Abstract
In chicken, peritoneal cystic lesions have not been clearly categorized. In this study, diffuse peritoneal multiple cysts were observed in two layer hens. The cysts in the serosa were lined with single layers of squamous or cuboidal cells. The papillary proliferations of columnar cells were also observed in one case. The smooth muscle layer or mass were observed around the cysts in both cases. The cystic lining cells were positive for pan-cytokeratin, vimentin, S100 and Wilms tumor 1. Ultrastructurally, they had sparsely microvilli on the luminal surface. The histological results indicated the present cases were multicystic mesothelioma, but also had characteristics of Mullerian epithelium. This is the first report describing the detailed pathological feature of unique multicystic tumor in chicken.
Keywords: chicken, electron microscopy, immunohistochemistry, mesothelioma, peritoneal cyst
In the layer hens, ovarian and oviductal adenocarcinoma occur at a high rate, and they can spread widely throughout the peritoneal cavity [8], but multicystic tumor is uncommon. In this study, we experienced two cases of peritoneal multiple cysts in layer hens which had different characteristics from adenocarcinoma. As long as we know, there was little information about pathological feature and classification of peritoneal cysts in chicken. In this study, we described histopathological, immunohistochemical and ultrastructural features of the multicystic lesion in two chicken and compared them with the avian and human peritoneal multicystic tumors.
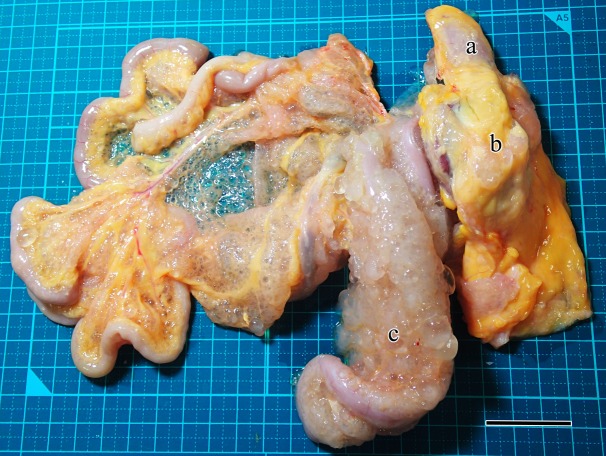
Peritoneal cystic lesions were incidentally detected from two white leghorn layer hens (case 1 and case 2) at routine chicken meat inspections. These hens had been raised at deferent farms and slaughtered on deferent days. They were uncertain age, but it was guessed that they were about 600 to 700 days old. Their abdominal organs were covered with multiple transparent cysts of less than 1 cm in diameter. The cysts contained clear, colorless and watery fluid. A larger number of the cysts were observed in case 1 than case 2. In case 1, the cysts diffusely spread almost throughout the mesentery (Fig. 1). Especially severe cyst formations were observed on the pancreas, duodenum, a part of liver and gizzard. Moderate cyst formations were also observed on the surface of the proventriculus, small intestine and spleen. A small number of the cysts were observed on the surface of the large intestine, kidney, oviduct and ovary. Similarly in case 2, the cysts had proliferated especially on the pancreas, duodenum and mesentery. A small number of the cysts were observed at the surface of the liver, proventriculus, gizzard, small and large intestine, spleen, kidney and ovary. The right degenerating oviduct dilated with viscous secretary fluid in both cases. Any conspicuous gross abnormalities were not observed on the parenchymal cross-sections of the organs, including the left oviductal mucosae.
Fig. 1.
Macroscopic feature of the gastrointestinal tract of case 1. There were multiple transparent cysts on the peritoneum, especially around the duodenum, pancreas and mesentery. a, proventriculus; b, gizzard; c, pancreas. Bar, 3 cm.
In both cases, heart, lung, liver, pancreas, proventriculus, gizzard, small and large intestine, mesentery, spleen, kidney, ovary and oviduct were collected and fixed by 10% phosphate-buffered formalin. The formalin-fixed tissues were embedded in paraffin after dehydration, cut into 4 µm paraffin sections, and stained with hematoxylin and eosin (H&E). Some selected sections with cystic lesions were also stained with periodic acid-Schiff (PAS), von Kossa, Masson’s trichrome (MT) or Alcian blue stain. Immunohistochemistry was also performed with anti-pan cytokeratin (CK), anti-vimentin, anti-alpha smooth muscle actin (SMA), anti-S100, anti-neuron specific enolase (NSE), anti-calretinin, anti-Wilms tumor 1 (WT1), anti-progesterone receptor (PR), anti-estrogen receptor (ER) and anti-avian leucosis virus (ALV) antibodies [27]. The details of the primary antibodies are shown in Table 1. For heat-induced epitope retrieval, the sections in distilled water were autoclaved for 10 min at 121°C. The staining was carried out with a polymer method (EnVision+ Single reagents, HRP, mouse or rabbit, Dako, Kyoto, Japan), followed by 3, 3′-diaminobenzidine-H2O2 solution as chromogen and hematoxylin as counterstain. The specificity of the antibodies was assessed by evaluating the normal tissues in the same individual (other than ALV) or tissues in ALV-affected chicken (ALV). Transmission electron microscopy was performed with formalin-fixed tissue samples according to methods described previously [17].
Table 1. Primary antibodies and result of immunohistochemistry.
| Antibody | Clone | Animal | Dilution | Source | Expression by the neoplastic cells | |
|---|---|---|---|---|---|---|
| Case 1 | Case 2 | |||||
| CK | AE1/AE3 | Mouse | × 40 | Abcam, Tokyo, Japan | + | + |
| Vimentin | VM452 | Mouse | × 400 | Novus Biologicals, Littleton, CO, USA | + | + |
| SMA | Polyclonal | Rabbit | × 200 | Abcam | - | - |
| S100 | Polyclonal | Rabbit | × 100 | Thermo Scientific, Fremont, CA, USA | + | + |
| NSE | Polyclonal | Rabbit | × 200 | Spring Bioscience, Pleasanton, CA, USA | - | - |
| Calretinin | Polyclonal | Rabbit | × 40 | Lsbio, Seattle, WA, USA | - (nuclear) | - (nuclear) |
| WT1 | Polyclonal | Rabbit | × 100 | Santa Cruz Bio, Santa Cruz, CA, USA | + | + |
| PR | hPRa 2 | Mouse | × 50 | Thermo Scientific | - | - |
| ER | Polyclonal | Rabbit | × 100 | Spring Bioscience | - | - |
| ALV | Antiserum | Rabbit | × 40,000 | Tsukamoto et al. [27] | - | - |
CK, pan-cytokeratin; SMA, smooth muscle actin; NSE, neuron specific enolase; WT1, Wilms tumor 1; PR, progesterone receptor; ER, estrogen receptor; ALV, avian leukosis virus.
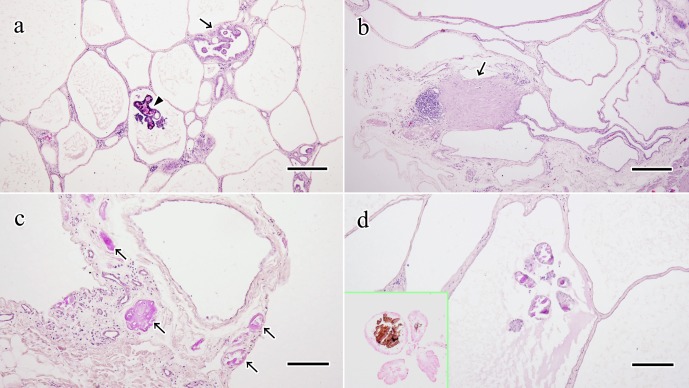
Histological examination revealed multiple cysts in the peritoneal serosa (Fig. 2a, 2b). The cysts in case 1 were lined with single layers of squamous or cuboidal epithelial cells. There were papillary proliferations of small columnar epithelial cells sporadically (Fig. 2a). The cysts in case 2 were lined with single layers of small squamous or cuboidal epithelial cells (Fig. 2b). The papillary proliferations were not observed in case 2. These neoplastic cells in both cases were almost uniform size and they had mildly eosinophilic cytoplasm, round or elongated nucleus and unclear nucleolus. Their mitosis and invasions into vessels or parenchyma of normal organs were not found. The cystic contents were stained mildly eosinophilic by H&E, mildly positive for PAS stain, and negative for Alcian blue stain (data not shown). In both cases, PAS-positive amorphous or circular structures were occasionally deposited in the stroma (Fig. 2c, 2d). The amorphous deposits seemed like a hyaline by H&E and showed bright magenta by MT stain (data not shown). The circular structures were mainly observed in the papillary lesion and calcified like psammoma body (Fig. 2d). Between the cysts, there was observed thin connective tissue with multifocal mild infiltration of lymphoid and plasma cells (Fig. 2b). Occasionally, focal proliferations of spindle cells were also observed in the stroma (Fig. 2b). Pyogenic inflammation occurred in a part of the right dilated oviductal mucosa. The other abnormal findings were not found in the oviducts of both cases. Lymphoid cells mildly infiltrated in the cardiac, renal, hepatic and gastrointestinal mucosal stroma in case 1.
Fig. 2.
Histological feature of cystic lesion. a: The neoplastic cells formed the cysts and papillary proliferation (arrow). Calcification (arrowhead) was occasionally observed in the stroma of papillary lesions. Case 1. H&E stain. Bar, 200 µm. b: Proliferation of spindle cells was observed in the stroma of cysts (arrow). Multifocal or diffuse mononuclear cell infiltration was also observed in the stroma. Case 2. H&E stain. Bar, 200 µm. c: PAS-positive amorphous deposits (arrows) were observed in the stroma of cysts. Case 2. PAS stain. Bar, 100 µm. d: Calcified circle structures in the stroma of papillary lesions were positive for PAS. Case 1. PAS stain. Bar, 100 µm. Insert, von Kossa stain.
Immunohistochemistry showed that the neoplastic cells were strongly positive for CK and vimentin in the cytoplasm (Fig. 3a, 3b). For S100, they were mildly positive in the cytoplasm and partially positive in the nucleus (Fig. 3c). For calretinin, neoplastic cells were strongly positive at the luminal surface, but negative in the nucleus (Fig. 3d). For WT1, positive reactions were partially observed in the nucleus (Fig. 3e). The neoplastic cells were negative for SMA, NSE, PR, ER and ALV in both cases. In the stroma, the SMA-positive layer surrounded the cysts, which was prominent in case 2 (Fig. 3f). In both cases, SMA-positive cellular masses were also observed in the stroma.
Fig. 3.
Immunohistochemistry of cystic lesion. a–e: The neoplastic cells were strongly positive for CK (a) and vimentin (b) in the cytoplasm; mildly positive for S100 in the cytoplasm and the nucleus (c); strongly positive for calretinin on the luminal surface, but negative in the nucleus (d); partially positive for WT1 in the nucleus (e). Inserts, high magnification. Case 1. Bars, 100 µm. f: The layer and mass of spindle cells surrounding the cysts were positive for SMA. Case 2. Bar, 100 µm.
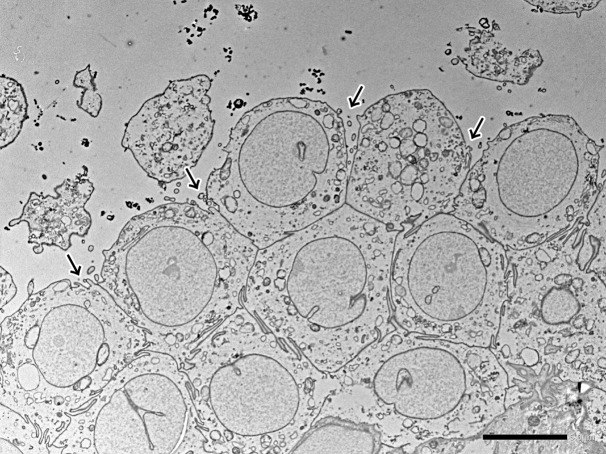
Ultrastructurally, the neoplastic cells had sparsely microvilli on the luminal surface (Fig. 4). The cell membranes were closely connected by desmosomes and interdigitations. In case 2, the interdigitations were observed more than case 1, but the microvilli and the desmosomes were less than case 1 (Fig. 4). Cilium and intracytoplasmic lumina were not found on the cells in either case. ALV-like viral particles were not observed in both cases.
Fig. 4.
Ultrastructure of the neoplastic cells in case 2. They had sparsely microvilli (arrows) on the luminal surface. The cell membranes were closely aligned with interdigitations. Bar, 5 µm.
The histological results indicated that the present cases were likely to benign mesothelioma. The tumors in the present cases were confined to the serous membrane, did not invade parenchyma. Additionally, mitosis or infiltrating proliferation were not found. The neoplastic cells were positive for cytokeratin and vimentin, as previously reported avian mesotheliomas [11, 13]. Morphologically, avian mesothelioma commonly develops multiple nodules or masses [5, 6, 11, 19, 24] and the neoplastic cells are present in gland-like or papillary arrangements [5,6,7, 19]. On the other hand, the present cases developed the multicystic lesion that was different from the previously reported avian mesotheliomas.
The present cases morphologically resembled human benign multicystic peritoneal mesothelioma (BMPM) which is a rare tumor developing during reproductive age [9, 14, 15, 21, 29]. In BMPM, the neoplastic cells are positive for cytokeratin, vimentin, calretinin and WT-1, and occasionally positive for PR or/and ER [14, 15, 22, 25, 28]. Their luminal surface is microvillous and cell boundaries are often tightly apposed with desmosomes and interdigitations [12, 23]. Therefore, the present cases also had immunohistological and ultrastructural similarities with BMPM.
As mentioned above, the present cases had histological features similar to mesothelioma. However, these cases showed different characteristics from mesothelioma on the following points: the expression of calretinin and the presence of smooth muscles. Calretinin is a useful marker for the definite diagnosis of human mesothelioma [10], and it was also expressed in both cytoplasm and nuclear in chicken normal mesothelium (Supplementary Fig. 1). The nuclear immunoreactivity with calretinin has high specificity for human mesothelioma [2, 10]. Our previous study also revealed that the neoplastic cells were strongly positive for calretinin in a malignant mesothelioma of a broiler breeder [13]. In contrast, the present cases showed negative reactions for calretinin in the nucleus. In addition, the smooth muscle layers in the stroma were revealed in the present cases, but mesothelioma commonly lacks them [21, 29]. Furthermore, almost the previously reported chicken mesotheliomas were associated with tumorigenic ALV infections [5, 7, 13], but the present cases might be spontaneous tumors without ALV infections.
The present cases also had characteristics of epithelial tumors. S100 which was positive in the present cases is positive for human ovarian or peritoneal serous carcinoma but negative for mesothelioma [30]. Ultrastructurally, microvilli of human serous carcinoma are fewer in number and length than mesothelioma [18]. The microvilli in the present cases were also fewer than previously reported chicken mesothelioma [7]. Additionally, the presence of smooth muscles might suggest the possibility that the tumor in the present cases originated from Mullerian duct. There are several sources presumed that human peritoneal serous adenocarcinoma arises from: mesothelium modified by various Mullerian influences, Mullerian inclusions in the peritoneal surfaces, and serous tubal intraepithelial carcinoma in the distal fallopian tubes [3, 4, 26]. Additionally, the mesothelium could exhibit a metaplastic process into Mullerian-type epithelium in human [16]. Those studies support the possibility that Mullerian epithelial tumors can occur even in chicken peritoneum. However, the present cases lacked cilia which found on oviductal epithelium and ovarian adenocarcinoma in human and chicken [1, 8, 18] and they were negative for ER and PR which indicate Mullerian differentiation [16].
In conclusion, this study showed unique chicken peritoneal multicystic tumor that was differentiated from ovarian and oviductal adenocarcinoma. This tumor had some characteristics of mesothelioma, but also contained notable differences. Mesothelioma is a rare tumor in birds [19, 20], and there were few studies about differential diagnoses of avian mesothelioma. The diagnosis of the present cases needs to differentiate benign mesothelioma and Mullerian epithelial tumor, but unfortunately difficult in this study. Further case studies in chickens will be required for definite diagnosis of this tumor. This is the first report describing the detailed pathological feature of the peritoneal multicystic tumor in chicken. We hope that this study will contribute to the classification of avian cystic tumors in the future.
Supplementary Material
Acknowledgments
The authors thank Prof. Kazuyuki Uchida (Laboratory of Veterinary Pathology, The University of Tokyo) for advices about diagnosis and manuscript, and Prof. Kenji Tsukamoto (Laboratory of Animal Health II, Azabu University) for provision of antibodies [27].
REFERENCES
- 1.Apperson K. D., Bird K. E., Cherian G., Löhr C. V.2017. Histology of the ovary of the laying hen (Gallus domesticus). Vet Sci 4: E66. doi: 10.3390/vetsci4040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attanoos R. L., Webb R., Dojcinov S. D., Gibbs A. R.2002. Value of mesothelial and epithelial antibodies in distinguishing diffuse peritoneal mesothelioma in females from serous papillary carcinoma of the ovary and peritoneum. Histopathology 40: 237–244. doi: 10.1046/j.1365-2559.2002.01352.x [DOI] [PubMed] [Google Scholar]
- 3.August C. Z., Murad T. M., Newton M.1985. Multiple focal extraovarian serous carcinoma. Int. J. Gynecol. Pathol. 4: 11–23. doi: 10.1097/00004347-198501000-00002 [DOI] [PubMed] [Google Scholar]
- 4.Carlson J. W., Miron A., Jarboe E. A., Parast M. M., Hirsch M. S., Lee Y., Muto M. G., Kindelberger D., Crum C. P.2008. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol. 26: 4160–4165. doi: 10.1200/JCO.2008.16.4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabot J. F., Beard D., Langlois A. J., Beard J. W.1970. Mesotheliomas of peritoneum, epicardium, and pericardium induced by strain MC29 avian leukosis virus. Cancer Res. 30: 1287–1308. [PubMed] [Google Scholar]
- 6.Cooper J. E., Pugsley S. L.1984. A mesothelioma in a ferruginous hawk (Buteo regalis). Avian Pathol. 13: 797–801. doi: 10.1080/03079458408418576 [DOI] [PubMed] [Google Scholar]
- 7.England J. M., Panella M. J., Ewert D. L., Halpern M. S.1991. Induction of a diffuse mesothelioma in chickens by intraperitoneal inoculation of v-src DNA. Virology 182: 423–429. doi: 10.1016/0042-6822(91)90583-W [DOI] [PubMed] [Google Scholar]
- 8.Fredrickson T. N.1987. Ovarian tumors of the hen. Environ. Health Perspect. 73: 35–51. doi: 10.1289/ehp.877335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gussago S., Spina P., Guerra A.2019. Benign Multicystic Peritoneal Mesothelioma (BMPM) as a rare cause of abdominal pain in a young male: case report and review of the literature. J. Surg. Case Rep. 2019: rjz057. doi: 10.1093/jscr/rjz057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husain A. N., Colby T. V., Ordóñez N. G., Allen T. C., Attanoos R. L., Beasley M. B., Butnor K. J., Chirieac L. R., Churg A. M., Dacic S., Galateau-Sallé F., Gibbs A., Gown A. M., Krausz T., Litzky L. A., Marchevsky A., Nicholson A. G., Roggli V. L., Sharma A. K., Travis W. D., Walts A. E., Wick M. R.2018. Guidelines for pathologic diagnosis of malignant mesothelioma. 2017 update of the consensus statement from the international mesothelioma interest group. Arch. Pathol. Lab. Med. 142: 89–108. doi: 10.5858/arpa.2017-0124-RA [DOI] [PubMed] [Google Scholar]
- 11.McCleery B., Jones M. P., Manasse J., Johns S., Gompf R. E., Newman S.2015. Pericardial mesothelioma in a yellow-naped amazon parrot (Amazona Auropalliata). J. Avian Med. Surg. 29: 55–62. doi: 10.1647/2014-017 [DOI] [PubMed] [Google Scholar]
- 12.Moore J. H., Jr., Crum C. P., Chandler J. G., Feldman P. S.1980. Benign cystic mesothelioma. Cancer 45: 2395–2399. doi: [DOI] [PubMed] [Google Scholar]
- 13.Murakami T., Sassa Y.2018. Pleomorphic malignant mesothelioma in a broiler breeder infected with avian leucosis virus subgroup J. J. Comp. Pathol. 160: 50–55. doi: 10.1016/j.jcpa.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 14.Murro D., Harbhajanka A., Mahon B., Deziel D.2014. Benign cystic mesothelioma associated with ipsilateral renal agenesis: a case report and review of literature. Pediatr. Dev. Pathol. 17: 487–490. doi: 10.2350/14-06-1510-CR.1 [DOI] [PubMed] [Google Scholar]
- 15.Myers D. J., Babiker H. M.2018. Cancer, mesothelioma, benign. Statpearls. https://www.ncbi.nlm.nih.gov/books/NBK531485/ [accessed on June 15, 2019].
- 16.Nakayama K., Masuzawa H., Li S. F., Yoshikawa F., Toki T., Nikaido T., Silverberg S. G., Fujii S.1994. Immunohistochemical analysis of the peritoneum adjacent to endometriotic lesions using antibodies for Ber-EP4 antigen, estrogen receptors, and progesterone receptors: implication of peritoneal metaplasia in the pathogenesis of endometriosis. Int. J. Gynecol. Pathol. 13: 348–358. doi: 10.1097/00004347-199410000-00009 [DOI] [PubMed] [Google Scholar]
- 17.Nakayama Y., Kamiie J., Watanabe G., Suzuki K., Murakami T.2017. Spontaneous, experimentally induced, and transmissible AA amyloidosis in Japanese quail (Coturnix japonica). Vet. Pathol. 54: 912–921. doi: 10.1177/0300985817723692 [DOI] [PubMed] [Google Scholar]
- 18.Ordóñez N. G.2006. The diagnostic utility of immunohistochemistry and electron microscopy in distinguishing between peritoneal mesotheliomas and serous carcinomas: a comparative study. Mod. Pathol. 19: 34–48. doi: 10.1038/modpathol.3800471 [DOI] [PubMed] [Google Scholar]
- 19.Reece R. L.1992. Observations on naturally occurring neoplasms in birds in the state of Victoria, Australia. Avian Pathol. 21: 3–32. doi: 10.1080/03079459208418815 [DOI] [PubMed] [Google Scholar]
- 20.Reece R. L.2008. Other tumors of unknown etiology. pp. 593–616. In: Diseases of Poultry, 12th ed. (Saif, Y. M., Fadly, A. M., Glisson, J. R., McDougald, L. R., Nolan, L. K. and Swayne, D. E. eds.), Blackwell, Ames. [Google Scholar]
- 21.Safioleas M. C., Constantinos K., Michael S., Konstantinos G., Constantinos S., Alkiviadis K.2006. Benign multicystic peritoneal mesothelioma: a case report and review of the literature. World J. Gastroenterol. 12: 5739–5742. doi: 10.3748/wjg.v12.i35.5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawh R. N., Malpica A., Deavers M. T., Liu J., Silva E. G.2003. Benign cystic mesothelioma of the peritoneum: a clinicopathologic study of 17 cases and immunohistochemical analysis of estrogen and progesterone receptor status. Hum. Pathol. 34: 369–374. doi: 10.1053/hupa.2003.31 [DOI] [PubMed] [Google Scholar]
- 23.Sienkowski I. K., Russell A. J., Dilly S. A., Djazaeri B.1986. Peritoneal cystic mesothelioma: an electron microscopic and immunohistochemical study of two male patients. J. Clin. Pathol. 39: 440–445. doi: 10.1136/jcp.39.4.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimonohara N., Holland C. H., Lin T. L., Wigle W. L.2013. Naturally occurring neoplasms in pigeons in a research colony: a retrospective study. Avian Dis. 57: 133–139. doi: 10.1637/10244-051012-Case.1 [DOI] [PubMed] [Google Scholar]
- 25.Søreide J. A., Søreide K., Körner H., Søiland H., Greve O. J., Gudlaugsson E.2006. Benign peritoneal cystic mesothelioma. World J. Surg. 30: 560–566. doi: 10.1007/s00268-005-0639-z [DOI] [PubMed] [Google Scholar]
- 26.Tong G. X., Chiriboga L., Hamele-Bena D., Borczuk A. C.2007. Expression of PAX2 in papillary serous carcinoma of the ovary: immunohistochemical evidence of fallopian tube or secondary Müllerian system origin? Mod. Pathol. 20: 856–863. doi: 10.1038/modpathol.3800827 [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto K., Hihara H., Kono Y.1991. Detection of avian leukosis virus antigens by the ELISA and its use for detecting infectious virus after cultivation of samples and partial characterization of specific pathogen-free chicken lines maintained in this laboratory. J. Vet. Med. Sci. 53: 399–408. doi: 10.1292/jvms.53.399 [DOI] [PubMed] [Google Scholar]
- 28.Uzüm N., Ozçay N., Ataoğlu O.2009. Benign multicystic peritoneal mesothelioma. Turk. J. Gastroenterol. 20: 138–141. [PubMed] [Google Scholar]
- 29.Watson H. I., Borovickova M., Shetty A.2014. The curious case of free-floating pelvic cysts. BMJ Case Rep. 2014: bcr2014205229. doi: 10.1136/bcr-2014-205229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J., Iwasa Y., Konishi I., Kan N., Kannagi R., Kobashi Y., Kim Y. C., Yamabe H.1995. Papillary serous carcinoma of the peritoneum in women. A clinicopathologic and immunohistochemical study. Cancer 76: 429–436. doi: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.