Abstract
Fluid responsiveness, defined as the response of stroke volume to fluid loading, is a tool to individualize fluid administration in order to avoid the deleterious effects of hypovolemia or hypervolemia in hospitalized patients. To evaluate the accuracy of two ultrasound indices, the caudal vena cava to abdominal aorta ratio (CVC/Ao) and the respiratory collapsibility of the caudal vena cava (cCVC), as independent predictors of fluid responsiveness in a heterogeneous population of spontaneously breathing, conscious, hospitalized dogs. A prospective, multicenter, observational, cross-sectional study was designed in twenty-five dogs. The accuracy of CVC/Ao and cCVC in predicting fluid responsiveness was evaluated by the area under the receiver operating characteristic curve (AUROC) in a group of hospitalized dogs after receiving a mini-fluid bolus of 4 ml/kg of Hartmann’s solution. Dogs with an increased aortic velocity time integral >15% were classified as fluid responders. Twenty-two dogs were finally included. Ten were classified as responders and 12 as non-responders. The AUROC curves were 0.88 for the CVC/Ao ratio (95% confidence interval, CI, 0.67–0.98; P=0.0001) and 0.54 for cCVC (95% CI 0.32–0.75; P=0.75). The CVC/Ao threshold optimized for best sensitivity (SE) and specificity (SP) values was 0.83 (SE 100%; SP 75%). In spontaneously breathing hospitalized dogs, the CVC/Ao measurement predicted stroke volume increase after a fluid bolus, while the respiratory variations in the cCVC did not discriminate between fluid responders and non-responders.
Keywords: dog, fluid, hemodynamic assessment, point-of-care, ultrasound
Administering appropriate intravenous fluid is a cornerstone of patient care during surgical perioperative periods and when managing various medical conditions. Despite years of medical research to determine the best dosing strategy for fluid therapy, published veterinary literature investigating intravenous fluid administration is mainly descriptive with little scientific evidence; thus, drawing solid, usable clinical values is difficult [31]. Fluid therapy protocols in small animal medicine are based predominantly on patient body weight and physical assessment [13], but clinical examination and vital signs, including arterial blood pressure, have little power to predict fluid responsiveness in humans [25] and are poorly correlated with intravascular volume status and cardiac output in dogs [35]. Several human studies have investigated the use of indices correlated with cardiac preload to administer fluids based on patient needs [24] since hypovolemia and fluid overload are detrimental [44]. Based on the Frank-Starling relationship between ventricular preload and stroke volume (SV), a patient whose SV increases by equal to or above 15% of their baseline solely in response to an intravenous (IV) fluid bolus is termed a ‘fluid responder’ and has a high probability of improved hemodynamic status after adequate fluid bolus therapy [10, 28, 32].
A lack of the extensive use of invasive and non-invasive cardiac output monitoring devices in veterinary medicine leads to difficulty in managing the preload. Central venous pressure measurement, a parameter widely proposed in the past for critically ill patients, is not a useful indicator of fluid responsiveness [26].
Recently, bedside point-of-care ultrasonography has become popular in assessing the preload for critically ill human patients [2]. Its main advantages include non-invasiveness, rapid execution and low cost. Ultrasonography use in the emergency room has also been proposed in veterinary medicine [5]. One proposed ultrasonographic index of human fluid responsiveness is the respiratory dimensional variations of the inferior vena cava (cIVC) [16]. Ultrasound evaluations of respiratory collapsibility of the caudal vena cava diameter (cCVC) have been proposed by some authors for assessing preload in conscious dogs, but evidence for this is lacking [22]. Validating the cCVC as a fluid responsiveness index should be investigated in a spontaneously breathing conscious canine population.
In a recent study on anesthetized ventilated dogs, the ratio between the caudal vena cava diameter and the aorta (CVC/Ao), measured by transcutaneous intercostal ultrasound at the porta hepatis level, was found to be well correlated with systolic pressure variation (SPV) [29], a validated measure of cardiac preload [35, 39]. CVC/Ao and cCVC are clinically advantageous, as they are also easily measured in conscious dogs.
This study evaluated the accuracy of the CVC/Ao ratio and the cCVC for predicting independent fluid responsiveness in a heterogeneous population of spontaneously breathing, conscious, hospitalized dogs.
MATERIALS AND METHODS
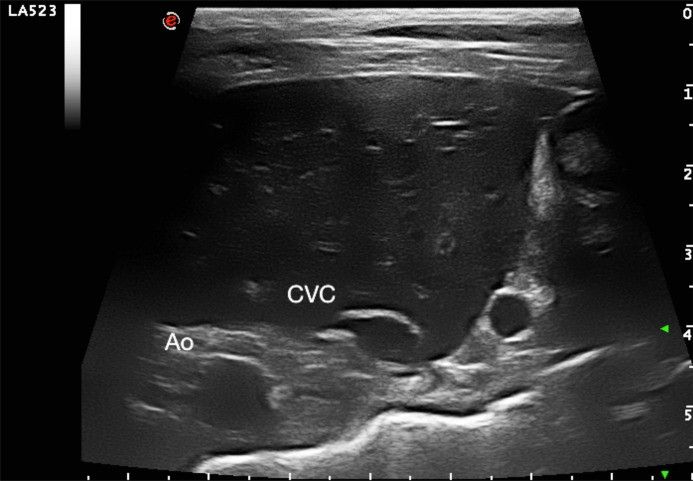
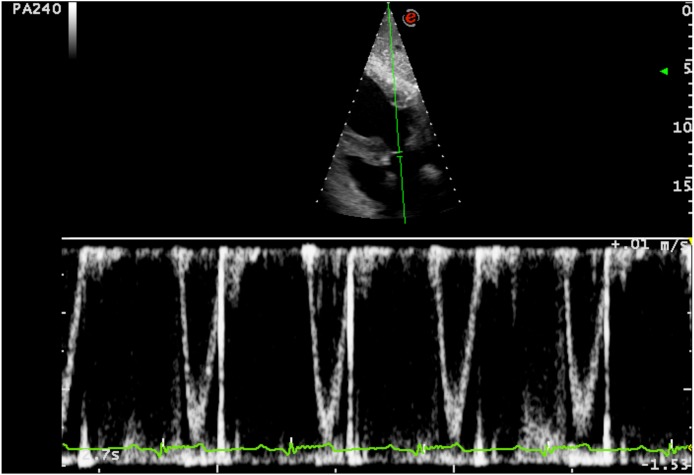
This prospective, multicenter, observational, cross-sectional study was approved by the Ethical Committee of the University of Padua (protocol number 89559) and all owners provided informed written consent. Twenty-five dogs that were hospitalized at the Policlinico Veterinario Roma Sud (Rome, Italy) and at Centro Veterinario Imperiese (Imperia, Italy) were included in the study. All animals included in the study underwent a physical examination and, at a minimum, blood test analyses were performed, including packed cell volume, plasma total protein, serum urea, and creatinine and electrolyte concentrations and were judged to require fluid therapy. Dogs were excluded from the study if there was owner refusal, a history and/or clinical signs of cardiovascular disease, cardiac arrhythmias, intrathoracic disease, abdominal hypertension, acute blood loss, tachypnea or liver disease, or if they were <1 year of age or required chemical sedation to achieve or improve patient compliance. Prior to formulating a plan for the individualized fluid therapy protocol in the hospital setting (maintenance rate or replacement rate), the caudal vena cava (CVC) and aorta (Ao) were assessed via transcutaneous ultrasound using two ultrasound systems (Mylab 70 CV, Esaote SpA, Genova, Italy and Mylab Class C, Esaote SpA, Genova, Italy) equipped with a 3–9 MHz curvilinear microconvex probe or a high-frequency 4–13 MHz linear array probe depending on the dog’s size. For this procedure, dogs were positioned in left lateral recumbency and gently manually restrained. The transducer was placed by one operator in each center (RR and SO) between the 10th and 13th intercostal space as described in a previously outlined study [29]. Both the two operators involved in this study had a minimum of 5 years of experience in focused ultrasound. A transverse image of the two vessels at the porta hepatis level was obtained. A long and short axis of the CVC was acquired just caudal to the hepatic vein inlet (Fig. 1). The Ao diameter (Ao) was measured at the minimum diameter during the cardiac cycle. Minimal pressure was applied to the skin to avoid changes in vessel diameters due to variation of intraabdominal pressure. After visualizing the CVC and Ao, the image was frozen and a cine-loop was used to take CVC measurements after frame-by-frame analysis to determine the maximal short axis length normally obtained during end-expiratory pause (CVCd-max) and the minimum short axis length normally obtained during the inspiratory phase (CVCd-min). Measurements were determined using electronic calipers incorporated in the ultrasound machine’s imaging software using the inner edge to inner edge technique and then stored for post hoc measurement. With the dog positioned in right lateral recumbency, an aortic Doppler study (Fig. 2) was performed using the same ultrasound machines with a phased array transducer with a 1–4 MHz frequency range. Aortic flow was recorded setting the higher pulsed wave Doppler sweep speed using a 1-lead electrocardiography (ECG) recording throughout the ultrasound examination; the faster speed allows greater precision of measurement, because time resolution improves [38]. An optimized subcostal standard view of the left ventricular outflow tract was used to acquire images. Two-dimensional cine-loops and Doppler tracings were recorded and stored on the internal hard drive of the ultrasound machine. The median heart rate was calculated over one respiratory cycle using an R–R interval on the ECG. The aortic velocity time integral (VTI) was obtained from recorded images and the median VTI value was calculated over one complete spontaneous respiratory cycle. All post-hoc measurements were made offline by two investigators (RR and SO) who were unaware of the patient’s medical history and hemodynamic status and who did also the preliminary inter and intra-rater variability assessment. Immediately after the basal measures were recorded, a mini-fluid bolus (MFB) of 4 ml/kg of Hartmann’s solution was administered manually by IV over 1 min using preloaded 50-ml syringes [1]. All ultrasonography measurements outlined above were repeated after MFB administration. Heart rate (HR), mean arterial pressure (MAP) and systolic blood pressure (SAP) were monitored and recorded before and after MFB administration. As previously reported in similar human studies, subjects were considered as responders (R group) to the MFB if their VTI increased by ≥15%; otherwise, they were defined as non-responders (NR group) [6, 17, 34].
Fig. 1.
Transverse intercostal ultrasound images of the liver at the level of the porta hepatis used to measure the caudal vena cava (CVC) and the abdominal aorta (Ao).
Fig. 2.
Subcostal standard echocardiographic view of the left ventricular outflow tract optimized to visualise the left ventricular outflow tract. The beat to beat values of the aortic velocity time integral (VTI) before and after the volume expansion were recorded. Median VTI was calculated over one respiratory cycle.
Once all the required images were obtained, the indices were calculated as follows:
CVC/Ao ratio=(CVCd-max) short axis maximal length/Ao diameter;
cCVC=(CVCd-max−CVCd-min)/CVCd-max; and cCVC is expressed as a percentage.
VTI variations (dVTI) were calculated as follows:
dVTI=(VTI post MFB-VTI preMFB)/VTI preMFB and is expressed as a percentage.
Statistical analysis
Categorical variables are expressed as frequencies and percentages. Continuous variables were checked for normal distribution using bar graphs, histograms and the Shapiro-Wilk test. Normally distributed variables are reported as the mean ± standard deviation (SD), whereas non-normally distributed variables are expressed as the median (range). Differences between non-normally distributed data were analyzed using the Mann-Whitney test. Inter-rater reliability was difficult to assess during the study protocol; thus, we made a preliminary inter- and intra-rater reliability assessment measuring CVC, and Ao in a preliminary study in 10 hospitalized dogs using three investigators. Inter-rater reliability was assessed using the single measure intra-class correlation coefficient (ICC) using a two-way mixed model (single measures) with absolute agreement for measurements, while intra-rater reliability was measured with a two-way mixed-effects reporting single measure ICC for test-retest (repeated measures) and the percentage of coefficient of variation (CV). VTI inter-rater and intra-rater reliability were evaluated with ICC on duplicated measurements of recorded images. During the study, we checked the intra-rater reliability of the aortic measurements with ICC, assuming no diameter modifications were made between pre-bolus and post-bolus. An ICC >0.7 commonly indicates sufficient reliability [18].
To assess whether the CVC/Ao ratio and the cCVC discriminated between fluid responders (R) (>15% increase in VTI after MFB administration) and non-responders (NR) (<15% increase in VTI after MFB administration), a receiver operating characteristics (ROC) curve for each parameter was generated. The Area Under the Receiver Operator curve (AUROC) ranges between 0 and 1, and a 95% Confidence Interval (CI) range equal to or less than 0.5 indicates that the discriminatory predictor ability is not better than chance, consistent with the null hypothesis. As such, an AUROC 95% CI of >0.5 indicates that the predictor has a significant discriminatory predictive reliability, with a value of 1 implying perfect performance of the prediction model. The difference between the predictive discriminatory accuracy of the two AUROC curves generated was evaluated and tested using the DeLong test. The optimal cutoff value for the ROC analysis of fluid responsiveness was explored using Youden’s index to minimize misclassification errors. The uncertainty interval for the predictive variables evaluated in the Sensibility vs Specificity Plot (“gray zone”) was defined as values with a sensitivity or specificity less than 90% (diagnosis tolerance of 10%), as previously suggested [34]. The inconclusive response range was evaluated only for significant independent predictors (P<0.05).
The study sample size for the best chance of obtaining a significant AUROC with a good discriminatory accuracy (>0.80), assuming a type I error of 0.05 with a power of 0.8, was calculated as a minimum of 20 ultrasound examinations. The significance level was set at 5%. For the statistical analysis, a commercial software was used (MedCalc Statistical Software ver. 18.11, MedCalc Software, Ostend, Belgium; http://www.medcalc.org; 2018).
RESULTS
This study was conducted on 25 dogs (13 males and 12 females). Three dogs were excluded from the analysis because of the quality of the image of the CVC and Ao ultrasound assessment (2 deep chested dogs and 1 uncooperative dog); thus, 22 dogs of different breeds were included in the analysis. The following breeds were represented in the study population, listed from the most frequent to the least: mixed-breed (12/22), Poodle (2/22), Beagle (2/22), Jack Russel terrier (2/22), Basset Hound (1/22), Yorkshire terrier (1/22), Boxer (1/22), Dobermann (1/22). The median age was 72 months (12–360 months) and the median weight was 7 kg (1.5–35 kg). Underlying disorders of the hospitalized dogs were subcategorized as acute gastrointestinal disease (8/22), acute kidney disease (4/22), bite wounds (4/22), urinary tract infection (2/22), traumatic brain injury (2/22), pancreatitis (1/22), peripheral neuropathy (1/22).
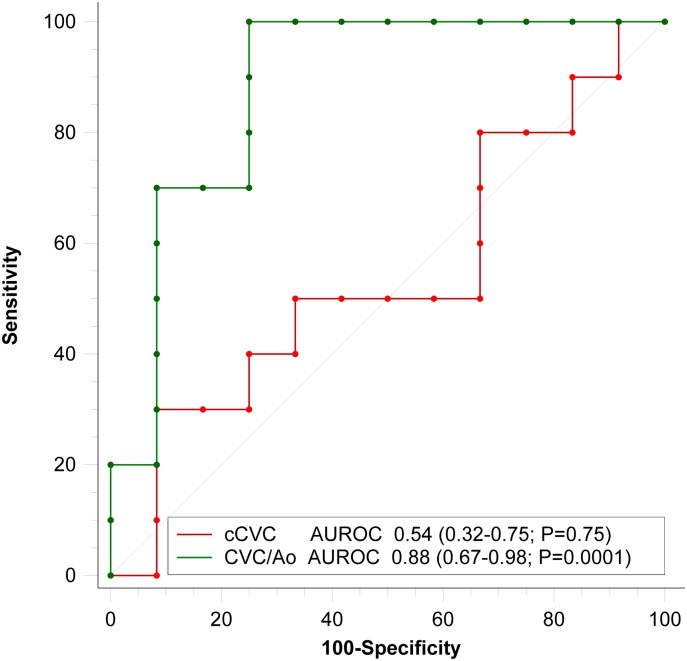
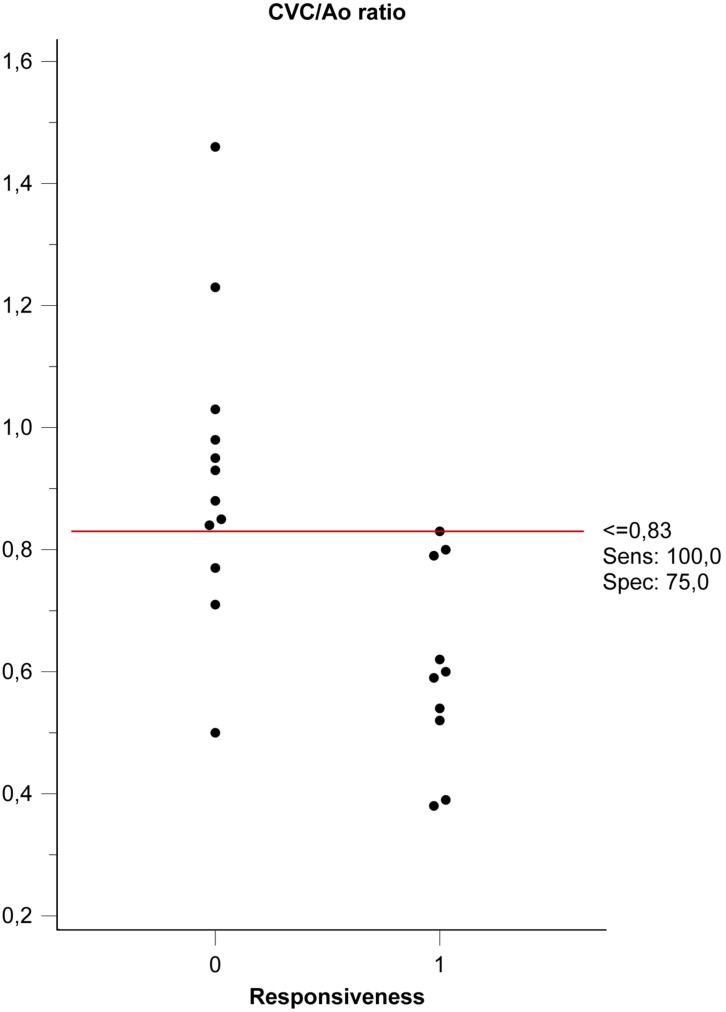
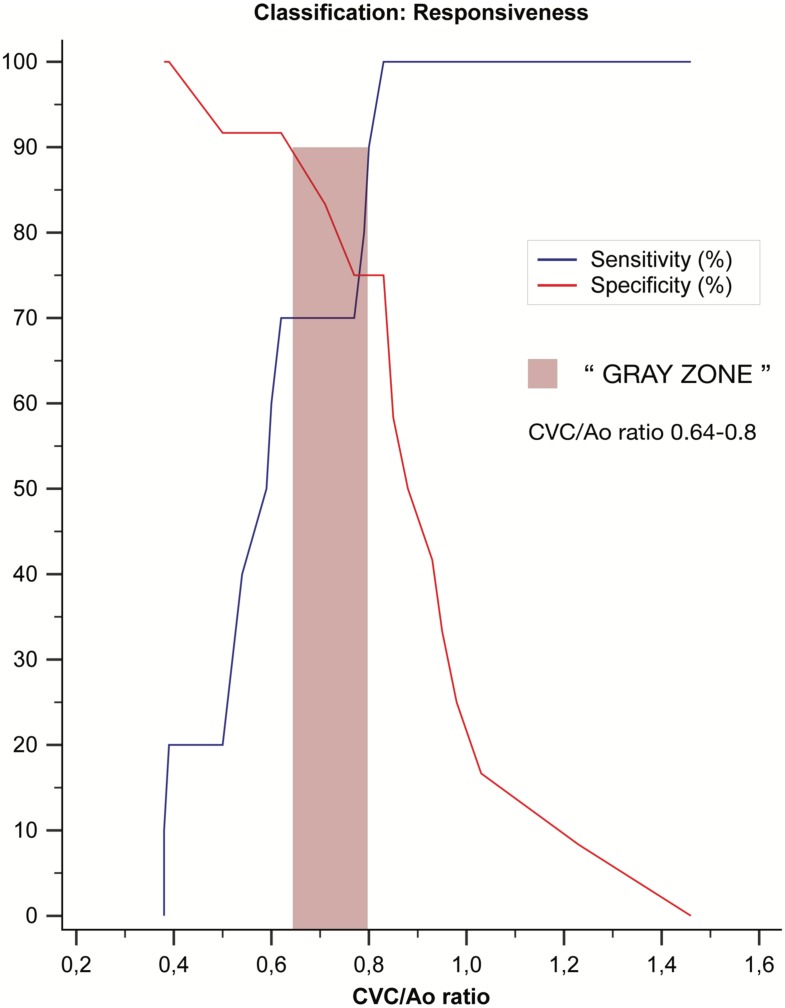
The median duration of the entire procedure (pre-bolus data collection, bolus administration and post-bolus data collection) was 7 min (5–8 min). The single measures (absolute agreement) ICCs for intra- and inter-rater reliability for CVC, Ao, and VTI were reported in Table 1. During the study, the ICC intra-rater reliability value for Ao pre- and post-bolus was 0.898 (95% CI 0.774–0.956). Ten patients were classified as responders (R) and 12 as non-responders (NR) according to the predefined classification criteria. Hemodynamic data, divided for groups R and NR, are expressed as non-parametric data due to the small sample size (Table 2), while the aggregate data of the groups used to evaluate ROC discriminatory power passed normality tests and were analyzed as parametric data. Neither responder nor non-responder subjects differed significantly in their pre- or post-bolus MAP and SAP values (P>0.05), while the HR differed significantly (P<0.05) (Table 2). The pre- and post-bolus CVC/Ao, and cCVC values are shown in Table 2. No difference was found in aortic dimension before the bolus (0.69 cm, 95% CI 0.59–0.79) or after the fluid bolus (0.67 cm, 95% CI 0.59–0.75; P=0.403). The responder pre-bolus VTI and CVC/Ao (P<0.05) differed significantly but the cCVC did not (Table 2). The overall performance of the study variables (CVC/Ao ratio and cCVC), when evaluated as independent predictors in response to an MFB (increase of VTI >15%), was studied by constructing ROC curves (Fig. 3). The Areas Under the ROC curves (AUROC) were 0.88 for the CVC/Ao ratio (95% CI 0.67–0.98; P=0.0001) and 0.54 for cCVC (95% CI 0.54; P=0.75). The CVC/Ao threshold optimized for best sensitivity (SE) and specificity (SP) values was 0.83 (SE 100%; SP 75%) (Fig. 4), with a “gray-zone” between 0.64 and 0.8 (Fig. 5). Three dogs were misclassified, 15 were correctly classified and 4 were in the “gray zone” (high probability of an inconclusive response). The CVC/Ao ratio had better diagnostic accuracy than the cCVC (P=0.009).
Table 1. Intra-rater and inter-rater reliability of the measure of caudal vena cava (CVC), abdominal aorta (Ao) and velocity time integral (VTI) using Intra-class Correlation Coefficient (ICC) reliability test and coefficient of variation (CV) for intra-rater variability.
| CV (%) logarithmic method | Single measures ICC (Absolute agreement) |
95% CI | |
|---|---|---|---|
| CVC (Intra-rater) | 2.60 | 0.989 | 0.959–0.997 |
| CVC (Inter-rater) | – | 0.930 | 0.817–0.980 |
| Ao (Intra-rater) | 5.92 | 0.943 | 0.797–0.985 |
| Ao (Inter-rater) | – | 0.791 | 0.529–0.937 |
| VTI (Intra-rater) | 3.52 | 0.989 | 0.958–0.997 |
| VTI (Inter-rater) | – | 0.979 | 0.959–0.997 |
Table 2. Comparison of haemodynamic variables (median and range) in dogs before and after a mini-fluid bolus (MFB) with 4 ml/kg Hartmann’s solution administered intravenously in 22 dogs.
| Group | Before fluid challenge |
After fluid challenge |
||||
|---|---|---|---|---|---|---|
| R | NR | P-valuea) | R | NR | P-valuea) | |
| Dogs (No.) | 10 | 12 | 10 | 12 | ||
| HR (beats/min) | 135 (110–180) | 108 (59–190) | 0.03 | 127 (83–182) | 98 (57–151) | 0.05 |
| SAP (mmHg) | 141 (85–175) | 152 (70–163) | 0.563 | 140 (117–170) | 148 (102–206) | 0.88 |
| MAP (mmHg) | 103 (70–159) | 110 (74–132) | 0.315 | 105 (75–113) | 110 (77–145) | 0.575 |
| VTI (cm) | 8.5 (3.4–12.7) | 12.1 (8–16.3) | 0.005 | 11.8 (6–18) | 12.3 (9–17) | 0.878 |
| CVC/Ao | 0.59 (0.38–0.83) | 0.90 (0.5–1.46) | 0.001 | 0.83 (0.46–1.24) | 0.97 (0.51–1.7) | 0.044 |
| cCVC | 33 (11–57) | 33 (10–71) | 0.771 | 32 (18–37) | 30 (6–77) | 0.871 |
Data are presented as median (range). a) Two Tailed probability Mann–Whitney U test. Responders (R), Non-responders (NR). Heart rate (HR), systolic arterial pressure (SAP), mean arterial pressure (MAP), velocity time integral (VTI), caudal vena cava diameter to abdominal aortic diameter ratio (CVC/Ao), collapsibility of the caudal vena cava (cCVC).
Fig. 3.
Area Under the Receiver Operating Characteristic curve (AUROC) comparing the discriminatory accuracy of caudal vena cava diameter to abdominal aortic diameter ratio (CVC/Ao) and collapsibility of the caudal vena cava (cCVC) to predict fluid responsiveness. Values expressed as AUROC (95% Confidence Interval; P value)
Fig. 4.
Dot plot of caudal vena cava diameter to abdominal aortic diameter ratio (CVC/Ao) threshold optimized for best sensitivity (SE) and specificity (SP) between Non-Responders (0) and Responders (1).
Fig. 5.
Plot of sensitivity and specificity versus criterion values. Sensitivity and specificity, values are displayed as percentages. “Gray zone” was defined as value with a sensitivity or specificity less than 90% (diagnosis tolerance of 10%).
DISCUSSION
This article supports using the CVC/Ao ratio as a new index for evaluating fluid responsiveness status in a heterogeneous population of hospitalized conscious dogs.
Routine preload assessment in non-collaborative, non-instrumented, conscious animals is a challenge to the clinician when treating critically ill subjects or during the perioperative period. Physical examination is crucial when treating these patients but routine measured hemodynamic parameters are insufficiently correlated with preload status [35]; thus, they may be unreliable predictors in discriminating between hypovolemic and hypervolemic subjects.
Over the last 20 years, our understanding of fluid responsiveness has changed drastically. New indices based on heart and lung interactions, known as “dynamic indices” were introduced and their superiority was demonstrated over the previous “static indices”, such as central venous pressure (CVP) [8, 27]. Examples of these new “dynamic” respiratory indices are the systolic pressure variation (SPV) [6, 41], pulse pressure variation (PPV) [15, 40, 41], stroke volume variation (SVV) [41], pleth variability index (PVI) [40] and the collapsibility of the vena cava (cIVC) [16], which are all based on cardiac response in terms of SV and cardiac output variations, to interactions between the heart and lungs during mechanical ventilation or spontaneous breathing. A dynamic index such as the SPV has been validated to preload in anesthetized dogs undergoing graded exsanguination [35]. SPV, defined as the difference between the maximum and minimum value of systolic blood pressure following a single positive pressure breath, has recently been studied as a dynamic index of fluid responsiveness in dogs anesthetized with isoflurane and mechanically ventilated with a peak airway pressure of 10 cm H2O with excellent diagnostic accuracy (AUROC 0.91) [6]. Bucci et al. tested two ultrasonographic indices of fluid responsiveness, the aortic flow peak velocity variation (ΔVpeak) and the caudal vena cava distensibility index (CVC-DI), in healthy anesthetized mechanically ventilated dogs, showing excellent discrimination for the ΔVpeak (AUROC 0.95) and lower discriminatory power for the CVC-DI (AUROC 0.78) in predicting fluid responsiveness. Unfortunately, SPV, PPV, PVI, SVV, and CVC-DI can have good discriminatory power only in anesthetized and mechanically ventilated subjects. To the best of our knowledge, this is the first study reported in the currently available literature, based on the evaluation of CVC as a predictor of fluid responsiveness in spontaneously breathing dogs.
In humans, the cIVC respiratory variation, which is expressed as the difference between the maximum and minimum respiratory variation in diameter divided by the maximum of the two values, is one of the most common parameters used to evaluate fluid responsiveness [16]. The IVC is highly compliant and changes in intravascular pressure easily cause size variations. Therefore, continuous variations in IVC size are produced by the respiratory cycle, which causes changes in the intrathoracic pressure and blood return to the heart. Chest expansion produces intrathoracic negative pressure and the IVC collapses. Two recently proposed meta-analyses of the cIVC in humans to predict fluid responsiveness have shown conflicting results, likely due to the wide heterogeneity in the analyzed studies, different methods of image acquisition and/or different proposed diagnostic cutoffs [23, 45]. Better diagnostic accuracy could be reached by lowering the cIVC threshold, as proposed more recently by other authors [11, 37]. The accuracy of the inferior vena cava maximal dimension (IVCd) measured at the end-expiratory phase to discriminate between hypovolemic status and euvolemic status were studied in the meta-analysis proposed by Dipti et al [14]. The clinical utility of both indices (cIVC and IVCd) in various critical care conditions correlated with preload assessment or cardio-respiratory disease (chronic heart failure, sepsis, respiratory failure, and pericardial effusion) was stated in the Guidelines for the Use of Echocardiography as a Monitor for Therapeutic Intervention in Adults, proposed by the American Society of Echocardiography [36].
The CVC/Ao ratio was first described in human pediatric patients [20] and, as a dimensionless index, is independent of body size; therefore, it could be used in dogs and pediatric subjects, where the wide variability in body size makes it difficult to establish a reference dimension range for major vascular structures. Until now, such a ratio has not been validated as an index of fluid responsiveness, precluding its clinical use. In a previous study, the CVC/Ao ratio was well correlated with SPV in anesthetized ventilated dogs [29] and a recent study reported the reliability of the CVC/Ao measure in dogs and normality parameters [12], however, this is the first time that it has been evaluated for predicting fluid responsiveness in a heterogeneous canine population. Our results provide a numerical cutoff value with good discriminatory power with an AUROC of 0.88. Cutoff value availability can make the CVC/Ao ratio a powerful monitoring tool to titrate fluid therapy on demand and tailor to the patients’ individual requirements in canine critical areas. This can be performed rapidly and non-invasively and can be easily used to monitor hemodynamic trends.
This study does not support the use of CVC collapsibility (cCVC) in conscious dogs because it showed an insignificant discriminatory power for fluid responsiveness (AUROC 0.51 P>0.05). Several reasons have been proposed to explain these poor results in dogs. The major factors could be that spontaneous breathing in an awake dog could have variable diaphragm excursion or different efforts and duty cycle on a breath-by-breath basis, as proposed in humans [19]. Furthermore, we evaluated different dog breeds presenting different thoracic morphologies, diaphragmatic excursion and respiratory patterns, which may have increased the abdominal-thoracic interaction variability between subjects, leading to a lack of cCVC predictability.
In this study, SV increase was measured by aortic VTI. In several human and animal studies, VTI has been used as a surrogate for SV to measure left ventricular ejection variations in the same subject [3, 4, 6, 17, 21, 33, 34, 42, 43]. Transthoracic echocardiography (TTE) measurements of aortic VTI for evaluating SV variation on the same subject as a repeated measure has several advantages for assessing hemodynamics in clinical settings; it is non-invasive, does not cause further pain or distress to conscious patients and monitors beat-to-beat SV. For VTI to be an appropriate SV surrogate, we must assume that the abdominal aortic diameter/area does not change with breathing or fluid challenge [4].
The CVC/Ao ratio can be used in clinical practice to avoid some limitations of dynamic index as SPV or PPV when used to assess preload status. These indexes do not provide reliable information on cardiac preload in spontaneous ventilation or when the R–R interval varies widely. In these subjects, the CVC/Ao ratio may be useful because it does not require invasive ventilation or arterial pressure monitoring.
Nevertheless, the CVC/Ao ratio has limitations and would not be reliable in some clinical situations. Patients with intrathoracic disease (e.g., right heart failure, cardiac tamponade, pneumothorax, pleural effusion or pulmonary thromboembolism) or in those with increased abdominal pressure (presence of fluid or masses), the CVC diameter would be altered and likely unrelated to preload status. To avoid gross mistakes in complex clinical cases, preload evaluation using the CVC/Ao should include a more comprehensive physical examination and echocardiographic assessment of cardiac preload and systolic-diastolic heart function. The presence of free fluid or a mass in the abdomen, along with large, deep-chested, polypneic dogs can cause further problems in obtaining sufficient quality ultrasound images, especially in large dogs. One limitation of all preload indices is that, while they predict fluid responsiveness, being a fluid responder does not automatically mean that the patient needs fluid, irrespective of their clinical condition [30]. For example, under general anesthesia, a patient with good cardiovascular status can be a fluid responder, but if the patient has a stable hemodynamic condition and organ perfusion, the short-term advantages of a fluid bolus should be weighed against the potential disadvantages of relative fluid overload during recovery [9]. All cutoffs evaluated with ROC analysis dichotomize a diagnostic test, providing a patient classification based on “positive” or “negative” results. However, the Frank-Starling law is continuous; therefore, it does not produce binary results. Applying the “gray-zone” approach should be considered when making clinical decisions in fluid management when a fluid responsiveness index is used and was already well described and adopted in humans [7] and in veterinary medicine [40]. The optimal threshold should also be interpreted in light of the patient status. For example, in a patient with respiratory disease who is more susceptible to the negative effects of fluid overload, the risk/benefit analysis should consider the sensitivity, and the best threshold for managing fluid administration should be more restrictive based on the gray zone approach. Over the last decades, fluid therapy research has focused on finding clinically easy-to-use indices correlated with preload in order to achieve an individualized fluid therapy protocol for managing volume expansion based on individual needs. We would like to stress that the decision to provide or not fluids in a fluid-responsive patient should always be based on the patient’s clinical condition and the underlying disease; not all the dogs responsive to fluid require and have a benefit to receive extra fluids.
The CVC/Ao ratio appears to be a good non-invasive index for preload assessment in conscious dogs, surpassing some of the limitations of other proposed preload indices. Our results should be confirmed with larger studies evaluating the diagnostic efficacy of CVC/Ao ratio in patients with multiple clinical conditions, evaluating different subgroups response rates. The wide availability of ultrasound machines and the simplicity of obtaining diagnostic images make this index an available and realistic option in clinical practice.
REFERENCES
- 1.Aya H. D., Rhodes A., Chis Ster I., Fletcher N., Grounds R. M., Cecconi M.2017. Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit. Care Med. 45: e161–e168. doi: 10.1097/CCM.0000000000002067 [DOI] [PubMed] [Google Scholar]
- 2.Bernier-Jean A., Albert M., Shiloh A. L., Eisen L. A., Williamson D., Beaulieu Y.2017. The diagnostic and therapeutic impact of point-of-care ultrasonography in the intensive care unit. J. Intensive Care Med. 32: 197–203. doi: 10.1177/0885066615606682 [DOI] [PubMed] [Google Scholar]
- 3.Biais M., Vidil L., Sarrabay P., Cottenceau V., Revel P., Sztark F.2009. Changes in stroke volume induced by passive leg raising in spontaneously breathing patients: comparison between echocardiography and Vigileo/FloTrac device. Crit. Care 13: R195. doi: 10.1186/cc8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco P., Aguiar F. M., Blaivas M.2015. Rapid ultrasound in shock (RUSH) velocity-time integral: a proposal to expand the RUSH protocol. J. Ultrasound Med. 34: 1691–1700. doi: 10.7863/ultra.15.14.08059 [DOI] [PubMed] [Google Scholar]
- 5.Boysen S. R., Lisciandro G. R.2013. The use of ultrasound for dogs and cats in the emergency room: AFAST and TFAST. Vet. Clin. North Am. Small Anim. Pract. 43: 773–797. doi: 10.1016/j.cvsm.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 6.Bucci M., Rabozzi R., Guglielmini C., Franci P.2017. Respiratory variation in aortic blood peak velocity and caudal vena cava diameter can predict fluid responsiveness in anaesthetised and mechanically ventilated dogs. Vet. J. 227: 30–35. doi: 10.1016/j.tvjl.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 7.Cannesson M., Le Manach Y., Hofer C. K., Goarin J. P., Lehot J. J., Vallet B., Tavernier B.2011. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology 115: 231–241. doi: 10.1097/ALN.0b013e318225b80a [DOI] [PubMed] [Google Scholar]
- 8.Carsetti A., Cecconi M., Rhodes A.2015. Fluid bolus therapy: monitoring and predicting fluid responsiveness. Curr. Opin. Crit. Care 21: 388–394. doi: 10.1097/MCC.0000000000000240 [DOI] [PubMed] [Google Scholar]
- 9.Cavallaro F., Sandroni C., Antonelli M.2008. Functional hemodynamic monitoring and dynamic indices of fluid responsiveness. Minerva Anestesiol. 74: 123–135. [PubMed] [Google Scholar]
- 10.Chaves R. C. F., Corrêa T. D., Neto A. S., Bravim B. A., Cordioli R. L., Moreira F. T., Timenetsky K. T., de Assunção M. S. C.2018. Assessment of fluid responsiveness in spontaneously breathing patients: a systematic review of literature. Ann. Intensive Care 8: 21. doi: 10.1186/s13613-018-0365-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corl K. A., George N. R., Romanoff J., Levinson A. T., Chheng D. B., Merchant R. C., Levy M. M., Napoli A. M.2017. Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J. Crit. Care 41: 130–137. doi: 10.1016/j.jcrc.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 12.Darnis E., Boysen S., Merveille A. C., Desquilbet L., Chalhoub S., Gommeren K.2018. Establishment of reference values of the caudal vena cava by fast-ultrasonography through different views in healthy dogs. J. Vet. Intern. Med. 32: 1308–1318. doi: 10.1111/jvim.15136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis H., Jensen T., Johnson A., Knowles P., Meyer R., Rucinsky R., Shafford H. and American Association of Feline PracticionersAmerican Animal Hospital Association. 2013. 2013 AAHA/AAFP fluid therapy guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 49: 149–159. doi: 10.5326/JAAHA-MS-5868 [DOI] [PubMed] [Google Scholar]
- 14.Dipti A., Soucy Z., Surana A., Chandra S.2012. Role of inferior vena cava diameter in assessment of volume status: a meta-analysis. Am. J. Emerg. Med. 30: 1414–1419.e1. doi: 10.1016/j.ajem.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 15.Fantoni D. T., Ida K. K., Gimenes A. M., Mantovani M. M., Castro J. R., Patrício G. C. F., Ambrósio A. M., Otsuki D. A.2017. Pulse pressure variation as a guide for volume expansion in dogs undergoing orthopedic surgery. Vet. Anaesth. Analg. 44: 710–718. doi: 10.1016/j.vaa.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 16.Feissel M., Michard F., Faller J. P., Teboul J. L.2004. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 30: 1834–1837. doi: 10.1007/s00134-004-2233-5 [DOI] [PubMed] [Google Scholar]
- 17.Jozwiak M., Depret F., Teboul J. L., Alphonsine J. E., Lai C., Richard C., Monnet X.2017. Predicting fluid responsiveness in critically Ill patients by using combined end-expiratory and end-inspiratory occlusions with echocardiography. Crit. Care Med. 45: e1131–e1138. doi: 10.1097/CCM.0000000000002704 [DOI] [PubMed] [Google Scholar]
- 18.Khan K. S., Chien P. F.2001. Evaluation of a clinical test. I: assessment of reliability. BJOG 108: 562–567. [DOI] [PubMed] [Google Scholar]
- 19.Kimura B. J., Dalugdugan R., Gilcrease G. W., 3rd., Phan J. N., Showalter B. K., Wolfson T.2011. The effect of breathing manner on inferior vena caval diameter. Eur. J. Echocardiogr. 12: 120–123. doi: 10.1093/ejechocard/jeq157 [DOI] [PubMed] [Google Scholar]
- 20.Kosiak W., Swieton D., Piskunowicz M.2008. Sonographic inferior vena cava/aorta diameter index, a new approach to the body fluid status assessment in children and young adults in emergency ultrasound—preliminary study. Am. J. Emerg. Med. 26: 320–325. doi: 10.1016/j.ajem.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 21.Lewis J. F., Kuo L. C., Nelson J. G., Limacher M. C., Quinones M. A.1984. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation 70: 425–431. doi: 10.1161/01.CIR.70.3.425 [DOI] [PubMed] [Google Scholar]
- 22.Lisciandro G. R.2014. Focused Ultrasound Techniques for the Small Animal Practitioner, Wiley-Blackwell, Ames. [Google Scholar]
- 23.Long E., Oakley E., Duke T., Babl F. E. and Paediatric Research in Emergency Departments International Collaborative (PREDICT). 2017. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness: a systematic review and meta-analysis. Shock 47: 550–559. doi: 10.1097/SHK.0000000000000801 [DOI] [PubMed] [Google Scholar]
- 24.Lopes M. R., Oliveira M. A., Pereira V. O. S., Lemos I. P. B., Auler J. O. C., Jr., Michard F.2007. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit. Care 11: R100. doi: 10.1186/cc6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie D. C., Noble V. E.2014. Assessing volume status and fluid responsiveness in the emergency department. Clin. Exp. Emerg. Med. 1: 67–77. doi: 10.15441/ceem.14.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marik P. E., Baram M., Vahid B.2008. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 134: 172–178. doi: 10.1378/chest.07-2331 [DOI] [PubMed] [Google Scholar]
- 27.Marik P. E., Cavallazzi R., Vasu T., Hirani A.2009. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit. Care Med. 37: 2642–2647. doi: 10.1097/CCM.0b013e3181a590da [DOI] [PubMed] [Google Scholar]
- 28.Marik P. E., Monnet X., Teboul J. L.2011. Hemodynamic parameters to guide fluid therapy. Ann. Intensive Care 1: 1. doi: 10.1186/2110-5820-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meneghini C., Rabozzi R., Franci P.2016. Correlation of the ratio of caudal vena cava diameter and aorta diameter with systolic pressure variation in anesthetized dogs. Am. J. Vet. Res. 77: 137–143. doi: 10.2460/ajvr.77.2.137 [DOI] [PubMed] [Google Scholar]
- 30.Monnet X., Marik P. E., Teboul J. L.2016. Prediction of fluid responsiveness: an update. Ann. Intensive Care 6: 111. doi: 10.1186/s13613-016-0216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muir W. W., Ueyama Y., Noel-Morgan J., Kilborne A., Page J.2017. A systematic review of the quality of IV fluid therapy in veterinary medicine. Front. Vet. Sci. 4: 127. doi: 10.3389/fvets.2017.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller L., Bobbia X., Toumi M., Louart G., Molinari N., Ragonnet B., Quintard H., Leone M., Zoric L., Lefrant J. Y. and AzuRea group. 2012. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit. Care 16: R188. doi: 10.1186/cc11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen H. B., Losey T., Rasmussen J., Oliver R., Guptill M., Wittlake W. A., Corbett S. W.2006. Interrater reliability of cardiac output measurements by transcutaneous Doppler ultrasound: implications for noninvasive hemodynamic monitoring in the ED. Am. J. Emerg. Med. 24: 828–835. doi: 10.1016/j.ajem.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 34.Pereira de Souza Neto E., Grousson S., Duflo F., Ducreux C., Joly H., Convert J., Mottolese C., Dailler F., Cannesson M.2011. Predicting fluid responsiveness in mechanically ventilated children under general anaesthesia using dynamic parameters and transthoracic echocardiography. Br. J. Anaesth. 106: 856–864. doi: 10.1093/bja/aer090 [DOI] [PubMed] [Google Scholar]
- 35.Perel A., Pizov R., Cotev S.1987. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology 67: 498–502. doi: 10.1097/00000542-198710000-00009 [DOI] [PubMed] [Google Scholar]
- 36.Porter T. R., Shillcutt S. K., Adams M. S., Desjardins G., Glas K. E., Olson J. J., Troughton R. W.2015. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 28: 40–56. doi: 10.1016/j.echo.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 37.Preau S., Bortolotti P., Colling D., Dewavrin F., Colas V., Voisin B., Onimus T., Drumez E., Durocher A., Redheuil A., Saulnier F.2017. Diagnostic accuracy of the inferior vena cava collapsibility to predict fluid responsiveness in spontaneously breathing patients with sepsis and acute circulatory failure. Crit. Care Med. 45: e290–e297. doi: 10.1097/CCM.0000000000002090 [DOI] [PubMed] [Google Scholar]
- 38.Quiñones M. A., Otto C. M., Stoddard M., Waggoner A., Zoghbi W. A. and Doppler quantification task force of the nomenclature and standards committee of the american society of echocardiography. 2002. Recommendations for quantification of doppler echocardiography: a report from the doppler quantification task force of the nomenclature and standards committee of the american society of echocardiography. J. Am. Soc. Echocardiogr. 15: 167–184. doi: 10.1067/mje.2002.120202 [DOI] [PubMed] [Google Scholar]
- 39.Rabozzi R., Franci P.2014. Use of systolic pressure variation to predict the cardiovascular response to mini-fluid challenge in anaesthetised dogs. Vet. J. 202: 367–371. doi: 10.1016/j.tvjl.2014.08.022 [DOI] [PubMed] [Google Scholar]
- 40.Sano H., Seo J., Wightman P., Cave N. J., Gieseg M. A., Johnson C. B., Chambers P.2018. Evaluation of pulse pressure variation and pleth variability index to predict fluid responsiveness in mechanically ventilated isoflurane-anesthetized dogs. J. Vet. Emerg. Crit. Care (San Antonio) 28: 301–309. doi: 10.1111/vec.12728 [DOI] [PubMed] [Google Scholar]
- 41.Sasaki K., Mutoh T., Yamamoto S., Taki Y., Kawashima R.2018. Comparison of noninvasive dynamic indices of fluid responsiveness among different ventilation modes in dogs recovering from experimental cardiac surgery. Med. Sci. Monit. 24: 7736–7741. doi: 10.12659/MSM.910135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sohn S., Kim H. S., Han J. J.2002. Doppler flow velocity measurement to assess changes in inotropy and afterload: a study in healthy dogs. Echocardiography 19: 207–213. doi: 10.1046/j.1540-8175.2002.00207.x [DOI] [PubMed] [Google Scholar]
- 43.Swenson J. D., Harkin C., Pace N. L., Astle K., Bailey P.1996. Transesophageal echocardiography: an objective tool in defining maximum ventricular response to intravenous fluid therapy. Anesth. Analg. 83: 1149–1153. doi: 10.1213/00000539-199612000-00003 [DOI] [PubMed] [Google Scholar]
- 44.Voldby A. W., Brandstrup B.2016. Fluid therapy in the perioperative setting-a clinical review. J. Intensive Care 4: 27. doi: 10.1186/s40560-016-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z., Xu X., Ye S., Xu L.2014. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med. Biol. 40: 845–853. doi: 10.1016/j.ultrasmedbio.2013.12.010 [DOI] [PubMed] [Google Scholar]