Abstract
As an opportunistic pathogen with high mortality rates, Cytomegalovirus (CMV) may lead to fatal disseminated CMV infection of the premature and newborn; thus necessitating the demonstration of CMV-DNA with clinical history and/or histopathological findings of CMV infection and defining other bacterial and viral infection agents with real-time polymerase chain reaction (RT-PCR) in udden unexpected death in infancy (SUDI) cases as we aimed in this study.
314 (144 female, 170 male) SUDI cases were prospectively investigated from January 2013 to January 2015 in Istanbul Forensic Medicine Institution. The study includes 87 tissue samples of 39 cases for post-mortem histopathological examination of interstitial pneumonia, myocarditis, meningitis, encephalitis, hepatitis, colitis or tubulointerstitial nephritis and/or accompanying chronic sialadenitis.
CMV-DNA was found positive in 35 (40.2%) salivary gland, 19 (21.8%) lung, 1 (1.1%) tonsil, and 1 (1.1%) brain tissues. CMV sialadenitis and/or CMV pneumonia associated with other viral and/or bacterial agents were detected in 23 (60%) of 39 infant cases.
The demonstration of CMV-DNA would significantly clarify the cause of death and collection of epidemiological data in SUDI cases with clinical history and histopathological findings of CMV infection accompanying chronic CMV sialadenitis. Furthermore, CMV suppresses the immune system, and may predispose to other bacterial and/or viral infections in these cases. Post-mortem molecular investigations are useful in explaining cause of death in SUDI with a suspicion of infection in forensic autopsies.
Keywords: Autopsy, Cytomegalovirus, SUDI, Paraffin embedded tissues, Post-mortem microbiology, Real-time PCR
Highlights
-
•
CMV may lead to fatal disseminated CMV infection of the premature and newborn.
-
•
Our study is the first comprehensive study investigating CMV infections in SUDI.
-
•
CMV suppresses the immune system, and may predispose to other infections.
-
•
Postmortem molecular investigations are useful in explaining cause of death in SUDI.
1. Introduction
Cytomegalovirus (CMV), a member of the betaherpesvirinae subfamily, is an opportunistic pathogen that remains latent in lymphocytes, polymorphonuclear leukocytes, renal epithelium cells, salivary glands and can be reactivated in case of immunosuppression.1, 2 CMV may result in asymptomatic infections in children and adults, and it may particularly cause infections with high mortality in immunocompromised individuals such as premature and neonatal infants. CMV may be transmitted through aspiration of mother's blood or infected genital secretion during birth or breast feeding and it generally remains latent in the infant.2, 3, 4 Disseminated CMV infections (pneumonia, myocarditis, colitis, tubulointerstitial nephritis, meningitis, etc.) may have a fatal progress in infants infected with this virus, accompanying chronic sialadenitis, which declines with age.5 Furthermore, CMV may also suppress the infant's immune system, thereby it may predispose the immune system to infections with other bacteria and viruses.1, 5
Demonstration of histopathologically-described CMV-DNA in infants with CMV infections and/or accompanying chronic sialadenitis, and definition of the other bacterial and viral infection agents in sudden unexpected death in infancy (SUDI) cases referred to the Council of Forensic Medicine of Istanbul for autopsy were the objectives of the study herein.
2. Materials and methods
2.1. SUDI cases
314 (144 female, 170 male) SUDI cases were prospectively investigated from January 2013 to January 2015 in Istanbul Forensic Medicine Institution. Samples were collected and histopathological examination was carried out with the routine hematoxyline and eosin staining method. The blood, CSF, lung, spleen, stool and tracheal swab samples were collected for microbiological examination. All of the cases were selected by the permission of Council of Forensic Medicine Istanbul Scientific Committee.
2.2. Bacterial cultures
Samples collected for microbiological examination were cultivated in appropriate media for aerobic and anaerobic bacteriological cultures. The identification of pathogenic bacteria was performed using conventional methods and a miniAPI (Biomerieux, France) semi-automated identification system.6
2.3. Detection of viruses
The multiplex PCR method was applied in order to designate the viral gastroenteritis agents (Adenovirus, Rotavirus, Astrovirus, Norovirus G1 and G2) in stool samples, and respiratory tract agents [Rhinovirus, Parainfluenzavirus (1,2,3,4), Influenza A and B Virus, Enterovirus, Human Bocavirus, Adenovirus, H1N1 Virus, Coronavirus (229,63,HKU,43), Human Metapneumovirus A/B, Parechovirus, Respiratory Syncytial Virus (RSV) A/B, Mycoplasma pneumoniae] in tracheal swab sample. The nucleic acids were extracted in the QIAsymphony device using a QIAsymphony DSP Virus/Pathogen midi kit, and were amplified in the Rotor GeneQ device (Qiagen, Germany) via the RT-PCR method using FTD Viral Gastroenteritis (Fast-track Diagnostics, Luxemburg) and FTD Respiratory 21 (Fast-track Diagnostics, Luxemburg) kits in accordance with the recommendations of the manufacturing company.
Viruses known as the causes of myocarditis (Enterovirus, Ebstein Barr Virus, CMV, Adenovirus, Parvovirus B19) were investigated in the tissue samples which were histopathologically considered to have myocarditis. The DNA isolation was carried out in the QIAsymphony device using a QIAsymphony DSP Virus/Pathogen midi kit, and the amplification procedures were performed in the Rotor GeneQ device (Qiagen, Germany) using an Artus (Qiagen, Germany) kit through the RT-PCR method in accordance with the recommendations of the manufacturing company.2, 3
2.4. Histopathological examination
Amongst the cases identified to have an ‘owl's eye’ appearance (Fig. 1 ) in the post-mortem histopathological examination of salivary glands, 87 tissue samples (37 salivary gland, 38 lung, 1 tonsil, 3 kidney, 3 myocardium, 1 brain, 1 liver, 3 bowel samples) from 39 cases demonstrating findings of interstitial pneumonia, myocarditis, meningitis, encephalitis, hepatitis, colitis or tubulointerstitial nephritis (Fig. 2, Fig. 3, Fig. 4 ) and/or accompanying chronic sialadenitis were included in the study.2
Fig. 1.
Intranuclear basophilic inclusions (owl's eye) in the ductal epithelial cells and interstitial mononuclear inflammatory infiltrate on the salivary gland (H&EX200).
Fig. 2.
Interstitial pneumonia with prominent septal mononuclear inflammatory infiltrate (H&EX200).
Fig. 3.
Prominent mononuclear inflammatory infiltrate in the interstitium of kidney (H&EX200).
Fig. 4.
Mononuclear inflammatory infiltrate with associated myocyte necrosis in the heart (H&EX400).
2.5. Detection of CMV-DNA
DNA was extracted from all 87 formalin-fixed paraffin-embedded tissues. Three or four 10-μm-thick sections from each block were cut by means of a microtome. Paraffin-embedded tissues underwent xylene and ethyl alcohol deparafinization procedure followed by incubation for 24-h with the addition of 20 ml proteinase K and 600 ml ATL buffer solution.7 CMV-DNA isolation was performed in the QIAsymphony device using a QIAsymphony DSP Virus/Pathogen midi kit and the DNA amplification procedure was performed in the Rotor GeneQ device (Qiagen, Germany) using an Artus (Qiagen, Germany) kit through the RT-PCR method in accordance with the recommendations of the manufacturing company. A 15 μl master mix was prepared, 10 μl DNA was added on top of it and the final volume of 25 μl was obtained. The PCR tubes were incubated for 40 cycles as follows: 10 min at 95 °C, 15 s at 95 °C, 30 s at 65 °C, 20 s at 72 °C.
3. Results
3.1. Patient profiles
Respectively 25 (64.1%) and 14 (35.9%) of the cases were male and female, they were aged between 25 days and 12 months and considered to have CMV infection. The age distributions of the infants were as follows: 3 infants at 0–3 months, 25 infants at 4–6 months, 11 infants at 7–12 months. The age, sex, clinical history, histopathological and microbiological findings of the cases are summarized in Table 1 .
Table 1.
Clinical, histopathological, microbiological findings of the SUDI cases (n:39).
| Sex | Age | Clinical history | Histopathological findings | Microbiological results |
|||
|---|---|---|---|---|---|---|---|
| Bacteriologic agent in lung | CMV-DNA results | Other viral results | |||||
| 1 | M | 5 m | Death in bed | Sialadenitis, interstitial pneumonia, myocarditis | SG and LG pos MY neg | ||
| 2 | M | 5 m | Runny nose, acute bronchitis, death in bed | Sialadenitis, interstitial and lobuler pneumonia | E. faecalis | SG and LG pos | RSV (RS) |
| 3 | M | 3 m | Death in bed | Sialadenitis, interstitial pneumonia, myocarditis | SG and LG pos, MY neg | Rhinovirus (RS) | |
| 4 | M | 9 m | Death in bed | Sialadenitis, interstitial pneumonia, | SG and LG pos | ||
| 5 | M | 6 m | Respiratory insufficiency, death before hospital admission | Sialadenitis, interstitial pneumonia, | SG and LG pos | Adenovirus (GIS) | |
| 6 | M | 4 m | Death before hospital admission | Sialadenitis, interstitial pneumonia, renal MNCI | S. aureus | SG and LG pos Kidney neg | Astrovirus (GİS) |
| 7 | F | 6 m | Death before hospital admission | Sialadenitis, interstitial pneumonia, myocarditis | SG and LG pos MY neg | Coronavirus HKU (RS) | |
| 8 | M | 4 m | Death in bed | Sialadenitis, interstitial pneumonia, renal MNCI | SG and LG pos Kidney neg | ||
| 9 | M | 11 m | ICU admission because of surgery, death at home | Sialadenitis, early stage of bronchopneumonia | No growth | SG pos, LG neg | |
| 10 | M | 6 m | Premature, ICU admission (lung infection), death at home | Sialadenitis, interstitial pneumonia | SG pos, LG neg | ||
| 11 | M | 12 m | Premature, ICU admission, death at home | Sialadenitis, interstitial pneumonia | SG pos, LG neg | Metapneumovirus (RS) | |
| 12 | M | 12 m | Death in bed | Sialadenitis, bronchopneumonia | M. pneumoniae (RS) | SG pos, LG neg | Adenovirüs (GIS) |
| 13 | F | 4 m | Death before hospital admission | Sialadenitis, interstitial pneumonia | SG pos, LG neg | Rhinovirus (RS) | |
| 14 | F | 6 m | Coughing, ICU admission, death at hospital | Sialadenitis, interstitial pneumonia, myocarditis | SG pos, LG neg | Enterovirus (myocard) | |
| 15 | F | 6 m | Death in bed | Sialadenitis, interstitial pneumonia | SG pos, LG neg | Influenza A (RS) | |
| 16 | F | 25 day | Death before hospital admission | Hyperemia(TB), bronchopulmonary dysplasia, | SG and LG neg | ||
| 17 | F | 8 m | Premature twin, ICU admission, death at hospital | Sialadenitis, pulmonary edema | No culture | SG and LG neg | |
| 18 | M | 5 m | Death before hospital admission | Sialadenitis, pulmonary edema,enteritis, colitis, | SG pos, LG and Intestine neg | ||
| 19 | M | 4 m | Down's syndrome, bronchiolitis and progressive dyspnea, death at hospital | Sialadenitis, interstitial pneumonia, bronchopneumonia | Enterobacter spp | SG and LG pos | Coronavirus 63 and RSV (RS) Norovirus G2 (GIS) |
| 20 | M | 6 m | Cold 1 month before death, respiratory insufficiency, death before hospital admission | Subarachnoidal MNCI, paranchymal microglial nodule (brain) | Tonsilla and Brain pos | ||
| 21 | F | 4 m | Premature, ICU admission, respiratory insufficiency, death at home | Sialadenitis, bronchopulmonary dysplasia | SG and LG pos | ||
| 22 | F | 7 m | Premature twin, ICU admission dyspnea, respiratory insufficiency, death before hospital admission | Sialadenitis, interstitial pneumonia, active chronic hepatitis | SG pos, LG and Liver neg | Adenovirus (RS) | |
| 23 | F | 6 m | Cold, respiratory insufficiency, hospital admission, death at home | Sialadenitis, interstitial and lobular pneumonia | No culture | SG poz, LG neg | |
| 24 | F | 12 m | Fever, hospital admission, death at home | Sialadenitis, interstitial pneumonia, | SG and LG poz | Rhinovirus (RS) Adenovirus (GIS) | |
| 25 | M | 5 m | Premature, bronchiolitis, hospital admission, death at hospital | Sialadenitis, comman atelectasis | No culture | SG poz, LG neg | |
| 26 | M | 7 m | Vomiting, diarrhea, hospital admission, death at home | Sialadenitis, interstitial pneumonia, colitis | SG and LG poz, Intestine neg | Rotavirus (GIS) | |
| 27 | M | 6 m | Respiratory insufficiency, hospital admission, death at home | Sialadenitis, interstitial pneumonia, | SG and LG poz | Rhinovirus, Adenovirus Coronovirus 43 (RS) Norovirus G2 (GIS) |
|
| 28 | M | 3 m | Cold, hospital admission, death at home | Sialadenitis, interstitial pneumonia, | SG poz, LG neg | ||
| 29 | M | 6 m | Fever, death before hospital admission | Sialadenitis, interstitial pneumonia, | SG and LG poz | Rhinovirus (RS) Rotavirus (GIS) | |
| 30 | F | 7 m | Death in bed | Sialadenitis, interstitial pneumonia, colitis | SG and LG poz, Intestine neg | Adenovirus (RS) Adenovirus (GIS) | |
| 31 | M | 4 m | Wheezing, death in bed | Sialadenitis, interstitial and lobular pneumonia | E. coli | SG and LG poz | |
| 32 | F | 5 m | Death before hospital admission | Sialadenitis and interstitial pneumonia | SG poz, LG neg | ||
| 33 | M | 6 m | Death before hospital admission | interstitial pneumonia and renal MNCI | LG and Kidney neg | ||
| 34 | M | 6 m | Premature, hospital admission, epilepsy, death at hospital | Sialadenitis, interstitial and lobular pneumonia | Streptococcus spp. | SG and LG poz | Rhinovirus (RS) |
| 35 | M | 7 m | Surgery because of congenital cardiac disease, death at home | Sialadenitis, interstitial and lobular pneumonia | E. coli | SG pos, LG neg | Adenovirus, Enterovirus and Parechovirus (RS) Norovirus G2 (GIS) |
| 36 | M | 4 m | Premature, hospital admission, death at home | Sialadenitis, atelectasis | SG pos, LG neg | ||
| 37 | F | 5 m | Death in bed | Sialadenitis and interstitial pneumonia | SG and LG pos | Rhinovirus (RS) | |
| 38 | F | 4 m | Death in bed | Sialadenitis, lobular pneumonia, hyaline membrane formation | Streptococcus spp. | SG and LG pos | Rhinovirus (RS) |
| 39 | M | 10 m | Congenital hydrocephalus, hospital admission, death at home | Sialadenitis, interstitial and lobular pneumonia. | Streptococcus spp. | SG pos, LG neg | Metapneumovirus (RS) |
F: female, M: male, m: month, ICU: Intensive Care Unit, MNCI Mono-Nukleer Cell Infiltration, MY: Myocardium, LG: Lung, SG: Salivary gland, GIS: Gastrointestinal System, RS: Respiratory System, pos: positive, neg: negative.
3.2. CMV-DNA results
CMV-DNA was detected in both the salivary gland and lung tissues of 19 infants. Only CMV-DNA was detected in the salivary gland tissues of 16 infants, while no CMV-DNA was detected in the lung tissues. CMV-DNA was found positive in the tonsil and brain tissues of an infant considered to have encephalitis. Additionally, CMV-DNA was found negative in 2 salivary gland, 2 lung, 3 kidney, 3 myocardium, 1 liver and 3 bowel tissues.
3.3. Accompanying bacterial and viral infections
Additional one or more viruses (Rhinovirus, Adenovirus, Metapneumovirus, RSV, Influenza A, Coronavirus 63, Coronavirus 43, Coronavirus HKU, Enterovirus, Parechovirus) were detected in the lung tissue samples of 15 out of 19 infants identified as CMV-DNA-positive, and in the respiratory tract samples of 6 out of 16 infants identified as CMV-DNA-negative. Furthermore, viruses causing gastroenteritis (Adenovirus, Norovirus G2, Rotavirus, Astrovirus), viruses causing myocarditis (Enterovirus), and bacteria causing pneumonia (M. pneumoniae, Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Streptococcus spp., Enterobacter spp.) were respectively detected in 10 infants, 1 infant and 9 infants (Table 1).
4. Discussion
In accordance with the objective of demonstrating CMV-DNA in tissues, and describing CMV infections and other accompanying infectious agents in SUDI cases, the study investigated CMV-DNA and other viral agents using the RT-PCR method in 87 paraffin-embedded tissue samples taken from 39 post-mortem SUDI cases. CMV-DNA was found positive in 35 (40.2%) salivary gland, 19 (21.8%) lung, 1 (1.1%) tonsil and 1 (1.1%) brain tissues in total under the study. Besides, other bacterial and/or viral infectious agents were detected in 23 (60%) out of 39 cases. The previous studies on post-mortem SUDI cases employed other microbiological methods (immunohistochemical methods, cell culture, etc.) in addition to histopathological examinations which were more commonly preferred for CMV diagnosis; however any comprehensive studies to detect CMV-DNA with a more precise RT-PCR method could not be identified. To the best of our knowledge, our study is the first comprehensive study investigating CMV infections in various tissues (salivary gland, lung, tonsil, kidney, myocardium, brain, liver, bowel samples) of SUDI cases in paraffin-embedded tissues using RT-PCR during the autopsy.
Initial studies on sudden and unexpected infant mortalities at home reported that these babies histopathologically demonstrated the findings of CMV infections.8, 9 Variend10 conducted a study on 51 infant autopsies, and pointed out to the appearance of CMV-type inclusions in the parotid gland of 24 infants and the presence of microglial nodules in the brains of 15 of these infants. In another study that he conducted years later, Variend described CMV parotitis findings in 40 out of 112 infants as well as microglial nodules in the brain and lymphocytic infiltration findings in the liver and kidney.11 Variend emphasized the necessity to employ more precise methods for future diagnosis of CMV infections at the end of that study.11 In the present study, a microglial nodule was observed in the brain tissue of a case diagnosed with encephalitis, and also CMV-DNA was found positive in the tonsil and brain tissues of the case (Fig. 5 ). CMV-DNA was demonstrated in the tonsil tissue as the salivary gland of the case was not sampled during the autopsy.
Fig. 5.
Small foci of microglial nodule forming aggregate in the brain (H&EX200).
Püschel et al.12 detected cytomegalic inclusions in the salivary glands of 24 out of 255 SUDI cases. In the consequent years, Weber et al.13, 14 demonstrated CMV infection in only 3 out of 546 SUDI cases using histopathological examination or immunofluorescent test in two separate studies. Andrade et al.15 described CMV infection using the immunohistochemical and in situ hybridization method in 37 post-mortem cases considered to have secondary interstitial pneumonia. In another study investigating 64 SUDI cases in the post-mortem phase, CMV infection was demonstrated in 3 cases using serologic method while they verified the presence of CMV in only one case using the PCR method.16 Dettmeyer et al. described CMV- induced pneumonia or myocarditis in three cases of suspected sudden infant death syndrome (SIDS) diagnosis by immunohistochemical techniques and molecular pathologic investigations (PCR).17 On the other hand, the CMV-DNA was positive in 35 salivary gland, 19 lung, 1 tonsil and 1 brain tissues of 87 samples from 39 infant cases out of 314 SUDI cases in our study, who were thought to have CMV infection based on histopathological results, with real-time PCR method, which has a high precision.
The histopathological examination is able to demonstrate intracytoplasmic inclusions and pathognomonic intranuclear basophilic inclusion with clear halo in the periphery (owl's eye) in case of CMV infection.2, 18 However, cells under typical CMV cytopathic effect may not be observed in during the onset of the infections (particularly in CMV pneumonia cases). As a result, it is rather useful to demonstrate CMV-DNA by means of molecular techniques in the cases considered to have histopathological CMV infections.18
Certain in vitro studies were carried out to demonstrate that CMV infections suppress the T-lymphocyte functions and activity of natural killer cells, thereby causing immunosuppression.3, 19, 20 Since the immune systems of infants infected by CMV are suppressed, infection with another accompanying virus that causes wheezing may also be seen.21 Doan et al.22 conducted a study on infants who developed CMV pneumonia, and reported a decrease in the number of CD4-positive T-cells in 69.4% of the infants.
CMV infections generally emerge in the first year of life. Such infections may have been perinatally or postnatally acquired since CMV-seropositivity is seen in the first 12 months in more than one third of the infants.23, 24 All of the post-mortem SUDI cases in this study were under the age of one.
Diffuse interstitial pneumonia may develop in infants or premature babies with perinatally acquired CMV infection and it may be life-threatening.5 Furthermore, CMV infections also suppress the immune system of an individual, hence predispose infections with other bacteria and viruses.1 For that reason, the infant's immunity is suppressed in cases with chronic CMV sialadenitis in the neonatal and early infancy periods, which causes the development of such infections. These agents could also contribute to identify clinical symptoms of cases with CMV-DNA positive and other bacterial and viral agents that cause pneumonia. The lung tissues of 19 cases with or without accompanying chronic sialadenitis based on histopathological results, diagnosed with interstitial pneumonia were found to be CMV-DNA positive as a result of our study. Other viral and/or bacterial infectious agents accompanying CMV infection were detected, as well in 15 of these cases. On the other hand, the presence of other viral agents were demonstrated in 6 infants with CMV-DNA positive in salivary gland whereas negative in lung, who were histopathologically diagnosed as interstitial pneumonia.
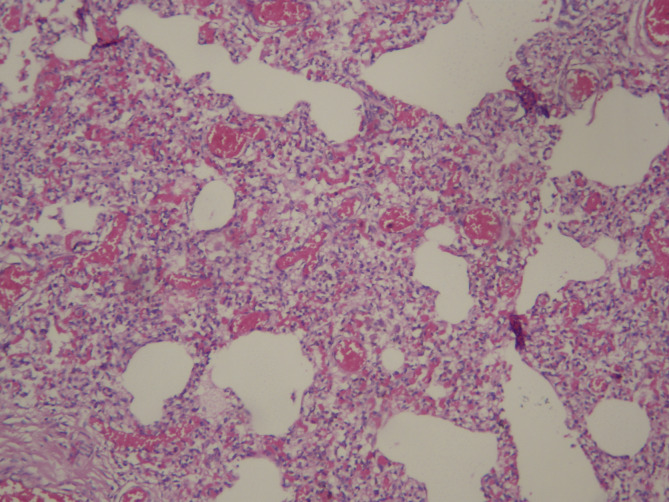
CMV pneumonia may be severe in premature infants and may especially progress to diffuse interstitial pneumonia, leading fibrosis and bronchopulmonary dysplasia in such infants.5, 25, 26 In this study, CMV-DNA was found positive in the salivary gland and lung tissues of a 4-month-old baby who was prematurely born, who was histopathologically identified to have bronchopulmonary dysplasia (Fig. 6 ) and intranuclear basophilic inclusion in the lung.
Fig. 6.
Atelectasis, overinflation in the small airways, interstitial fibroplasia and smooth muscle cell proliferation in the small airways as a finding of late-stage broncopulmonary displasia (H&EX200).
CMV infections have a silent clinical progress in most infants while non-specific lower respiratory tract symptoms or an infectious mononucleosis-like disease may be noted in some of them.21 Furthermore, poor socio-economic conditions also contribute to high CMV infection rates. Turkey is listed among the countries with a high seroprevalence (85–95%) with respect to CMV seropositivity.1 The fact that some of the infants in this study had a history of being found dead in bed or were exitus when taken to the hospital is important for noting that CMV infections may have non-specific signs and remain undetected until death.1 Some of the infants with another underlying predisposing factors (premature delivery, Down syndrome, having twin, congenital diseases, past surgery) had more prominent clinical signs and a history of hospitalization. Although it was not possible to access the discharge reports of all these cases, it is believed that the majority of those hospitalized cases were not monitored and treated for CMV infection. Predisposing factors are known for their effects of increasing the probability of secondary infections compared to an otherwise healthy infant.1, 5 Cases histopathologically diagnosed as CMV infection were included the study regardless of predisposing factor existence as the clinical history was not completely known for almost half of the cases. The facts that salivary gland was not sampled in 2 out of 39 cases during autopsy and clinical history was not completely known in approximately 50% of the cases are the limitations of the study.
Certain clinical studies emphasized that CMV infections (with pneumonia ranking the first) in immunocompetent infants may be treated with ganciclovir.27, 28 However, there are reservations regarding the use of this drug in the treatment of CMV infection due to its toxicity.3, 27 CMV infection -primarily pneumonia-should definitely be considered in infancy periods (with or without immunocompromise) and CMV-DNA should be checked in babies without improved clinical conditions.22, 23 Even though the natal and perinatal transmission of CMV cannot be prevented, an assessment of the CMV-DNA in infants with unresolved disease would significantly decrease infant deaths due to CMV infections.1, 3
In conclusion, CMV infections that accompany chronic CMV sialadenitis and accompanying bacterial and viral infections may be seen in SUDI cases. Moreover, the demonstration of CMV-DNA and other infectious agents in SUDI cases with clinical history and/or histopathological findings of CMV infection would significantly clarify the cause of death.14 Post-mortem molecular investigations are useful in explaining the cause of death in SUDI with a suspicion of infection in forensic autopsies. Multidisciplinary studies with the participation of microbiologists, pathologists and forensic medicine experts would obviously contribute for further detection of the cause of death in such cases.29
Conflict of interests
The authors declare no conflict of interest.
Funding
None declared.
Ethical approval
None.
References
- 1.Us T. Cytomegalovirus (HHV5) In: Topçu A.W., Söyletir G., Doğanay M., editors. Infectious diseases and Microbiology. 2nd ed. Nobel Tip; Istanbul: 2008. pp. 1679–1689. [Google Scholar]
- 2.Winn W., Allen S., Janda W., Koneman E., Procop G., Schreckenberger P., Woods G., editors. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Lippincott Williams and Wilkens; 2006. Diagnosis of Infections Caused by Viruses, Chlamydia, Rickettsia and Related Organisms. Chapter 23; pp. 1327–1419. [Google Scholar]
- 3.Murray P.R., Rosenthal K.S., Pfaller M.A., editors. Medical Microbiology. 5th ed. Pennsylvania; Philadelphia: 2005. Human Herpesviruses; pp. 558–562. [Google Scholar]
- 4.Gandhi M.K., Khanna R. Human cytomegalovirus: clinical aspects, immun regulation and emerging treatments. Natale Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 5.Coclite E., Di Natale C., Nigro G. Congenital and perinatal cytomegalovirus lung infection. J Matern Fetal Neonatal Med. 2013;26(17):1671–1675. doi: 10.3109/14767058.2013.794207. [DOI] [PubMed] [Google Scholar]
- 6.Murray P.R., Rosenthal K.S., Pfaller M.A., editors. Medical Microbiology. 5th ed. 2005. Laboratory Diagnosis of Bacterial Diseases; pp. 213–219. Philadelphia, Pennsylvania. [Google Scholar]
- 7.Chan P.K.S., Chan D.P.C., To K.-F., Yu M.Y., Cheung J.L.K., Cheng A.F. Evaluation of extraction methods from paraffin wax embedded tissues for PCR amplification of human and viral DNA. J Clin Pathol. 2001;54:401–403. doi: 10.1136/jcp.54.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potencz H., Diosi P. Cytomegalovirus infection and sudden death in infants. J Pediatr. 1974;85(2):281–283. doi: 10.1016/s0022-3476(74)80417-8. [DOI] [PubMed] [Google Scholar]
- 9.Smith S.D., Cho C.T. Letter: Cytomegalovirus pneumonia in sudden infant death syndrome. JAMA. 1975;233(8):861. [PubMed] [Google Scholar]
- 10.Variend S. Infant mortality, microglial nodules and parotid CMV-type inclusions. Early Hum Dev. 1990;21(1):31–40. doi: 10.1016/0378-3782(90)90108-u. [DOI] [PubMed] [Google Scholar]
- 11.Variend S., O'Neill D., Arnold P. The possible significance of cytomegaloviral parotitis in infant and early childhood deaths. Arch Pathol Lab Med. 1997;121(12):1272–1276. [PubMed] [Google Scholar]
- 12.Püschel K., Hashimoto Y., Löning T., Lignitz E. Cytomegalic inclusion disease of the salivary glands in sudden infant death syndrome. Z Rechtsmed. 1988;99(4):281–289. doi: 10.1007/BF00204439. [DOI] [PubMed] [Google Scholar]
- 13.Weber M.A., Hartley J.C., Ashworth M.T., Malone M., Sebire N.J. Virological investigations in sudden unexpected deaths in infancy (SUDI) Forensic Sci Med Pathol. 2010;6(4):261–267. doi: 10.1007/s12024-010-9181-x. [DOI] [PubMed] [Google Scholar]
- 14.Weber M.A., Ashworth M.T., Risdon R.A., Hartley J.C., Malone M., Sebire N.J. The role of post-mortem investigations in determining the cause of sudden unexpected death in infancy. Arch Dis Child. 2008;93(12):1048–1053. doi: 10.1136/adc.2007.136739. [DOI] [PubMed] [Google Scholar]
- 15.Andrade Z.R., Garippo A.L., Saldiva P.H., Capelozzi V.L. Immunohistochemical and in situ detection of cytomegalovirus in lung autopsies of children immunocompromised by secondary interstitial pneumonia. Pathol Res Pract. 2004;200(1):25–32. doi: 10.1016/j.prp.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Rodríguez A., Ballesteros S., de Ory F., Echevarría J.E., Alvarez-Lafuente R., Vallejo G. Virological analysis in the diagnosis of sudden children death: a medico-legal approach. Forensic Sci Int. 2006;161(1):8–14. doi: 10.1016/j.forsciint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Dettmeyer R., Sperhake J.P., Müller J., Madea B. Cytomegalovirus-induced pneumonia and myocarditis in three cases of suspected sudden infant death syndrome (SIDS): diagnosis by immunohistochemical techniques and molecularpathologic methods. Forensic Sci Int. 2008;174(2-3):229–233. doi: 10.1016/j.forsciint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Kradın R.L., editor. Diagnostic Pathology of Infections Disease. Saunders Elsevier; Philadelphia: 2010. Cytopathology of Infectious and Inflammatory Diseases. Chapter 4. Schnadig VJ; p. 62. [Google Scholar]
- 19.Rinaldo C.R., Jr., Carney W.P., Richter B.S., Black P.H., Hirsch M.S. Mechanisms of immunosuppression in cytomegaloviral mononucleosis. J Infect Dis. 1980;141(4):488–495. doi: 10.1093/infdis/141.4.488. [DOI] [PubMed] [Google Scholar]
- 20.Schrier R.D., Rice G.P., Oldstone M.B. Suppression of natural killer cell activity and T cell proliferation by fresh isolates of human cytomegalovirus. J Infect Dis. 1986;153(6):1084–1091. doi: 10.1093/infdis/153.6.1084. [DOI] [PubMed] [Google Scholar]
- 21.Morisawa Y., Maeda A., Sato T., Hisakawa H., Fujieda M., Wakiguchi H. Cytomegalovirus infection and wheezing in infants. Pediatr Int. 2008;50(5):654–657. doi: 10.1111/j.1442-200X.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 22.Doan T.T., Phung T.T., Pham H.V., Pham S.H., Nguyen L.T. Effect of ganciclovir for the treatment of severe cytomegalovirus-associated pneumonia in children without a specific immunocompromised state. BMC Infect Dis. 2013;13:424. doi: 10.1186/1471-2334-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov I.S., Popov N.I., Moshe R.I., Terzieva D.D., Stefanov R.S., Panova M.V. Prevalence of cytomegalovirus infection in hospitalized infants. Folia Medica. 2012;54(4):45–52. doi: 10.2478/v10153-012-0005-5. [DOI] [PubMed] [Google Scholar]
- 24.Ataman S., Colak D., Günseren F,Senol Y., Colak T., Aktekin M.R. Investigation of cytomegalovirus seroepidemiology in Antalya with population-based cross sectional study and review of related data in Turkey. Mikrobiyol Bul. 2007;41(4):545–555. [PubMed] [Google Scholar]
- 25.Sawyer M.H., Edwards D.K., Spector S.A. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. Am J Dis Child. 1987;141(3):303–305. doi: 10.1001/archpedi.1987.04460030081030. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi R., Tagawa M., Sanjo M., Chiba H,Ito T., Yamada M. Severe postnatal cytomegalovirus infection in a very premature infant. Neonatology. 2007;92(4):236–239. doi: 10.1159/000103982. [DOI] [PubMed] [Google Scholar]
- 27.Avila-Agüero M.L., Paris M.M., Alfaro W., Avila-Agüero C.R., Faingezicht I. Ganciclovir therapy in cytomegalovirus (CMV) infection in immunocompetent pediatric patients. Int J Infect Dis. 2003;7(4):278–281. doi: 10.1016/s1201-9712(03)90107-x. [DOI] [PubMed] [Google Scholar]
- 28.Siret D., David V. Treatment of cytomegalovirus pneumonia with ganciclovir in an immunocompetent infant. Arch Pediatr. 2002;9(5):499–502. doi: 10.1016/s0929-693x(01)00832-6. [DOI] [PubMed] [Google Scholar]
- 29.Turner G.D., Bunthi C., Wonodi C.B., Morpeth S.C., Molyneux C.S., Zaki S.R. The role of post-mortem studies in pneumonia etiology research. Clin Infect Dis. 2012;54(Suppl 2):S165–71. doi: 10.1093/cid/cir1062. [DOI] [PMC free article] [PubMed] [Google Scholar]