Abstract
Isolated hepatocytes can be obtained from the liver by collagenase infusion, a procedure that could affect cell isolation as well as the integrity of membrane receptors. In this respect we compared metabotropic glutamate subtype 5 receptor (mGluR5) protein expression and activity in rat hepatocytes isolated by two collagenases, type I and type IV. Hepatocytes were isolated from male Wistar rats (200-250 g) using collagenase I or collagenase IV and after isolation, viability and morphology of rat hepatocytes were assessed measuring mGluR5 protein expression by Western blot analyses. mGluR5 activation was evaluated by inositol-1-phosphate (IP-1) accumulation after treatment with the mGluR5 orthosteric agonist ACPD or the selective antagonist MPEP. No difference in cellular viability and morphology was observed using collagenase I when compared with collagenase IV. An increase in mGluR5 protein expression was observed in hepatocytes isolated using collagenase IV with respect to collagenase I. Moreover, hepatocytes treated with ACPD and with MPEP presented higher levels of IP-1 when isolated using collagenase IV compared to collagenase I. These results indicate that collagenase IV better preserves the activity of surface proteins such as mGluR5 in isolated rat hepatocytes, an in vitro model useful to reduce the use of experimental animals, in line with the 3R ethical framework.
Key words: Collagenase, isolated hepatocytes, mGluR5, IP-1
Introduction
Isolated hepatocytes are an important model for studying cellular metabolism, pharmacokinetics and toxicology of xenobiotics, liver regeneration and translational research. The process of cell isolation involves the infusion with collagenase from Clostridium hystoliticum.1 Collagenase, being multi-modular zinc-metalloproteinase, cleaves the triple α-helical amino acid chain structure of native collagen, which characterizes up to 90% of the extracellular matrix of a tissue.2,3 When collagen is degraded, the integrity of tissue membranes is lost and cells can be isolated. Seven types of clostridial collagenases have been identified to belong to Class I or Class II, according to their respective gelatinase or peptidase activity. 2 It is known that a specific collagenase type is needed for each tissue. For example, type II collagenase is suitable for mouse cardiomyocytes, 4 while for rat pancreatic acinar cells the best collagenase is type III.5 Collagenase IV was recently used for the isolation of highly viable thymic epithelial cells.6 For liver investigations, type I is the collagenase of choice because the majority of pharmacological studies on isolated hepatocytes are aimed to evaluate the function of intracellular cytochromes.7
As part of the scientific community, following the publication of the book by Russell and Burch in 1959 “The Principles of Humane Experimental Technique”, we now fully adopted what is known as the principle of the “Three Rs: Replacement, Refinement and Reduction”.8 According to these ethical guidelines, animals should be used only when it is not possible to replace them with inanimate models, such as in silico research, or with the employment of in vitro models. Moreover, if animal models are needed, their number should be minimized to maximize information and obtain sufficient data to satisfy the research question. Finally, “refinement” refers to the adoption of husbandry methods and experimental procedures aimed at reducing as much as possible animal suffering and pain, from the moment of birth to the sacrifice. 9 For liver investigation usually focusing on the function of intracellular cytochromes, type I is the collagenase of choice. We hypothesized that the infusion of collagenase could partially affect the integrity and function of metabotropic glutamate receptor subtype 5 (mGluR5).10,11 The mGluR5, together with mGluR1, belongs to the mGluR family group I. Coupled to Gq proteins, it activates phospholipase C, which in turn hydrolyzes phosphatidylinositol 4,5-bisphosphate to inositol trisphosphate (IP-3) and diacylglycerol, leading to calcium release from intracellular stores and kinases activation.10 It has been demonstrated by Storto and colleagues that mGluR5 is expressed on the surface of primary rat hepatocytes and that the receptor activation through agonist administration worsens hepatocytes damage when they are exposed to ischemic conditions.12 On the contrary, the receptor blockade protects cells against hypoxic injury12 and plays a crucial role in the reduction of the inflammatory process in different experimental models, such as in rats intoxicated by paracetamol,13 in parkinsonian rats11 and in ex vivo models of hepatic cold and warm ischemia.14
Our goal was to find the best method to isolate primary rat hepatocytes preserving both the mGluR5 integrity and its downstream activity and, according to this aim, we tested two collagenase types, collagenase I versus collagenase IV. In this way, the Three Rs principle could also be satisfied.
Materials and Methods
Materials
Collagenase I and collagenase IV were purchased from Worthington Biochemical Corporation (Lakewood, NJ, USA). The mouse monoclonal anti-Tubulin was acquired at Sigma Aldrich (Milan, Italy), while the rabbit polyclonal anti-mGluR5 at EMD Millipore (Burlington, MA, USA). IP-One ELISA was provided by Cisbio (Codolet, France). Male Wistar rats were from Charles River Laboratories (Lecco, Italy).All other reagents were purchased from Sigma Aldrich (Milan, Italy). 1-Amino-1,3-dicarboxycyclopentane (ACPD) and 2-Methyl-6-(phenylethynyl)pyridine (MPEP) were purchased from Tocris Bioscience (Bristol, UK).
Hepatocyte isolation
Hepatocytes were isolated from male Wistar rats (200-250 g) (n=8). Animals had free access to food and water. The use and care of animals in this experimental study was approved by the Italian Ministry of Health. Rats were anesthetized intraperitoneally using 500 μL of pentobarbital (25 mg/mL dissolved in sodium chloride solution). The peritoneal cavity was open and the hepatic portal vein was cannulated as described by Ferrigno and colleagues.15 Hepatocytes were isolated by collagenase perfusion of the liver as previously described.14 Liver cells were layered on the top of a 36% Percoll suspension and centrifuged at 100 g for 1 min. The pellets containing hepatocytes were suspended in KRH buffer (NaCl 114.9 mM, KCl 4.98 mM, KH2PO4 0.94 mM, MgSO4 ・ 7 H2O 1.2 mM, HEPES 25 mM) and incubated in rotavapor at 37°C for 30 min (final cell density: 2x106/mL). Afterward, cells were seeded in 96-well plates, as described below, or were treated for western blotting analysis.
Hepatocyte viability
After cell isolation, hepatocyte viability was assessed by Trypan Blue exclusion method.14 Briefly, 20 μL of cell suspension was added to a solution containing 160 μL of PBS and 20 μL of Trypan Blue and counted using a Burker chamber and an optical microscope (Nikon Ys). The viability of cells was calculated as the percentage of live cells.
Cell culture
Ninety six-well plates were coated with 100 μL Poly-L-Lysine 0.01% (from Sigma Aldrich). After 5 minutes Poly-L-Lysine was removed, wells were rinsed with ultrapure water. Isolated rat hepatocytes were seeded at 80,000 in 200 μL of William’s Medium (Sigma Aldrich) supplemented with 2.2 g/L sodium bicarbonate and incubated overnight at 37°C at 5% CO2.
Inositol-1-phosphate assay
IP-1 accumulation was evaluated using an ELISA kit. The cells were treated according to the manufacturer’s instructions. To trigger the activation of inositol phosphates, cells were pharmacologically treated to stimulate or inhibit the mGluR5, using the agonist ACPD at 10 μM or the negative allosteric modulator MPEP at 30 μM. Inositol 1 phosphate (IP-1) accumulation occurred as a result of LiCl-mediated inhibition of inositol monophosphatase.16
Western blotting analysis
Cells were centrifuged at 50 g for 30 s, then lysed in mammalian cell lysis reagent plus 10 μL/mL of protease inhibitor cocktail and kept on ice for 10 min. Successively, cell lysates were centrifuged at 13,000 g for 15 min and after Lowry protein assay, the supernatant protein content was brought to 2 μg/μL in SDS-bromophenol blue and 2% β-mercaptoethanol. Samples were boiled at 96°C for three min and centrifuged at 13,000 rpm for 30 s. Forty μg of proteins were loaded for each sample and gel electrophoresis was performed onto 7.5% SDS polyacrylamide gels. Gels were electroblotted for 2 h onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA) and then PVDF membranes were blocked in tris-buffered saline buffer containing 5% bovine serum albumin for 2 h at 4°C. Blots were then incubated at 4°C overnight with primary antibodies all at 1:1000 dilution. The following primary antibodies were used: mouse monoclonal anti-Tubulin; rabbit polyclonal anti-mGluR5. Then, blots were incubated for 1 h with peroxidase-coupled secondary antibodies anti-mouse and anti-rabbit, respectively, diluted 1:2000 in PBS. Immunostaining was revealed by ECL using a Chemidoc XRS Plus (Biorad Laboratories). The signal intensity of the immunoreactive bands was quantified using Image Lab software (Bio-Rad Laboratories), both for mGluR5 and for its respective tubulin, used to check for equal loading. The ratio between the quantification of the mGluR5 and tubulin intensity bands was then calculated, obtaining the normalized receptor expression values.
Statistics
Differences between groups were tested by Student’s independent group t-test. Results are expressed as means value ± tandard error (SE). The value of P<0.05 was considered the criterion for statistical significance. Statistical analysis was performed using MedCalc Statistical Software version 18.11.3 (MedCalc Software bvba, Ostend, Belgium, 2019; https://www.medcalc.org).
Results
Hepatocyte viability
Cell viability was measured after isolation of rat hepatocytes and no significant difference was detected using both collagenases: high cell viability was found (Figure 1A). The number of hepatocytes was comparable using collagenase I or collagenase IV (Figure 1B). The morphology of hepatocytes isolated by digestion of collagenase I or IV was similar: the cells appeared round and with well-defined contours. Only a limited number of blebs were observed but they were reabsorbed following the recovery period on rotavapor (Figure 2).
Metabotropic glutamate receptor type 5 (mGluR5) protein expression
Isolated rat hepatocytes obtained by digestion with collagenase IV showed a higher mGluR5 protein expression, with respect to cells isolated using collagenase I (Figure 3).
IP-1 production
Since the activation of mGluR5 triggers the accumulation of inositol phosphates within the cells, we also assessed the amount of functioning mGluR5 by measuring the accumulation of inositol monophosphates after mGluR5 activation, by the orthosteric agonist ACPD, or mGluR5 blockade, using the negative allosteric modulator MPEP. Hepatocytes treated with ACPD 10 μM and isolated using collagenase I showed a lower amount of IP-1 when compared to hepatocytes isolated by collagenase IV. By blocking the receptor with MPEP 30 μM, the concentration of IP-1 was lower in cells obtained by collagenase I digestion when compared with collagenase IV isolated hepatocytes (Figure 4).
Discussion
This study documented the superiority of collagenase IV over collagenase I when primary rat hepatocytes are used for pharmacological studies such as membrane receptor modulation. Both collagenases produced cells with similar morphology and viability but they affect in different ways surface protein expression and function. In fact, collagenase I, containing different lytic enzymes, such as collagenase, caseinase, clostripain, is suitable for liver dissociation, since it presents stronger enzymatic activity respect to collagenase IV.17 On the contrary, the low tryptic activity of collagenase IV is supposed to affect in a limited way transmembrane proteins, preserving their integrity.17 To verify this hypothesis, we evaluated the mGluR5 protein expression and function in primary rat isolated hepatocytes. Cells isolated by digestion with collagenase IV showed a higher mGluR5 protein expression when compared with cells isolated by using collagenase I. The effective maintenance of mGluR5 functioning in isolated hepatocytes was tested by IP-1 production in cells isolated by the two collagenase types. After 10 μM ACPD stimulation of mGluR5, higher production of IP-1 in collagenase IV isolated cells was observed, when compared with collagenase I isolated hepatocytes.
Figure 1.
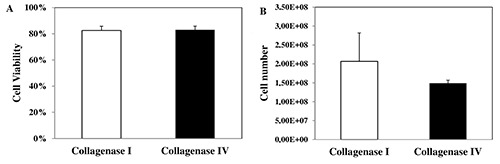
Hepatocyte viability and cell number in cell suspensions using collagenase I or collagenase IV. A) No differences in cell viability were found between the two groups. B) The number of rat hepatocytes isolated by collagenase I or collagenase IV was comparable. Results are expressed as means value ± SE.
Figure 2.
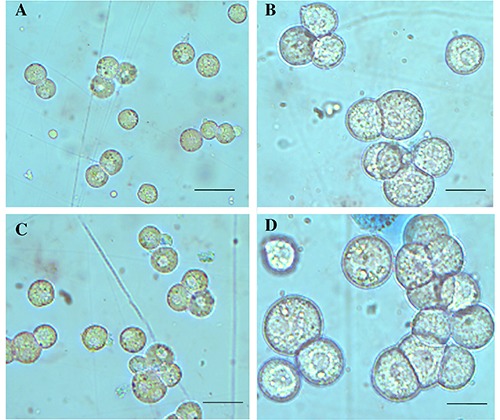
Morphological appearance of isolated hepatocytes. No morphological changes were observed in cells isolated by collagenase I (A,B) versus collagenase IV (C,D). Scale bars: A,C) 40 μm; B,D) 20 μm.
Figure 3.
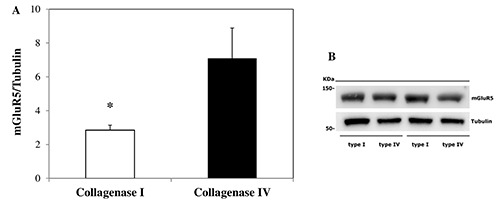
Metabotropic glutamate receptor type 5 (mGluR5) protein expression. A) The image analysis software Image Lab (Biorad Laboratories) was used for the quantification of immunoreactive bands of mGluR5 and Tubulin; mGluR5 expression values were presented as mGluR5/Tubulin ratio; higher mGluR5 protein expression was found in rat hepatocytes isolated by collagenase IV, when compared to cells digested by collagenase I. B) Immunoreactive bands of mGluR5 in isolated primary rat hepatocytes by digestion with collagenase I and collagenase IV. Results are expressed as means value ± SE. *P<0.05 obtained employing Student's independent group t-test.
Figure 4.
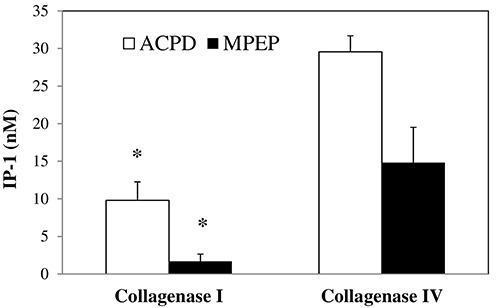
IP-1 accumulation within the hepatocytes. The use of collagenase I resulted in a reduced IP-1 concentration in 10 mM-ACPDstimulated rat hepatocytes as compared to collagenase IV group, as well as for IP-1 accumulation using the antagonist MPEP at 30 mM. Results are expressed as means value ± SE. *P<0.05 obtained by means of Student's independent group t-test.
The blockade of the receptor by the allosteric antagonist MPEP 30 μM, and the consequent reduction of IP-1 concentration, was higher in collagenase IV-isolated hepatocytes. Our data confirm and reinforce the idea that specific collagenase types can act differently, preserving in a better way membrane-associated proteins, like mGlu5 receptor
In compliance with the Three Rs guidelines for animal welfare, scientists must replace the use of animals with other techniques, reduce the number of animals used and refine the way experiments are carried out, to reduce animal suffering.9 Thus, in this perspective, our study was focused on the setup of rat hepatocyte isolation to obtain suitable cells for several assays, including those related to membrane receptor function, minimizing the number of animal sacrifices. Moreover, by the comparison of two different collagenases, our work has clarified that the choice of the correct type of this enzyme is fundamental for the successful outcomes of experiments. The enzymatic activity of bacterial collagenases, extracted as a poorly purified mixture of enzymes, could significantly change within the different commercialized lots, affecting the quality and the number of isolated cells.18
Therefore, the choice of one collagenase over another is very crucial depending on the type of investigation that will be carried out on the isolated hepatocytes obtained. In studies where a large number of cells is requested or performed to evaluate intracellular proteins, type I collagenase is the gold standard.7 On the contrary, when both the integrity of surface and transmembrane proteins and their downstream signaling are requested, the use of more gentle collagenases that preserve the functionality of membrane receptors is more suitable, as demonstrated by our results on mGluR5. In this way, it is feasible to minimize the number of experimental animals, accomplishing also the Three Rs principle.
Acknowledgements
This work was carried out using the internal resources of the Department of Internal Medicine and Therapeutics, Cellular and Molecular Pharmacology and Toxicology Unit. We thank Mr. Massimo Costa for his skillful technical assistance, Mrs. Nicoletta Breda for her editing assistance and Micaela Ascoli for her administrative support.
References
- 1.Williams SK, Mckenney S, Jarrell BE. Collagenase lot selection and purification for adipose tissue digestion. Cell Transplant 1995;4:281-9. [DOI] [PubMed] [Google Scholar]
- 2.Green ML, Breite AG, Beechler CA, Dwulet FE, Mccarthy RC. Effectiveness of different molecular forms of C. histolyticum class I collagenase to recover islets. Islets 2017;9:177-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckhard U, Huesgen PF, Brandstetter H, Overall CM. Proteomic protease specificity profiling of clostridial collagenases reveals their intrinsic nature as dedicated degraders of collagen. J Proteomics 2013;100:102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Wu J, Bai Y, Zhao X, Liu L. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp 2014;e51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang C, Zhao X, Han J. Primary culture of porcine pancreatic acinar cells. Pancreas 2002;25:68-70. [DOI] [PubMed] [Google Scholar]
- 6.Kim MJ, Serwold T. Isolation of highly viable thymic epithelial cells for use in in vitro and in vivo experiments. Methods in Molecular Biology. Humana Press; 2019; p. 143-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CJ, Charlton CA, Michael LW, Morten KJ, Hodson L. The isolation of primary hepatocytes from human tissue : optimising the use of small non-encapsulated liver resection surplus. Cell Tissue Bank 2017;18:597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boo J, Hendriksen C. Reduction strategies in animal research: a review of scientific approaches at the intra-experimental, supra-experimental and extra-experimental levels. Altern Lab Anim 2005;33:369-77. [DOI] [PubMed] [Google Scholar]
- 9.Fenwick N, Griffin G, Gauthier C. The welfare of animals used in science: How the “Three Rs” ethic guides improvements. Can Vet J 2009;50:523-30. [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrigno A, Berardo C, Di Pasqua LG, Siciliano V, Richelmi P, Vairetti M. Localization and role of metabotropic glutamate receptors subtype 5 in the gastrointestinal tract. World J Gastroenterol 2017;23:4500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrigno A, Vairetti M, Ambrosi G, Rizzo V, Richelmi P, Blandini F, et al. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against hepatic mitochondrial dysfunction in 6-OHDA lesioned Parkinsonian rats. Clin Exp Pharmacol Physiol 2015;42:695-703. [DOI] [PubMed] [Google Scholar]
- 12.Storto M, de Grazia U, Knopfel T, Canonico PL, Copani A, Richelmi P, et al. Selective blockade of mGlu5 metabotropic glutamate receptors protects rat hepatocytes against hypoxic damage. Hepatology 2000;31:649-55. [DOI] [PubMed] [Google Scholar]
- 13.Storto M, Ngomba RT, Battaglia G, Freitas I, Griffini P, Richelmi P, et al. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against acetaminophen hepatotoxicity in mice. J Hepatol 2003;38:179-87. [DOI] [PubMed] [Google Scholar]
- 14.Ferrigno A, Berardo C, Di Pasqua LG, Siciliano V, Richelmi P, Nicoletti F, et al. Selective blockade of the metabotropic glutamate receptor mGluR5 protects mouse livers in in vitro and ex vivo models of ischemia reperfusion injury. Int J Mol Sci 2018;19:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrigno A, Richelmi P, Vairetti M. Troubleshooting and improving the mouse and rat isolated perfused liver preparation. J Pharmacol Toxicol Methods 2013;67:107-14. [DOI] [PubMed] [Google Scholar]
- 16.Ackermann KE, Gish BG, Honchar MP, Sherman WR. Evidence that inositol 1-phosphate in brain of lithium-treated rats results mainly from phosphatidylinositol metabolism. Biochem J 1987;242:517-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worthington Biochemical Corporation. [Internet] Available from: http://www.worthington-biochem.com/cls/default.html [Google Scholar]
- 18.Mitry RR, Hughes RD, Dhawan A. Progress in human hepatocytes: Isolation, culture & cryopreservation. Semin Cell Dev Biol 2002;13:463-7. [DOI] [PubMed] [Google Scholar]


