Abstract
Background
Intestinal tuberculosis (ITB) is a fraction of extrapulmonary TB, and its diagnosis often pose a significant challenge due to nonspecific presentation. Several methods have been utilized to diagnosed ITB, including findings of specific inflammatory process on histopathological examination. We hereby report three cases of ITB that manifested as caecal and adnexal mass.
Case report
First case, a 22-year-old male, presenting with abdominal pain, underwent exploratory laparotomy, biopsy, right hemicolectomy, and anastomosis end-to-side to the transverse ileocolical region due to partial ileus obstruction from caecal tumor. The second and third cases, a 27-year-old and 39-year-old females, both presenting with abdominal pain and distension, underwent exploratory laparotomy, adhesiolysis and biopsy. Histopathological examination in all three cases showed chronic granulomatous inflammation caused by TB. All three patients were diagnosed as ITB and received 6 months of anti-tuberculosis drug (ATD).
Discussion
Intestinal TB most commonly affected region is the ileocaecal, accounts for 64% of the incidence of gastrointestinal TB. The main reasons for the predilection of ileocaecal region are due to relatively longer faecal static, the abundant of lymphoid tissue, a neutral pH environment and absorptive transport mechanisms that allow swallowed mycobacterium to be absorbed. Intestinal TB may pose similar symptoms as those found in pulmonary TB, yet patients most commonly presenting with abdominal pain. Bacteriological signs and histopathological findings are gold standard for ITB diagnosis. Therapy for ITB includes pharmacological ATD and surgical therapy.
Keywords: Intestinal, Extrapulmonary, Tuberculosis
1. Introduction
Tuberculosis (TB) typically affects the lungs, but may involve any other sites which referred as extrapulmonary TB. Extrapulmonary TB represented 14% of the 6.4 million incident cases worldwide that were notified in 2017, and 15% in South-East Asia [1]. As a fraction of extrapulmonary TB that manifest in gastrointestinal tract, intestinal TB (ITB) accounts for 2% of TB cases globally [2].
Diagnosis of ITB often pose a significant challenge, patients frequently suffer from delays in diagnosis and initiation of anti-tuberculosis drug (ATD) leading to high morbidity and mortality. Several methods have been utilized to diagnosed ITB, including findings of specific inflammatory process on histopathological examination that may consider as a practical methods. A more advanced method such as polymerase chain reaction (PCR) analyses of mucosal biopsy specimens from endoscopy have been reported to be a valuable tool, with high specificity, 95% [[3], [4], [5]]. The PCR has also been found to be more sensitive than acid-fast bacilli (AFB) stain and Mycobacterial tuberculosis culture [4,5]. Recent study on various TB patients in Makassar, Indonesia found that multiplex PCR should be suitable for a rapid and correct diagnosis of patients suspected of having mycobacterial disease [5]. Regardless of the progress in diagnosis of ITB, numerous cases may still be misdiagnosed or overlooked due to nonspecific presentation. We hereby report three cases of ITB that manifested as caecal and adnexal mass.
2. First case
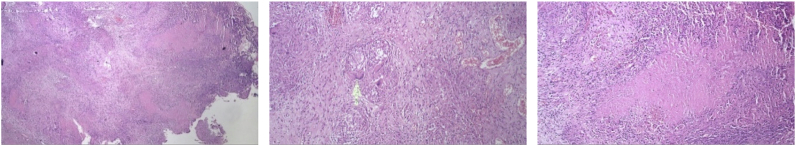
A 22-year-old male underwent exploratory laparotomy after presenting with abdominal pain, fever, anorexia, night sweats, and weight loss, over one month (Fig. 1). He provided no history of close contact with TB patients, although he had history of chronic recurrent cough. This information raised a high suspicion of TB. A diagnosis of small bowel obstruction secondary to a caecal mass was made and histopathology of omental biopsy confirmed a chronic granulomatous inflammation caused by TB without any signs of malignancy (Fig. 2). He underwent a right hemicolectomy and anastomosis end-to-side to the transverse ileocolical region. The physical examination on subsequent presentation showed no adventitious breath sounds, an abdominal scar from the laparotomy, without organomegaly. However, infiltrates in both apices was shown in his chest x-ray, and an abdominal CT-scan prior his surgery was remarkable for signs of bowel obstruction. He was anemic with a haemoglobin of 9.4 g%. Sputum gram-stain revealed positive results of AFB and Diplococcus. He was then diagnosed with pulmonary and intestinal TB, therefore treated with 6 months of ATD.
Fig. 1.
Patient profile of first case.
Fig. 2.
Histopathological findings on first case.
3. Second case
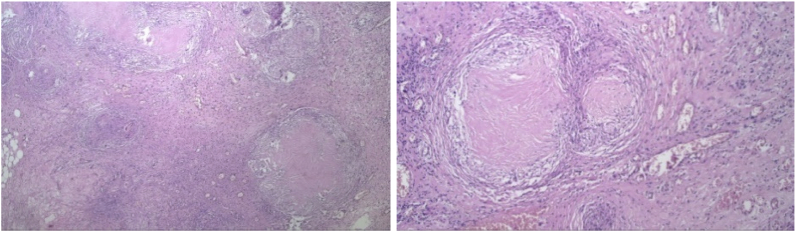
A 27-year-old female underwent exploratory laparotomy after presenting with one-month history of continuous abdominal pain and distension, accompanied by fever, night sweat, and significant weight loss. She had a history of close contact with active TB patient, which completed ATD over 10 years before. This information ultimately raised a suspicion of TB. She underwent an adhesiolysis of sigmoid and fallopian tubes, also omental biopsy. The physical examination on subsequent presentation showed normal breath sounds, an abdominal scar from the laparotomy, without organomegaly (Fig. 3). Her chest x-ray showed minimal pleural effusion on right hemithorax, and abdominal ultrasonography revealed right ovarian mass with signs of malignant ascites. Whole abdominal CT-scan before her surgery confirmed a mass on right adnexal with signs of malignant ascites. She also had a high serum level of Ca-125 > 600 IU/mL (reference range from 0 to 35 IU/mL). Nonetheless, histopathology of omental biopsy confirmed a chronic granulomatous inflammation caused by TB without signs of malignancy (Fig. 4). She was then diagnosed with ITB and received 6 months of ATD.
Fig. 3.
Patient profile of second case.
Fig. 4.
Histopathological findings on second case.
4. Third case
A 38-year-old female underwent exploratory laparotomy after presenting with two-months history of abdominal distension and intermittent pain, along with constipation, anorexia, and significant weight loss (Fig. 5). She provided no history of close contact with TB patient. She underwent an adhesiolysis of intestinal and omental, also peritoneal biopsy which was confirmed a chronic granulomatous inflammation caused by TB without any signs of malignancy (Fig. 6). The physical examination on subsequent presentation showed no adventitious breath sounds, an abdominal scar from the laparotomy, without organomegaly. Her chest x-ray was showed no abnormalities, while abdominal ultrasonography revealed adnexal cystic mass, and minimal ascites. However, her whole abdominal CT-scan prior surgery just confirmed the signs of malignant ascites. She was slight anemia with a haemoglobin of 9.8 g%, and her serum level of Ca-125 was slightly increase 74.48 IU/mL. She was then diagnosed with ITB and received 6 months of ATD.
Fig. 5.
Patient profile of third case.
Fig. 6.
Histopathological findings on third case.
5. Discussion
Intestinal TB may pose similar clinical symptoms as those found in pulmonary TB, which includes fever, significant weight loss, anorexia, and night sweats [6]. Our three patients in this cases report presenting with such symptoms.
The postulated mechanisms by which the mycobacterium reach the gastrointestinal tract are: (1) ingestion of bacilli in sputum from active pulmonary focus; (2) hematogenous or lymphatic spread from a primary lung focus, with later reactivation; (3) direct spread from adjacent organs; and (4) ingestion of milk products infected with Mycobacterium bovis. [4,7] In the first case which presented with specific findings on chest x-ray and positive sputum AFB, it was possible due to ingestion of infected sputum from active pulmonary focus. In the second case, which has a history of close contact with active TB patients, maybe due to hematogenous or lymphatic spread from a primary lung focus, with later reactivation. As for the third case, TB spread mechanism remains unclear.
Interestingly, apart from the extensive evidence of the involvement of pulmonary TB as the entry pathway to gastrointestinal TB, chest x-ray showed abnormality findings in only 25% of the patients, and among them only 14.3% have positive sputum AFB [8]. Unfortunately, the mechanism regarding this discrepancy is scarce.
Intestinal TB most commonly affected region is the ileocaecal, accounts for 64% of the incidence of gastrointestinal TB, followed by jejunum and large bowel. The main reasons for the predilection of ileocaecal region are due to a relatively longer faecal static, high density of lymphoid tissue, a neutral pH environment and absorptive transport mechanisms that allow swallowed mycobacterium to be absorbed [6].
The manifestations of ITB can be divided into three categories: ulcerative form (60%), hypertrophic form (10%) and lesions such as mass (ulcero-hypertrophic, 30%) that mimic malignancies leading to a common misdiagnosis. Manifestations depend on host's immune system. The ulcerative forms occur in those with reduced immune responses, whereas the hyperthrophic form occurs in those with an enhanced immune system. Manifestations may be nonspecific and show similarities to other gastrointestinal disorders, such as Crohn's disease, peptic ulcer, malignancy, sarcoidosis, fungal infections or idiopathic granulomatous gastritis [9,10]. In this cases report, the patients were all mistakenly diagnosed as a malignancy process, until proven to be ITB by histopathological findings which may be consider as a practical diagnosis method.
Because TB can affect any part of the gastrointestinal tract, the presenting symptoms often vary depending on the affected anatomic location of the disease. Based on study by Tripathi et al., patients most commonly presented with abdominal pain, fever, weight loss regardless of the anatomical involvement [7]. In accordance with this, review by Choi and Coyle showed that the most common symptoms were abdominal pain (81%), followed by weight loss (62%), fever (51%), nausea and vomiting (42%), diarrhea (29%), and constipation (22%) based on numerous studies [4]. In the first patient there were symptoms of abdominal pain and bowel obstruction. The second and third patients there were an abdominal enlargement with palpable mass. Based on history taking, all three cases were a new case of TB. This finding is consistent with other TB studies, including one that conducted in Makassar, Indonesia, among 225 TB patients with and without diabetes mellitus, the majority of patients were a new case of TB [11]. This is a reflection of Indonesia as one of high TB burden countries in the world.
Bacteriological signs and histopathological findings are gold standard to establish ITB. Sharma et al. studied 70 cases of abdominal TB and found evidence of active or cured lesions in chest X-ray in 22 cases (46%). Biopsy methods include endoscopy, gastrointestinal mucosal biopsy, percutaneous biopsy, guided endoscopic ultrasound biopsy, and surgery (open or laparoscopic). In all three of these patients a biopsy was performed and histopathological examination confirmed a chronic granulomatous inflammation with caseous necrosis caused by TB. In ITB, granulomas are presented as larger amount and larger size (>200 μm) in the mucosa and submucosa. Positive PCR and ascites fluid tests were found in 72% and 87.5% of ITB patients [[12], [13], [14]]. Among bacteriological examination, PCR analyses of biopsy specimens have been shown to be a valuable tool in improving diagnostic yield, with a high specificity, 95%. It has also been found to be more sensitive than AFB stain and Mycobacterium tuberculosis culture [4]. However, drawback of PCR utilization is the uncommon availability, notably on developing countries such as Indonesia.
Therapy for ITB includes pharmacological anti-tuberculosis drugs and surgical therapy. The first choice for ITB management is anti-tuberculosis drugs. When patients are suspected of ITB, ATD can be given in a full dose. Drugs administration is the same as for pulmonary TB. Conventional antituberculosis therapy for at least 6 months including the initial two months combination of isoniazid, rifampicin, ethambutol and pyrazinamide followed by four months combination of isoniazid and rifampicin, and this therapy is recommended in all patients with ITB in Indonesia [13]. Although there are some controversies where some clinicians treat for longer periods due to concerns that six months is inadequate to achieve cure and prevent relapse of the disease after the end of treatment, recent review from Cochrane found that six-month and nine-month regimens are probably similarly effective in terms of the chances of achieving cure and no evidence to suggest that six-month regimens are less safe for gastrointestinal and peritoneal TB than nine-month regimens [14]. In all three cases, after 6 months therapy with ATD demonstrated significant clinical improvement. Specifically for the first case, improvement also be found in negative conversion of sputum smear.
Alongside pharmacological therapy, surgery is the second choice for complications such as free perforation, significant bleeding, complete obstruction, abscess formation, large fistulae, and refractory to antimicrobial drugs. Obstruction is the most common complication; patients with multiple and/or long strictures are less likely to respond to medical therapy. Colonoscopic balloon dilation, which shown may become one of the alternatives, may be used to manage readily accessible, short and fibrous tuberculous ileal strictures causing subacute obstructive symptoms. Although the experience is very limited, this technique appears safe and may obviate the need for surgery [15]. In our cases report, surgeries were performed prior to a diagnosis of ITB, due to suspicions of malignancy. Nevertheless, each surgery helped to establish ITB diagnosis.
6. Conclusion
Nonspecific clinical manifestations mimic some other gastrointestinal conditions including malignancy may pose a significant challenge in ITB diagnosis. Histopathological findings could help to confirm bacteriological signs and associated tissue changes, therefore guiding the clinicians in establishing accurate diagnosis and prescribing proper management and therapy.
Contributor Information
Irawaty Djaharuddin, Email: irawatymuzakkir@gmail.com.
Mochammad Hatta, Email: hattaram@yahoo.com.
Nur Ahmad Tabri, Email: nurahmad_59@yahoo.com.
Eliana Muis, Email: elianamuis@gmail.com.
Safriadi Safriadi, Email: safriadi.musa@gmail.com.
Muhammad Reza Primaguna, Email: rezzprima@yahoo.com.
References
- 1.WHO . World Health Organization; Geneva: 2018. Global Tuberculosis Report 2018.https://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Miah A.R. Clinicopathological profile of patients with abdominal tuberculosis. J Nepal Health Res Counc. 2011;9(2):169–175. PMID: 22929848. [PubMed] [Google Scholar]
- 3.Parmer J., Allen L., Walton W.C.E. Tuberculosis: a new screening recommendation and an expanded approach to elimination in the United States. Am. J. Nurs. 2017;117(8):24–34. doi: 10.1097/01.NAJ.0000521946.45448.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi E.H., Coyle W.J. Gastrointestinal tuberculosis. Microbiol. Spectr. 2016;4(6) doi: 10.1128/microbiolspec.TNMI7-0014-2016. [DOI] [PubMed] [Google Scholar]
- 5.Hatta M., Sultan A.R. Detection and identification of mycobacteria in sputum from suspected tuberculosis patients. BMC Res. Notes. 2010;3:72. doi: 10.1186/1756-0500-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debi U. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J. Gastroenterol. 2014;20(40):14831–14840. doi: 10.3748/wjg.v20.i40.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripathi P.B., Amarapurkar A.D. Morphological spectrum of gastrointestinal tuberculosis. Trop. Gastroenterol. 2009;30(1):35–39. PMID: 19624086. [PubMed] [Google Scholar]
- 8.Rathi P., Gambhire P. Abdominal tuberculosis. J. Assoc. Phys. India. 2016;64(2):38–47. PMID: 27730779. [PubMed] [Google Scholar]
- 9.Chong V.H., Lim K.S. Gastrointestinal tuberculosis. Singap. Med. J. 2009;50(6):638–645. quiz 646. PMID: 19551320. [PubMed] [Google Scholar]
- 10.Niitsu H., Tanabe K., Tokumoto N. Idiopathic granulomatous gastritis resembling a gastrointestinal stromal tumor. Case Rep Gastroenterol. 2012;6(2):502–509. doi: 10.1159/000341806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fachri M., Hatta M., Abadi S. Comparison of acid fast bacilli smear for Mycobacterium tuberculosis on adult pulmonary tuberculosis patients with type 2 diabetes mellitus and without type 2 diabetes mellitus. Resp Med Case Report. 2018;23:158–162. doi: 10.1016/j.rmcr.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weledji E.P., Pokam B.T. Abdominal tuberculosis: is there a role for surgery? World J. Gastrointest. Surg. 2017;9(8):174–181. doi: 10.4240/wjgs.v9.i8.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma M.P., Bhatia V. Abdominal tuberculosis. Indian J. Med. Res. 2004;120(4):305–315. PMID: 15520484. [PubMed] [Google Scholar]
- 14.Indonesia M.o.H.R.o., editor. Regulation of the Minister of Health Republic of Indonesia No.67. 2016. http://hukor.kemkes.go.id/uploads/produk_hukum/PMK_No._67_ttg_Penanggulangan_Tuberkulosis_.pdf [Google Scholar]
- 15.Jullien S. Six-month therapy for abdominal tuberculosis. Cochrane Database Syst. Rev. 2016;11:CD012163. doi: 10.1002/14651858.CD012163.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]