Abstract
Neonicotinoid pesticides (NNs) act as agonists on nicotinic acetylcholine receptors (nAChRs) of insects, and there have been concerns about the effects of NNs on the health of mammals. Since nAChRs are expressed in immune cells, it is possible that NNs disturb the immune system. However, few reports have examined the immunotoxicity of clothianidin (CLO), a widely-used NN. Here, we report the effects of CLO on immune organs and type IV allergic reactions in ear auricles. We orally administered CLO at 0, 30 and 300 mg/kg/day (CLO-0, 30 and 300) to Sprague-Dawley rats for 28 days. The effects were evaluated by organ and body weights, histopathology, and immunohistochemistry (TCRαβ, CD4, CD8, CD11b, CD68, CD103). In addition, some cecal contents were subjected to preliminary gut microbiota analysis, because microbiota contribute to host homeostasis, including the immunity. Our results showed loose stool, suppression of body weight gain, significant changes in organ weights (thymus: decreased; liver: increased) and changes of the gut microbiota in the CLO-300 group. There were no obvious histopathological changes in immune organs. Granulomas of the ear auricles were found in one rat of each of the CLO-30 and 300 groups, but CLO had no apparent effect on the thickness or immunohistochemistry in the ear auricles. We present new evidence that CLO affects the thymus and intestine, and might enhance the local inflammatory response. These findings should contribute to the appropriate evaluation of the safety of NNs in the future.
Keywords: allergic reaction, clothianidin, gut microbiota, rat, thymus
Pesticides have made a major contribution to the improved productivity in modern agriculture, but at the same time, there are serious concerns about their impact on ecosystems and human health. Neonicotinoid pesticides (NNs) were developed in the 1980s as an alternative to conventional pesticides. The domestic shipment of NNs tripled in the 10 years between 2004 and 2014 [43], and NNs are now among the most widely used pesticides, accounting for over 25% of the world market share with rapidly increasing sales. NNs, like nicotine, have significant agonistic effects on the nicotinic acetylcholine receptors (nAChRs) of insects, and disrupt their neurotransmission. They are systemic pesticides characterized by high water solubility, residual activity and selective toxicity to insects. Since the binding ability of NNs to mammalian nAChRs is several hundred to several thousand times lower than that of NNs to insect nAChRs [39, 61], they have been considered to have low toxicity to mammals. However, several studies have reported the disappearance and mass death of honeybees in Europe, as well as in America and other countries, occurring since the 1990s [18, 21, 65], which has raised concerns about the secondary effects of these agents on non-targeted insects. It has also recently been reported that NNs have adverse effects on vertebrates for example, on the reproductive organs in reptiles [9], birds [26, 60] and mammals [67], and the emotional behavior in mice [23, 24, 59, 68]. In order to assess the risk of NNs, therefore, there is an urgent need for definitive information about their effects on mammals.
In recent years, the incidence of immune disorders such as allergies and autoimmune diseases has been increasing in developed countries, including Japan [3, 4, 42, 49]. These diseases are caused by an imbalance of immune cells and abnormal immune responses to external or self-antigens: impairment of immune tolerance. In Japan, the number of patients with atopic dermatitis, ulcerative colitis and systemic lupus erythematosus in particular has been increasing since the 1980s. Some reports have shown that these immune disorders are caused by not only genetic factors [32, 34, 55], but also environmental factors such as hygiene and smoking [2, 45, 50]. NNs may contribute to the high frequency of these diseases, because the domestic shipments of NNs have been increasing, as mentioned above [43], and people are routinely exposed to NNs through diet.
Some studies have shown that nAChRs are expressed in non-neural cells, including immune cells [31, 64], and immune function in mammals is regulated by acetylcholine (ACh) via autocrine/paracrine pathways [30]. In particular, it has been well established that α7 nAChR plays an important role in immune response [38, 51, 63, 69, 70]. ACh acts at α7 nAChRs to modulate cytokine synthesis in T cells, dendritic cells, macrophages and B cells, and α7 nAChRs on antigen-presenting cells interfere with antigen presentation by inhibiting antigen processing. NNs are likely to disrupt the mammal immune response via nAChRs, because NNs have low affinity to mammalian nAChRs but bind to them and induce neuronal excitation [22, 33]. To date, there have been reports on immunotoxicity in vivo by the NNs imidacloprid, thiamethoxam and acetamiprid [5, 52, 56].
Considering the increasing number of patients with allergic diseases, the present study focused on contact dermatitis, a type IV allergic reaction. We hypothesized that contact dermatitis in NN-exposed rats arises from a novel inflammatory reaction, rather than the normal inflammation via nAChRs. Clothianidin (CLO) is one of the most recently developed NNs and is widely used. It has been reported that CLO suppress cytokine production and activation of the transcription factor NF-κB in THP-1 cells [11], but there are no animal studies on its involvement in immunotoxicity. We conducted the present study to investigate the effects of CLO on rat immune responses, particularly allergic reactions, by using mainly histological analysis.
MATERIALS AND METHODS
Animals
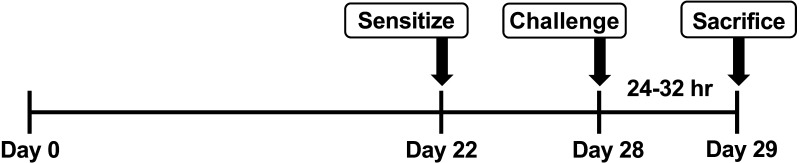
Eighteen male Sprague-Dawley (SD) rats aged 5 weeks from Japan SLC (Hamamatsu, Japan) were group-housed (3 rats per an individual ventilated cage of 40.5 × 35.5 × 23.0 cm, Green Line IVC Sealsafe PLUS Rat; Tecniplast, Buguggiate, Italy) under controlled temperature (23 ± 2°C) and humidity (50 ± 10%) on a 12-hr light/dark cycle at the Kobe University Life-Science Laboratory with ad libitum access to water and a pellet diet (DC-8; Clea Japan, Tokyo, Japan). We used SD rats characterized by gentle, in order to reduce the administration stress, and actually, rats have been often used in general toxicity tests [14]. After one week acclimation, all rats (body weight: 190 ± 20 g) were gently orally administered CLO (purity: over 95%, extracted from Dantotsu® Sumitomo Chemical Co., Tokyo, Japan, [23]) or vehicle (0.5% carboxymethylcellulose, 7.5 ml/kg) at a dose of 0, 30 or 300 mg/kg body weight for 28 days. The dose concentrations were chosen with reference to the no-observed-adverse-effect level (NOAEL) of 27.9 mg/kg from a 90-day dietary subchronic toxicity study in male rats [14]. These groups were defined as the CLO-0, 30 and 300 groups, respectively. The experimental design of present study is summarized in Fig. 1. The present study was approved by the Institutional Animal Care and Use Committee (Permission #30-01-01) and carried out according to the Kobe University Animal Experimentation Regulations.
Fig. 1.
Scheme showing the overall experimental design. Clothianidin (0, 30, 300 mg/kg/day) was administered to 6-week-old male Sprague-Dawley rats for 28 days. Three weeks after administration (Day 22), rats were sensitized to 2,4-dinitrofluoribenzene (DNFB) solution on their shaved abdomen. On the last administration day (Day 28), rats were challenged with DNFB solution on the right ear auricles. Within 24 to 32 hr after challenge, rats were sacrificed for analysis.
Induction of contact dermatitis
Contact dermatitis, one of the cell-mediated immune responses, was induced by using 2,4-dinitrofluoribenzene (DNFB; Wako Pure Chemical Co., Tokyo, Japan) as described in previous studies [6, 41, 58, 66]. After 3 weeks of administration (Day 22), the rats were sensitized on the shaved abdomen by applying 50 µl of 0.5% DNFB solution (dissolved in a mixture of acetone and sesame oil, 4:1) with a micropipette. Six days later (Day 28), the outer surfaces of the right ear auricles were challenged with 50 µl of 0.5% DNFB, and vehicle alone was applied in the same manner to the left ear auricles as a negative control. Within 24–32 hr after induction of contact dermatitis, all rats were deeply anesthetized with a mixture of pentobarbital and medetomidine, and the ear auricles, other organs (thymus, femoral bone marrow, spleen, mandibular lymph node, ileum including Peyer’s patch, and liver) and cecal contents were collected and subjected to analysis. The inflammatory response by DNFB was assessed by ear thickness, general histology and immunohistochemistry.
Measurement of ear thickness
To assess the degree of inflammatory response, we measured the thickness of the vertically cut right ear auricle using a micrometer dial thickness gauge (G-1 A type, Peacock; Ozaki MFG, Osaka, Japan). The measured value 10 sec after holding the ear auricles with the dial thickness gauge was evaluated. The ear auricle was stored at −80°C until measurement, and a single investigator performed the measurement throughout any one experiment to minimize variation due to technique.
Tissue preparation
We measured the organ weights of the thymus, spleen and liver after trimming. We cut the ear auricles vertically by using razors (FEATHER Safety Razor Co., Osaka, Japan), then embedded one cross section in Tissue-Tek® OCT compound and fresh-froze it (Sakura Finetek, Tokyo, Japan). The other cross sections of the ears and small blocks of other organs were fixed in 4% paraformaldehyde in phosphate buffer for 24 hr at 4°C, dehydrated through a graded series of ethanol followed by xylene, and embedded in paraffin. Finally, 4-µm-sections were cut by a cryostat (CM1950; Leica Microsystems, Wetzlar, Germany, for frozen-sections) or a sliding microtome (SM2000R; Leica Microsystems, for paraffin-sections), and mounted on a glass slide precoated with 0.2% 3-aminopropyltriethoxysilane (Shin-Etsu Chemical Co., Tokyo, Japan).
Histopathological analysis
For general histological analysis, the sagittal sections of ear auricles and the transverse sections of the thymus, femoral bone marrow, spleen, mandibular lymph node, ileum including the Peyer’s patch, and liver were stained with hematoxylin and eosin (HE; Merck KGaA, Darmstadt, Germany) after their deparaffinization and hydration, following the manufacturer’s instructions. To assess the degree of inflammatory response on the auricles, we examined the tissues for epidermal atrophy or thickening and changes in the extent of cellular infiltration in the dermis. In the case of the thymus, we measured the cortical and medullary areas as a histological assessment of the immunological status of this organ by using ImageJ software.
Immunohistochemistry
To determine the localization of immune cells (helper T cells, cytotoxic T cells, macrophages, dendritic cells and granulocytes), antigens were detected using the indirect method of enzyme immunohistochemistry, following the manufacturer’s instructions. Briefly, the sections of the ear auricles were dehydrated in acetone for 10 min at −18°C, then crosslinking fixed in 2% periodate lysine paraformaldehyde for 1 min at 4°C. After being rinsed three times in 0.01 M phosphate-buffered saline with 0.05% Tween-20 (TPBS; pH 7.4), they were incubated with Blocking One Histo (Nacalai Tesque Inc., Kyoto, Japan) for 1 hr at room temperature (RT), followed by incubation with anti-TCRαβ antibody (R73, diluted at 1:400; Serotec Ltd., Oxford, UK), anti-CD4 antibody (OX-35, diluted at 1:800; ThermoFisher Scientific, Waltham, MA, USA), anti-CD8 antibody (MRC OX-8, diluted at 1:800; ThermoFisher Scientific), anti-CD11b antibody (ED7, diluted at 1:800; ThermoFisher Scientific), anti-CD68 antibody (ED1, diluted at 1:800; Bio-Rad Laboratories, Inc., CA, USA) and anti-CD103 antibody (OX-62, diluted at 1:200; Abcam, Cambridge, MA, USA) for 18 hr at 4°C. Then all sections were immersed in 3% H2O2 for 1 hr at RT to quench the endogenous peroxidase activity. After being rinsed three times in TPBS, the sections were reacted with horseradish peroxidase rat anti-mouse IgG (H+L) (diluted at 1:200; Jackson ImmunoResearch Inc., West Grove, PA, USA) for 1 hr at RT. After an additional three rinses in TPBS, the immunoreactivities of the primary antibodies were examined with 3,3-diaminobenzidine tetrahydrochloride solution (EnVision®+ kit/HRP [DAB], Dako, Glostrup, Denmark). Mayer’s hematoxylin was used as a counterstain. The sections on which immunoreactivity was detected with the DAB system were placed in a graded series of ethanol, dehydrated with absolute ethanol, cleared in xylene and mounted in Eukitt® mounting medium (O. Kindler, Freiburg, Germany). We observed these glass slides paying attention to the extent of positive reaction in the auricles, in order to assess the degree of inflammatory response on the auricles.
16S rRNA sequencing
The cecal contents from individual rats (n=1: the CLO-0 and 30 groups, n=2: the CLO-300 group) were collected for 16S rRNA analysis [8, 20] after euthanasia, and stored under –80°C until preliminarily analysis. The cecal samples were sent to GENEWIZ (South Plainfield, NJ, USA), and DNA extraction, quality control, rDNA variable region amplification, library construction, high-throughput sequencing and data analysis were conducted. DNA samples were quantified using a Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA). 30–50 ng DNA was used to generate amplicons using a MetaVxTM Library Preparation kit (GENEWIZ). The v3 and v4 hypervariable regions of 16S rDNA were amplified using forward primers containing the sequence CCTACGGRRBGCASCAGKVRVGAAT and reverse primers containing the sequence GGACTACNVGGGTWTCTAATCC. Indexed adapters were added to the ends of the 16S rDNA amplicons to generate indexed libraries ready for downstream NGS sequencing on Illumina Miseq. DNA libraries were validated by Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA), and quantified by a Qubit 2.0 Fluorometer. DNA libraries were multiplexed and loaded on an Illumina MiSeq instrument according to manufacturer’s instructions (Illumina, San Diego, CA, USA). Sequencing was performed using a 2 × 300 paired-end (PE) configuration; image analysis and base calling were conducted by the MiSeq Control Software embedded in the MiSeq instrument. The QIIME data analysis package was used for 16S rRNA data analysis. After quality filtering and the removal of chimeric sequences, the sequences were grouped into operational taxonomic units (OTUs) using the clustering program VSEARCH (1.9.6) against the Silva 119 database pre-clustered at 97% sequence identity. The Ribosomal Database Program (RDP) classifier was used to assign a taxonomic category to all OTUs at confidence threshold of 0.8. The RDP classifier uses the Silva 132 database which has taxonomic categories predicted to the species level.
Statistical analysis
Statistical analysis was performed with Excel statistics 2012 (Version 1.00, SSRI, Tokyo, Japan). All data were analyzed by one-way ANOVA followed by the Dunnett’s post hoc test. The results were considered significant when the P-value was less than 0.05.
RESULTS
General findings
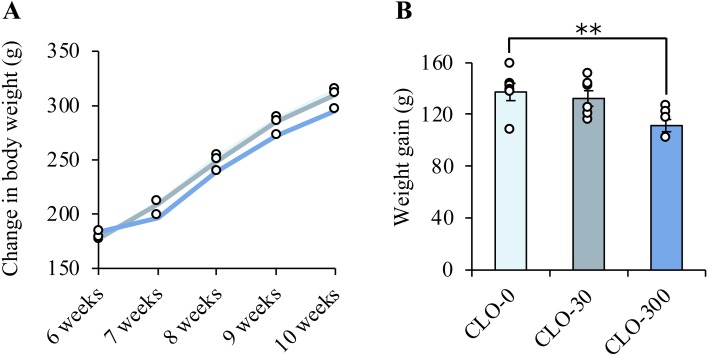
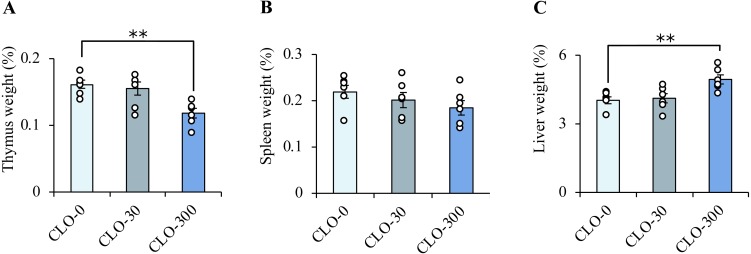
Three weeks after initiation of the administration experiment, loose stools were observed in some rats of the CLO-300 group. The mean values of final body weight were the largest in the CLO-0 group and the smallest in the CLO-300 group (Fig. 2A). Weight gain was significantly suppressed in the CLO-300 group (Fig. 2B). The relative organ weights (/body weight) of the thymus, spleen and liver of rats are shown in Fig. 3A–C. Compared with the CLO-0 group, the thymus weights were significantly lower, and the spleen weights approached significance (P=0.12) in the CLO-300 group. The liver weights were significantly higher in the CLO-300 group.
Fig. 2.
Effect of clothianidin (CLO) on the pattern of body weight gain. A: Observation of the changes in body weight over 28 days revealed that the average final body weight was suppressed in a CLO concentration-dependent manner. The circles represent the average value for each group. B: Weight gain was significantly lower in the CLO-300 group. Data represent means ± SE of each group and circles show the values for individual rats (n=6 in each). **P<0.01 vs. the CLO-0 group (one-way ANOVA followed by Dunnett’s post-hoc test).
Fig. 3.
Effect of clothianidin (CLO) on relative organ weight gain (/body weight). The weights of the thymus (A), spleen (B) and liver (C) of rats exposed to subchronic administrations are shown. The thymus weight was significantly lower and the change in the spleen weights approached significance in the CLO-300 group. The liver weight was significantly higher in the CLO-300 group. Data represent means ± SE of each group and circles show the values for individual rats (n=6 in each), **P<0.01 vs. the CLO-0 group (one-way ANOVA followed by Dunnett’s post-hoc test).
Assessment of inflammatory response
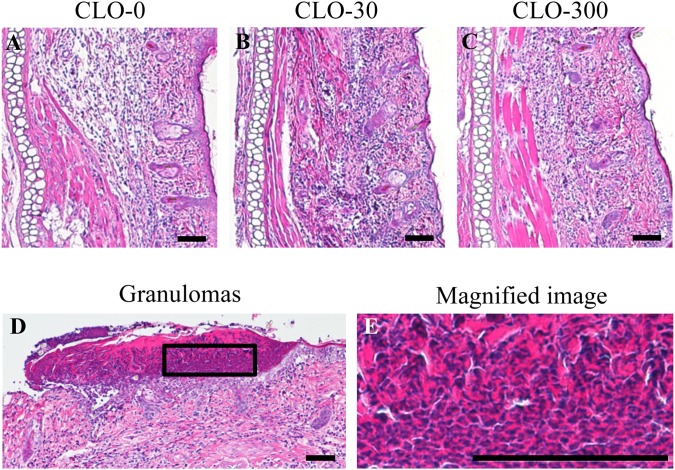
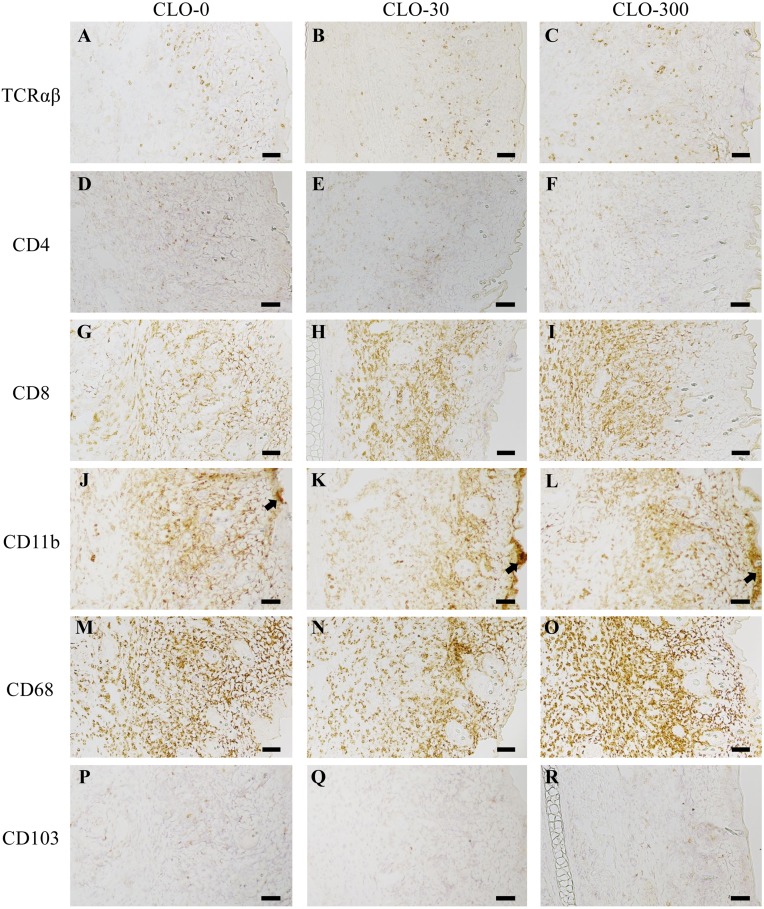
We induced contact dermatitis in rats using DNFB in order to investigate the effect of CLO on the immune response. The inflammatory response of contact dermatitis was observed in the right ear auricles of the animals and assessed by ear thickness, general histology and immunohistochemistry. The median value of the ear thickness was 0.612 mm (mean value: 0.619 mm; range: 0.481-0.765 mm). There was no significant effect of CLO on ear thickness (Table 1). The general histological analyses of the ear auricles by HE staining are shown in Fig. 4. Marked inflammatory cell infiltrations were observed in the right ear auricles (inflammatory skin), although no infiltrations were observed in the left ear auricles (normal skin), in all experimental groups. No significant effect of CLO on the degree of epidermal atrophy or thickening or the extent of inflammatory cell infiltration in the dermis was observed (Fig. 4A–C), although granulomas, areas of intense epidermal hyperplasia, were found in one rat of each of the CLO-30 and 300 groups (Fig. 4D). The magnified image of the granulomas (Fig. 4E) shows eosinophilic and the density of the flattened nucleus. The results of the immunohistochemical analyses of the ear auricles visualizing TCRαβ, CD4, CD8, CD11b, CD68 and CD103 are shown in Fig. 5. In all experimental groups, some degree of TCRαβ, CD4, CD8, CD11b and CD68 immunoreactivity was observed in the right ear auricles, whereas there were few positive cells in the left ear auricles. We assessed the degree of inflammation in the ear using the following 2 parameters as indices: (1) the distribution of immunoreactive cells in the vertical (the epidermis, dermis and area around the cartilage) and horizontal (base to tip) range, and (2) the immunoreactive intensity. Scattered TCRαβ immunoreactive cells and only a small number of CD4 immunoreactive cells were observed throughout the ear auricle in all experimental groups (Fig. 5A–F). There were a large number of CD8 (Fig. 5G–I) and CD68 (Fig. 5M–O) immunoreactive cells throughout the ear auricle, especially in the dermis, and these markers showed strong immunoreactivity in all experimental groups. The number of CD11b immunoreactive cells that showed strong immunoreactivity was large in all experimental groups, and dense regions of immunoreactive cells were observed in the epidermis in some rats regardless of CLO administration (Fig. 5J–L). There were almost no CD103 immunoreactive cells, and the intensity of CD103 was lower compared with the intensities of the other markers (Fig. 5P–R). Taken together, these results showed that CLO had no significant effect on the distribution of immunoreactive cells, and the immunoreactive intensity.
Table 1. Effect of clothianidin (CLO) on contact dermatitis and thymic structure.
| Groups | Ear thickness (µm) | M/C ratio (%) |
|---|---|---|
| CLO-0 | 62.23 ± 3.23 | 30.87 ± 6.48 |
| CLO-30 | 60.70 ± 2.88 | 33.85 ± 3.32 |
| CLO-300 | 62.68 ± 3.35 | 30.74 ± 2.63 |
Median values for ear thickness and medulla/cortical area (M/C) ratio. Each values were used to assess contact dermatitis or the immunological status of thymus. Data represent mean ± SE of each group (n=6 in each) and evaluated by one-way ANOVA followed by Dunnett’s post-hoc test. There was no significant effect of CLO.
Fig. 4.
General histology of contact dermatitis. The results of HE staining are shown for the sagittal sections of the right ear auricle in the rats of the clothianidin (CLO)-0 (A), CLO-30 (B) and CLO-300 (C) groups. The left side of each panel is the cartilage area, the central part is the dermis, and the right side is the epidermis. The granulomas found in a portion of the epidermis in one rat of each of the CLO-30 and 300 groups (D) and a magnified image (E) are also shown.
Fig. 5.
Representative immunohistochemistry for TCRαβ (A–C), CD4 (D–F), CD8 (G–I), CD11b (J–L), CD68 (M–O) and CD103 (P–R) of the right ear auricles. The left, central and right panels show results for the clothianidin (CLO)-0, 30 and 300 groups, respectively. In addition to the HE staining, the left side of each panel shows the cartilage area, the central part the dermis, and the right side the epidermis. As for CD11b, the densities of immunoreactive cells (arrow) were observed in a portion of the epidermis in some rats regardless of CLO administration. Scale bars=100 µm.
General histological findings excluding the ear auricle
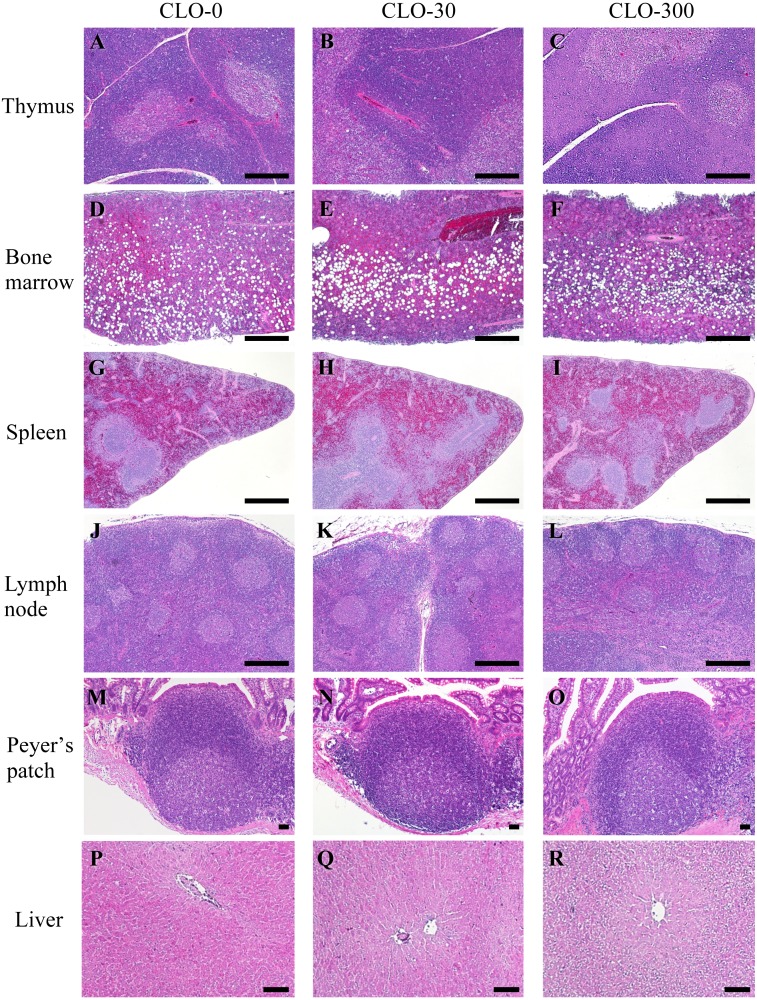
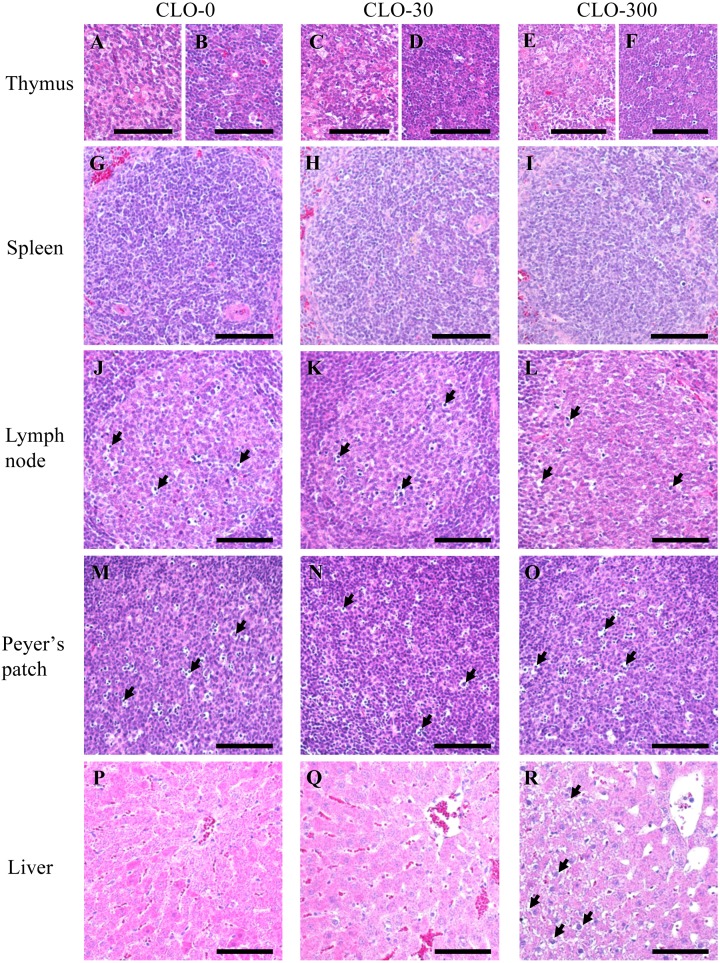
Next, we investigated whether subchronic oral administration of CLO affects the histological structure of the immune organs and liver. The general histological analyses of thymus, femoral bone marrow, spleen, mandibular lymph node, ileum including the Peyer’s patch, and liver by HE staining are shown in Figs. 6 and 7. In the thymus, there were no histological differences in any of the structures—such as the number of nuclear pyknosis and Hassall bodies—among the experimental groups (Figs. 6A–C, 7A–F). No significant effect of CLO on the cortical and medullary areas was observed (Table 1). In the femoral bone marrow of all experimental groups, adipocyte formation was significantly observed, but blood cells, which are indicators of hematopoietic function, appeared normal (Fig. 6D–F). In the spleen, abnormal histological changes such as increased fibrosis and hemosiderin deposition were not observed in any of the groups (Fig. 6G–I). There were no histological changes in the structures, such as the area occupied by the white pulp and the number of nuclear pyknosis, in any of the experimental groups (Fig. 7G–I). In the mandibular lymph node of all experimental groups, developed lymphoid follicles and a certain number of nuclear pyknosis in the germinal center were observed. There was no significant effect of CLO in the structure of the mandibular lymph node (Figs. 6J–L, 7J–L). In the Peyer’s patch, there were no histological changes in the structures, such as the number of nuclear pyknosis, in any of the experimental groups (Figs. 6M–O, 7M–O). In the liver, there was no significant effect of CLO on the nucleus and cell membrane, but cytoplasmic vacuolation of the hepatocytes was found in most rats of CLO-300 group (Figs. 6P–R, 7P–R). It is known that NNs induce oxidative stress and cause fatty liver in Quails by depositing free fatty acid [53, 60], while other pesticides influence body glucose homeostasis and cause glycogen storage [46, 48]. It is possible that our results reflect the deposition of fat drops or glycogen by CLO.
Fig. 6.
Effect of clothianidin (CLO) on the histology of immune organs and liver. Oral administration of CLO induced no marked alterations in histopathology of the thymus (A–C), femoral bone marrow (D–F), spleen (G–I), mandible lymph node (J–L) and Peyer’s patch (M–O), except for liver (P–R). The left, central and right panels show results for the CLO-0, 30 and 300 groups, respectively. HE staining, transverse section and scale bars=500 µm (A–L), 100 µm (M–R).
Fig. 7.
Magnified images of the immune organs and liver (A–R). The left, central and right panels show results for the clothianidin (CLO)-0, 30 and 300 groups, respectively. Representative examples of the general histology of the medulla of the thymus are shown in (A, C, E), and representative examples of the general histology of the cortex of the thymus are shown in (B, D, F). Representative general histology for the white pulp of spleen (G–I). Representative general histology for the germinal center of mandible lymph node. A certain number of nuclear pyknosis (arrows) were observed among all experimental groups (J–L). Representative general histology for the follicles’ germinal centers of Peyer’s patch. Some nuclear pyknosis (arrows) were similarly observed (M–O). Representative general histology for around central vein of liver (P–R). Cytoplasmic vacuolation of the hepatocytes (arrows) was found in most of CLO-300 group (R). Scale bars=100 µm.
Analysis of differential gut microbiota
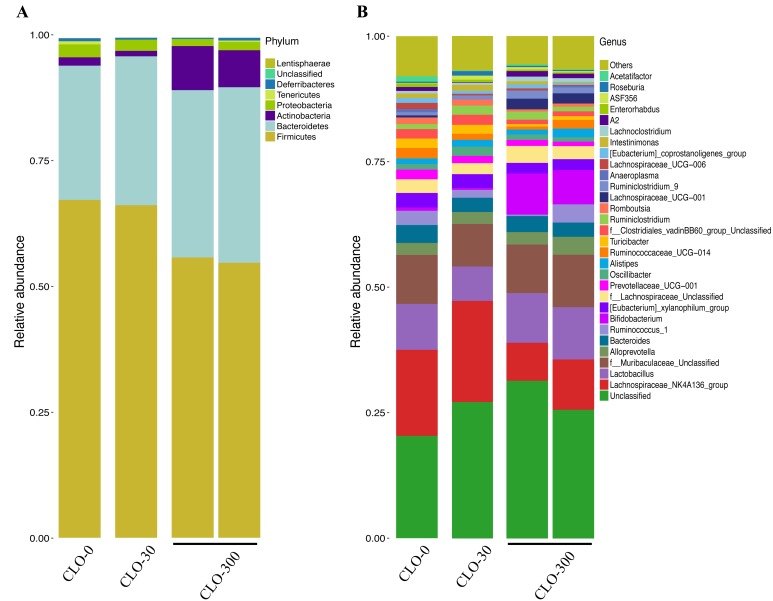
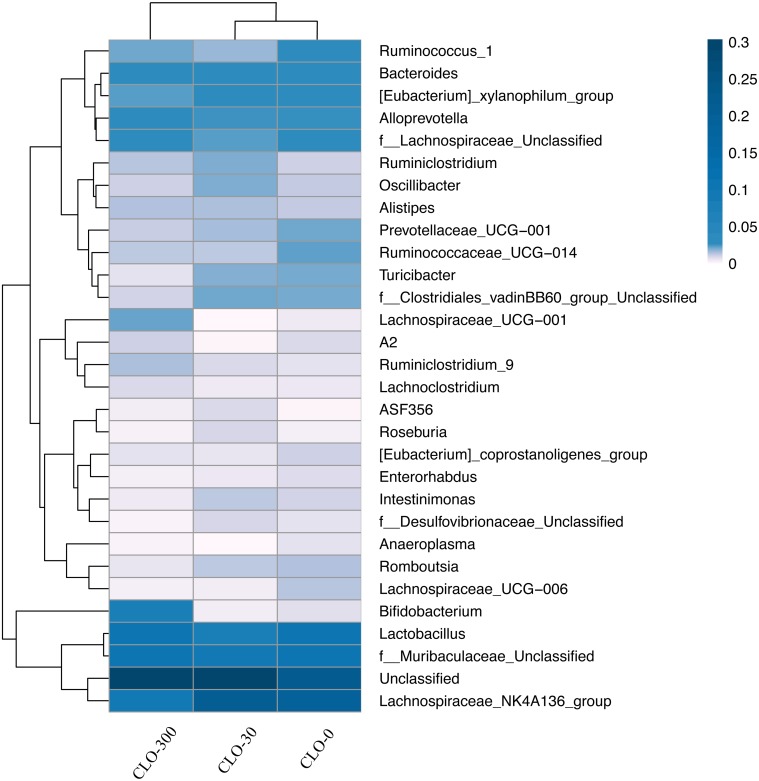
Illumina MiSeq sequencing was conducted at GENEWIZ in order to evaluate the effect of CLO on the gut microbiota preliminarily. The cecal samples from individual rats of the administration experiment were subjected to analysis. The numbers of OTUs identified in the cecal samples were 445 (the CLO-0 group), 442 (the CLO-30 group) and 447 and 440 (the CLO-300 group), respectively; thus, there were no obvious differences among groups. In the CLO-0 and 30 groups, the phyla Firmicutes and Bacteroidetes were dominant in terms of relative abundance, followed by Proteobacteria and Actinobacteria. In the CLO-300, the phyla Firmicutes and Bacteroidetes were dominant, but compared with the other groups, Firmicutes was clearly decreased and Bacteroidetes was increased. The dominance of Actinobacteria and Proteobacteria were reversed in the CLO-300 group (Fig. 8A). At the genus level, the Lachnospiraceae_NK4A136_group was the most dominant bacterial group in the CLO-0 and 30 groups. On the other hand, in the CLO-300 group, Lachnospiraceae_NK4A136_group was the third or fourth most dominant bacterial group, and Bifidobacterium was much more abundance than in the CLO-0 and 30 groups (Fig. 8B). The distributions of the 30 most abundant species in each group on the Genus level were clustered and plotted in a heatmap (Fig. 9). The heatmap shows that the abundance of Lachnospiraceae_NK4A136_group was lower, and that of Bifidobacterium was higher in the CLO-300 group than in the CLO-0 and 30 groups. Moreover, there were some effects of CLO on the relative abundance of microbiota on the genus level, such as for [Eubacterium]_xylanophilum_group, Prevotellaceae_UCG-001, Ruminococcaceae_UCG-014, Turicibacter, Lachnospiraceae_UCG-001 and Unclassified. The cluster results indicate that the composition of microbiota of the CLO-300 group was distinct from those of the CLO-0 and 30 groups, although this result was not definitive due to the small number of samples.
Fig. 8.
Effect of clothianidin (CLO) on the gut microbiota. The relative abundance of various phyla and genera in the gut microbiomes of rats is shown. Group names are given (CLO-0: n=1, CLO-30: n=1, CLO-300: n=2). Abundance at the phylum level is shown in panel (A), and that at the genus level is shown in panel (B). “Other” indicates the relative abundance of all genus-level classifications other than the top 30.
Fig. 9.
The distributions of the 30 most abundant species of each group at the Genus level were clustered and plotted in a heatmap. Similarities and differences in each species are visualized by the color scheme in the heat map. The columns represent groups and the rows represent species. The dendrogram above the heatmap shows the cluster results for the samples and/or groups, and the dendrogram to the left shows the species cluster. The colors in the heat map represent the relative abundance of the corresponding species in the corresponding group.
DISCUSSION
We conducted experiments to investigate the effects of CLO on the immune system, including the immune response, in order to evaluate the causal association between the high frequency of allergic diseases and routine exposure to NNs. In contact dermatitis, no significant effect of CLO was found on ear swelling and immune cell infiltration. The concentration and amount of DNFB solution used for sensitization were almost the same as in the previous studies mentioned above [6, 41, 58, 66]. It is unlikely that we caused an excessive inflammatory response and failed to detect faint CLO effects. Therefore, our results suggest that CLO had only a small or no effect on the cellular immune response. There are also the following possibilities in regard to our results on ear swelling and immune cell infiltration. We showed that CLO decreased the thymus weight. Thus, the reason that a normal cellular immune response was observed may be that CLO significantly affected the primary lymphoid organs, but not the secondary ones. Previous studies reported that immune cells in peripheral tissues generally migrate to specific nearby lymph nodes (regional lymph nodes), and the mandibular lymph nodes are considered to be regional lymph nodes of the ear, nose and throat [7, 40]. Since people are routinely exposed to CLO via food consumption, the present study was conducted using an oral exposure route. CLO may have had no effect on the regional lymph nodes of ear auricles, which are more distant than the exposure sites. General histological findings of the ear auricles also showed no significant effect of CLO on the degree of epidermal atrophy or thickening and the extent of inflammatory cell infiltration in the dermis. However, granulomas, areas of intense epidermal hyperplasia, were found in one test rat of each of the CLO-30 and 300 groups. Granulomas are masses resulting from chronic inflammation. ACh reduces inflammatory cytokines from macrophages via α7 nAChR [44]. It is possible that subchronic CLO exposure might suppress cholinergic anti-inflammatory effects by reducing the expression of nAChRs. Thus, it was suggested that CLO might enhance the local inflammatory response, even if this does not result in severe inflammation.
Although there was little effect on the cellular immune response, thymus weight loss was found in the CLO-300 group. The general histological analysis showed no abnormal structures, including with respect to the percentages of cortical and medullary areas. In addition, no significant general histological changes were observed in the femoral bone marrow, spleen, mandibular lymph node or Peyer’s patch. Our results indicate that there were no toxicity-related effects on the structure of these organs involved in immunity. Further studies are needed to determine the functional impact of CLO. The thymus is a specialized immune organ that plays an important role in the increase of TCR repertoires recognizing antigens, and the differentiation to CD4+ or CD8+ T cells. Since CD4+ T cells play a central role in the acquired immune system, it is possible that CLO had some effects on immune functions, such as the humoral immune response, that were dependent on decreased cells in the thymus. It is generally known that the thymus is highly sensitive to various stresses such as aging, sound, light and chemicals, and exposure to those stressors causes thymic atrophy [19]. Nicotine, which is an agonist of nAChRs, can activate the α7 nAChR on thymocytes and induce upregulation of the Fas apoptotic pathway [36]. It has been demonstrated that nicotine can mediate cell-cycle progression through the α7 nAChRs [54]. Taken together, these results suggest that CLO may have induced stress and/or disrupted the signaling downstream of nAChRs. Currently, there are few studies regarding thymic atrophy caused by NNs. In the present study, we provide new evidence that CLO causes thymic atrophy in only 10 times the amount of NOAEL [14].
Loose stool and suppression of body weight gain were observed in the CLO-300 group, suggesting that CLO may have some effect on the intestine. The intestine is considered to be the largest immune organ in the body, accounting for about 60–70% of host immune cells. ACh, which is a ligand of nAChRs, is one of the neurotransmitters in the nervous system, and the peristaltic movement is controlled by innervation of the enteric nervous system and autonomic nerves. Thus, it is possible that peristaltic movement was activated by CLO, leading to inadequate absorption of nutrition and water during feeding. In fact, anticholinergic agents are used for the treatment of diarrhea, because they slow colonic transit and improve stool consistency and frequency [35]. Increasing evidence indicates that the immune and nervous system maintain extensive interactions [12, 57]. Our present results further may suggest that the homeostasis of the immune system may be disrupted via influencing the intestinal functions.
In addition, recent studies have shown that the mammalian immune system plays an essential role in maintaining homeostasis with resident microbial communities, thus ensuring that the mutualistic nature of the host-microbial relationship is maintained [13, 25]. Immune maturation is likely influenced directly and/or indirectly by the presence of commensal microbes [17, 47]. The microbiota is influenced by a series of environmental factors, such as antibiotics and heavy metals [10, 29, 37, 71]. It has been increasingly investigated that several pesticides, including NNs, can influence gut microbiota [16, 28]. In consideration of the results of these previous studies, we investigated the direct and/or indirect effects of CLO on the immune system that are mediated by the gut microbiota. We found that Firmicutes was clearly decreased, Bacteroidetes was increased and the dominance of Proteobacteria and Actinobacteria was reversed in the CLO-300 group. There was also some effect of CLO on the relative abundance of microbiota at the genus level, such as on the levels of [Eubacterium]_xylanophilum_group and Bifidobacterium, which are related to production of short-chain fatty acids (SCFAs). SCFAs are known to be involved in inducing Treg cells, macrophages or dendritic cells [15, 62]. In spite of the small number of samples, this result shows that CLO changes the composition of the gut microbiota and may influence the immune system.
In summary, we found no significant effect of CLO on the inflammatory response in histological analyses, but we observed thymus weight loss, loose stool, suppression of body weight gain, and changes in the gut microbiota in rats exposed to CLO for 28 days. Although these results were observed at a dose 10 times greater than the NOAEL dose, they suggested that CLO may have effects on the immune system. Further studies are needed in regard to specific effects on inflammatory cytokines, immune cells and nAChRs. Several studies have already demonstrated that exposure to a combination of multiple pesticides, or a combination of pesticide(s) plus stress, elicits more serious effects than exposure to either a single pesticide or stress alone [1, 18, 23, 27]. Multiple pesticides are often used in agriculture and people are exposed to multiple environmental factors in real-world settings. The present study provides evidence that subchronic exposure to CLO alone affects the immune system, which should contribute to the accuracy of future evaluations of the safety of NNs.
Acknowledgments
This work was partly supported by Grants-in-Aid for Scientific Research A (#18H04132) and B (19H04277) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Abou-Donia M. B., Khan W. A., Dechkovskaia A. M., Goldstein L. B., Bullman S. L., Abdel-Rahman A.2006. In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult offspring rats. Arch. Toxicol. 80: 620–631. doi: 10.1007/s00204-006-0077-1 [DOI] [PubMed] [Google Scholar]
- 2.Arnson Y., Shoenfeld Y., Amital H.2010. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 34: J258–J265. doi: 10.1016/j.jaut.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Bach J. F.2001. Protective role of infections and vaccinations on autoimmune diseases. J. Autoimmun. 16: 347–353. doi: 10.1006/jaut.2000.0478 [DOI] [PubMed] [Google Scholar]
- 4.Bach J. F.2005. Infections and autoimmune diseases. J. Autoimmun. 25 Suppl: 74–80. doi: 10.1016/j.jaut.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 5.Badgujar P. C., Jain S. K., Singh A., Punia J. S., Gupta R. P., Chandratre G. A.2013. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ. Toxicol. Pharmacol. 35: 408–418. doi: 10.1016/j.etap.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 6.Bian R., Tang J., Hu L., Huang X., Liu M., Cao W., Zhang H.2018. (E)-phenethyl 3-(3,5-dihydroxy-4-isopropylphenyl) acrylate gel improves DNFB-induced allergic contact hypersensitivity via regulating the balance of Th1/Th2/Th17/Treg cell subsets. Int. Immunopharmacol. 65: 8–15. doi: 10.1016/j.intimp.2018.09.032 [DOI] [PubMed] [Google Scholar]
- 7.Bruneton J. N., Roux P., Caramella E., Demard F., Vallicioni J., Chauvel P.1984. Ear, nose, and throat cancer: ultrasound diagnosis of metastasis to cervical lymph nodes. Radiology 152: 771–773. doi: 10.1148/radiology.152.3.6463260 [DOI] [PubMed] [Google Scholar]
- 8.Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J., Holmes S. P.2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13: 581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardone A.2015. Imidacloprid induces morphological and molecular damages on testis of lizard (Podarcis sicula). Ecotoxicology 24: 94–105. doi: 10.1007/s10646-014-1361-0 [DOI] [PubMed] [Google Scholar]
- 10.Chi L., Mahbub R., Gao B., Bian X., Tu P., Ru H., Lu K.2017. Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem. Res. Toxicol. 30: 2110–2119. doi: 10.1021/acs.chemrestox.7b00162 [DOI] [PubMed] [Google Scholar]
- 11.Di Prisco G., Iannaccone M., Ianniello F., Ferrara R., Caprio E., Pennacchio F., Capparelli R.2017. The neonicotinoid insecticide Clothianidin adversely affects immune signaling in a human cell line. Sci. Rep. 7: 13446. doi: 10.1038/s41598-017-13171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dou Y., Luo J., Wu X., Wei Z., Tong B., Yu J., Wang T., Zhang X., Yang Y., Yuan X., Zhao P., Xia Y., Hu H., Dai Y.2018. Curcumin attenuates collagen-induced inflammatory response through the “gut-brain axis”. J. Neuroinflamm. 15: 6. doi: 10.1186/s12974-017-1047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Aidy S., Dinan T. G., Cryan J. F.2014. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 5: 146. doi: 10.3389/fmicb.2014.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Agriculture Organization of the United Nations. 2016. FAO Specifications and Evaluations for Agricultural Pesticide Clothianidin http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/Clothianidin2011.pdf [accessed on January 10, 2020].
- 15.Furusawa Y., Obata Y., Fukuda S., Endo T. A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., Takahashi M., Fukuda N. N., Murakami S., Miyauchi E., Hino S., Atarashi K., Onawa S., Fujimura Y., Lockett T., Clarke J. M., Topping D. L., Tomita M., Hori S., Ohara O., Morita T., Koseki H., Kikuchi J., Honda K., Hase K., Ohno H.2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. doi: 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 16.Gao B., Bian X., Mahbub R., Lu K.2017. Sex-specific effects of organophosphate diazinon on the gut microbiome and its metabolic functions. Environ. Health Perspect. 125: 198–206. doi: 10.1289/EHP202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geuking M. B., Cahenzli J., Lawson M. A., Ng D. C., Slack E., Hapfelmeier S., McCoy K. D., Macpherson A. J.2011. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34: 794–806. doi: 10.1016/j.immuni.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 18.Gill R. J., Ramos-Rodriguez O., Raine N. E.2012. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491: 105–108. doi: 10.1038/nature11585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruver A. L., Sempowski G. D.2008. Cytokines, leptin, and stress-induced thymic atrophy. J. Leukoc. Biol. 84: 915–923. doi: 10.1189/jlb.0108025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handelsman J.2004. Metagenomics: application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 68: 669–685. doi: 10.1128/MMBR.68.4.669-685.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry M., Béguin M., Requier F., Rollin O., Odoux J. F., Aupinel P., Aptel J., Tchamitchian S., Decourtye A.2012. A common pesticide decreases foraging success and survival in honey bees. Science 336: 348–350. doi: 10.1126/science.1215039 [DOI] [PubMed] [Google Scholar]
- 22.Hirano T., Minagawa S., Furusawa Y., Yunoki T., Ikenaka Y., Yokoyama T., Hoshi N., Tabuchi Y.2019. Growth and neurite stimulating effects of the neonicotinoid pesticide clothianidin on human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 383: 114777. doi: 10.1016/j.taap.2019.114777 [DOI] [PubMed] [Google Scholar]
- 23.Hirano T., Yanai S., Omotehara T., Hashimoto R., Umemura Y., Kubota N., Minami K., Nagahara D., Matsuo E., Aihara Y., Shinohara R., Furuyashiki T., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2015. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J. Vet. Med. Sci. 77: 1207–1215. doi: 10.1292/jvms.15-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirano T., Yanai S., Takada T., Yoneda N., Omotehara T., Kubota N., Minami K., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2018. NOAEL-dose of a neonicotinoid pesticide, clothianidin, acutely induce anxiety-related behavior with human-audible vocalizations in male mice in a novel environment. Toxicol. Lett. 282: 57–63. doi: 10.1016/j.toxlet.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 25.Hooper L. V., Littman D. R., Macpherson A. J.2012. Interactions between the microbiota and the immune system. Science 336: 1268–1273. doi: 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshi N., Hirano T., Omotehara T., Tokumoto J., Umemura Y., Mantani Y., Tanida T., Warita K., Tabuchi Y., Yokoyama T., Kitagawa H.2014. Insight into the mechanism of reproductive dysfunction caused by neonicotinoid pesticides. Biol. Pharm. Bull. 37: 1439–1443. doi: 10.1248/bpb.b14-00359 [DOI] [PubMed] [Google Scholar]
- 27.Iwasa T., Motoyama N., Ambrose J. T., Roe R. M.2004. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 23: 371–378. doi: 10.1016/j.cropro.2003.08.018 [DOI] [Google Scholar]
- 28.Jones J. C., Fruciano C., Hildebrand F., Al Toufalilia H., Balfour N. J., Bork P., Engel P., Ratnieks F. L., Hughes W. O.2017. Gut microbiota composition is associated with environmental landscape in honey bees. Ecol. Evol. 8: 441–451. doi: 10.1002/ece3.3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaminogawa S.2010. Effects of food components on intestinal flora, intestinal immune system and their mutualism. Biosci. Microflora 29: 69–82. doi: 10.12938/bifidus.29.69 [DOI] [Google Scholar]
- 30.Kawashima K., Fujii T., Moriwaki Y., Misawa H., Horiguchi K.2015. Non-neuronal cholinergic system in regulation of immune function with a focus on α7 nAChRs. Int. Immunopharmacol. 29: 127–134. doi: 10.1016/j.intimp.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 31.Kawashima K., Yoshikawa K., Fujii Y. X., Moriwaki Y., Misawa H.2007. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 80: 2314–2319. doi: 10.1016/j.lfs.2007.02.036 [DOI] [PubMed] [Google Scholar]
- 32.Kawahito Y.2012. [Rheumatoid arthritis: progress in diagnosis and treatment. Topics: I. pathogenesis; 2. environmental factor]. Nippon Naika Gakkai Zasshi 101: 2824–2829 (in Japanese). doi: 10.2169/naika.101.2824 [DOI] [PubMed] [Google Scholar]
- 33.Kimura-Kuroda J., Komuta Y., Kuroda Y., Hayashi M., Kawano H.2012. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS One 7: e32432. doi: 10.1371/journal.pone.0032432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kono T.2011. Recent issues in the etiology of atopic dermatitis. J. Nippon Med. Sch. 7: 83–87. [Google Scholar]
- 35.Lee K. J.2015. Pharmacologic agents for chronic diarrhea. Intest. Res. 13: 306–312. doi: 10.5217/ir.2015.13.4.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H. X., Liu S., Qu W., Yan H. Y., Wen X., Chen T., Hou L. F., Ping J.2017. α7 nAChR mediated Fas demethylation contributes to prenatal nicotine exposure-induced programmed thymocyte apoptosis in mice. Oncotarget 8: 93741–93756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchesi J. R., Adams D. H., Fava F., Hermes G. D., Hirschfield G. M., Hold G., Quraishi M. N., Kinross J., Smidt H., Tuohy K. M., Thomas L. V., Zoetendal E. G., Hart A.2016. The gut microbiota and host health: a new clinical frontier. Gut 65: 330–339. doi: 10.1136/gutjnl-2015-309990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mashimo M., Komori M., Matsui Y. Y., Murase M. X., Fujii T., Takeshima S., Okuyama H., Ono S., Moriwaki Y., Misawa H., Kawashima K.2019. Distinct roles of α7 nAChRs in antigen-presenting cells and CD4+ T cells in the regulation of T cell differentiation. Front. Immunol. 10: 1102. doi: 10.3389/fimmu.2019.01102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda K., Buckingham S. D., Kleier D., Rauh J. J., Grauso M., Sattelle D. B.2001. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 22: 573–580. doi: 10.1016/S0165-6147(00)01820-4 [DOI] [PubMed] [Google Scholar]
- 40.McMaster P. D., Hudack S. S.1935. The formation of agglutinins within lymph nodes. J. Exp. Med. 61: 783–805. doi: 10.1084/jem.61.6.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mondal S., Ghosh R. C., Mate M. S., Karmakar D. B.2009. Effects of acetamiprid on immune system in female Wistar rats. Proc. Zool. Soc. 62: 109–117. doi: 10.1007/s12595-009-0012-6 [DOI] [Google Scholar]
- 42.Nakazawa D. J.2008. The Autoimmune Epidemic: Bodies Gone Haywire in a World Out of Balance. Touchstone/ Simon & Schuster, New York. [Google Scholar]
- 43.National Institute for Environmental Studies Environmental Risk Research Center. 2018. https://www.nies.go.jp/kisplus/dtl/chem/NT300118 [accessed on January 10, 2020].
- 44.Pavlov V. A., Wang H., Czura C. J., Friedman S. G., Tracey K. J.2003. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 9: 125–134. doi: 10.1007/BF03402177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phipatanakul W., Cronin B., Wood R. A., Eggleston P. A., Shih M. C., Song L., Tachdjian R., Oettgen H. C.2004. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann. Allergy Asthma Immunol. 92: 420–425. doi: 10.1016/S1081-1206(10)61777-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahimi R., Abdollahi M.2007. A review on the mechanisms involved in hyperglycemia induced by organophosphorus pesticides. Pestic. Biochem. Physiol. 88: 115–121. doi: 10.1016/j.pestbp.2006.10.003 [DOI] [Google Scholar]
- 47.Rakoff-Nahoum S., Medzhitov R.2008. Innate immune recognition of the indigenous microbial flora. Mucosal Immunol. 1 Suppl 1: S10–S14. doi: 10.1038/mi.2008.49 [DOI] [PubMed] [Google Scholar]
- 48.Rezg R., Mornagui B., El-Arbi M., Kamoun A., El-Fazaa S., Gharbi N.2006. Effect of subchronic exposure to malathion on glycogen phosphorylase and hexokinase activities in rat liver using native PAGE. Toxicology 223: 9–14. doi: 10.1016/j.tox.2006.02.020 [DOI] [PubMed] [Google Scholar]
- 49.Rheumatology/Allergy Prevention Committee report. 2011. https://www.mhlw.go.jp/stf/shingi/2r9852000001nes4-att/2r9852000001newa.pdf [accessed on January 10, 2020].
- 50.Romagnani S.2004. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunology 112: 352–363. doi: 10.1111/j.1365-2567.2004.01925.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosas-Ballina M., Olofsson P. S., Ochani M., Valdés-Ferrer S. I., Levine Y. A., Reardon C., Tusche M. W., Pavlov V. A., Andersson U., Chavan S., Mak T. W., Tracey K. J.2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101. doi: 10.1126/science.1209985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salema L. H., Alwan M. J., Yousif A. A.2016. Immunotoxic effect of thiamethoxam in immunized mice with Brucella abortus cultural filtrate antigen. Vet. World 9: 1407–1412. doi: 10.14202/vetworld.2016.1407-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato H.1999. Hepatotoxicity. pp. 111–118. In: Toxicology—Living Organism, Environment, Ecosystem— (Fujita, S. ed.), Asakura Publishing Co., Ltd., Tokyo (in Japanese). [Google Scholar]
- 54.Schaal C., Chellappan S. P.2014. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 12: 14–23. doi: 10.1158/1541-7786.MCR-13-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz Larsen F.2000. Genetic epidemiology of atopic dermatitis. pp. 113–124. In: Atopic Dermatitis (Williams, H. C. ed.), Cambridge University Press, Cambridge. [Google Scholar]
- 56.Shakthi Devan R. K., Prabu P. C., Panchapakesan S.2015. Immunotoxicity assessment of sub-chronic oral administration of acetamiprid in Wistar rats. Drug Chem. Toxicol. 38: 328–336. doi: 10.3109/01480545.2014.966382 [DOI] [PubMed] [Google Scholar]
- 57.Stanisz A. M., Befus D., Bienenstock J.1986. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer’s patches, mesenteric lymph nodes, and spleen. J. Immunol. 136: 152–156. [PubMed] [Google Scholar]
- 58.Tahara E., Satoh T., Watanabe C., Shimada Y., Itoh T., Nagai H., Terasawa K., Saiki I.1999. A third-phase cutaneous (very late phase) response after elicitation with dinitrofluorobenzene in passively or actively sensitized mice. Allergol. Int. 48: 265–273. doi: 10.1046/j.1440-1592.1999.00143.x [DOI] [Google Scholar]
- 59.Takada T., Yoneda N., Hirano T., Yanai S., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Hoshi N.2018. Verification of the causal relationship between subchronic exposures to dinotefuran and depression-related phenotype in juvenile mice. J. Vet. Med. Sci. 80: 720–724. doi: 10.1292/jvms.18-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tokumoto J., Danjo M., Kobayashi Y., Kinoshita K., Omotehara T., Tatsumi A., Hashiguchi M., Sekijima T., Kamisoyama H., Yokoyama T., Kitagawa H., Hoshi N.2013. Effects of exposure to clothianidin on the reproductive system of male quails. J. Vet. Med. Sci. 75: 755–760. doi: 10.1292/jvms.12-0544 [DOI] [PubMed] [Google Scholar]
- 61.Tomizawa M., Casida J. E.2003. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 48: 339–364. doi: 10.1146/annurev.ento.48.091801.112731 [DOI] [PubMed] [Google Scholar]
- 62.Trompette A., Gollwitzer E. S., Yadava K., Sichelstiel A. K., Sprenger N., Ngom-Bru C., Blanchard C., Junt T., Nicod L. P., Harris N. L., Marsland B. J.2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20: 159–166. doi: 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 63.Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J.2003. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421: 384–388. doi: 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- 64.Wessler I., Kirkpatrick C. J.2008. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 154: 1558–1571. doi: 10.1038/bjp.2008.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitehorn P. R., O’Connor S., Wackers F. L., Goulson D.2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336: 351–352. doi: 10.1126/science.1215025 [DOI] [PubMed] [Google Scholar]
- 66.Wu W., Takemasa M., Nagano T.2015. Effect of dinitrofluorobenzene on contact hypersensitivity in mice. Kawasaki J. Med. Welf. 25: 113–119. [Google Scholar]
- 67.Yanai S., Hirano T., Omotehara T., Takada T., Yoneda N., Kubota N., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2017. Prenatal and early postnatal NOAEL-dose clothianidin exposure leads to a reduction of germ cells in juvenile male mice. J. Vet. Med. Sci. 79: 1196–1203. doi: 10.1292/jvms.17-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoneda N., Takada T., Hirano T., Yanai S., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Hoshi N.2018. Peripubertal exposure to the neonicotinoid pesticide dinotefuran affects dopaminergic neurons and causes hyperactivity in male mice. J. Vet. Med. Sci. 80: 634–637. doi: 10.1292/jvms.18-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanetti S. R., Ziblat A., Torres N. I., Zwirner N. W., Bouzat C.2016. Expression and functional role of α7 nicotinic receptor in human cytokine-stimulated NK cells. J. Biol. Chem. 291: 16541–16552. doi: 10.1074/jbc.M115.710574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zdanowski R., Krzyżowska M., Ujazdowska D., Lewicka A., Lewicki S.2015. Role of α7 nicotinic receptor in the immune system and intracellular signaling pathways. Cent. Eur. J. Immunol. 40: 373–379. doi: 10.5114/ceji.2015.54602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L., Nichols R. G., Correll J., Murray I. A., Tanaka N., Smith P. B., Hubbard T. D., Sebastian A., Albert I., Hatzakis E., Gonzalez F. J., Perdew G. H., Patterson A. D.2015. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ. Health Perspect. 123: 679–688. doi: 10.1289/ehp.1409055 [DOI] [PMC free article] [PubMed] [Google Scholar]