in recurrent epithelial ovarian cancer: benefits on Survival for patients with residual disease of 0.1-1 cm after secondary cytoreduction. J. Surg. Oncol. 2010;101:244–250. doi: 10.1002/jso.21491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.