Abstract
Autopsy imaging (Ai) was performed for a King Penguin. Ai-computed tomography (CT) revealed air sac membrane thickening, multiple nodules in the cranial air sac, suspected abscess, lung infiltration, and air sac contraction. Based on these findings, respiratory disorder was concerned. Aspergillosis, which is the highly observed in penguins, was considered as the primary differential diagnosis. The cultured sample showed characteristic conidial head of Aspergillus spp., the DNA of which was 100% identical to that of A. fumigatus. The cause of death was determined to respiratory failure due to aspergillosis. Ai-CT findings facilitated the dissection workflow and alerted the pathologist to potential hazards during the autopsy. Ai is useful to determine the cause of death and for readiness and safe pathological dissection.
Keywords: aspergillosis, Aspergillus fumigatus, autopsy imaging, fungal culture, King Penguin
Necropsy is an important procedure to determine the cause of death. Autopsy imaging (Ai), which is conducted postmortem and before necropsy, is primarily used in human forensic medicine [8]. However, the term “autopsy” refers to human necropsy and is used even in the veterinary field [12]. In humans, Ai has been widely used to determine the cause of death from any medical treatment, to investigate suspicious deaths, and for public health survey purposes. Various imaging modalities such as computed tomography (CT) and magnetic resonance imaging [14] and technique including contrast agent injection have been applied [3]. Generally, it is difficult to determine the cause of death only on the basis of body surface observation. Therefore, autopsy is indispensable; however, it is not always performed because of opposition from bereaved families. Thus, Ai is considered as a noninvasive procedure that is important for inspecting dead bodies. Ai does not simply refer to postmortem imaging, but pathologists refer to imaging findings and provide feedback to radiologists; therefore, imaging and pathological dissection are complementarily used to determine the cause of death. In the veterinary field, to date, the cause of death is often not determined, and thus, Ai is less commonly performed. The cost and availability of CT before the dissection add to hesitation and difficulty in performing Ai [11]; thus, there have been few reports of forensic veterinary medicine [2, 10] and Ai of economically valuable animals [6, 13]. We had experienced an advantage of Ai for the economically valuable zoo animal, King Penguin.
A 6-year-old male King Penguin (Aptenodytes patagonicus) kept in an aquarium was observed to intermittently mouth-breathe for 6 months; during this time, its body weight decreased from 16.59 kg to 12.92 kg. A blood test performed 7 days before its death revealed the white blood cell count (10,360/µl), red blood cell count (185 × 104/µl), hemoglobin level (14.8 g/dl), total protein (7.0 g/dl), albumin (2.9 g/dl), and globulin (4.1 g/dl); slightly low red blood cell count suggested anemia, low albumin level suggested poor nutritional status, and high globulin level suggested inflammation [11]. Aspergillus antigen and antibody were both negative. From these symptoms and blood test, antemortem diagnosis could not be determined. Ai-CT was performed 1 day after death (Fig. 1). CT images were obtained using a four-row multidetector CT scanner (Asteion Super 4, Cannon, Tokyo, Japan) with 80 kV, 150 mA, 0.75 sec/rotation, 2.0-mm slice thickness, and 320-mm field of view. Digital Imaging and Communications in Medicine data were sent to a viewer (Newton OsiriX, Newton-Graphics, Sapporo, Japan) to read the images. The transverse CT images revealed membrane thickening, multiple nodules in the cranial air sac (Fig. 2A), suspected abscess (Fig. 2B), lung infiltration, and air sac contraction (Fig. 2C). From these imaging findings, respiratory disorder including aspergillosis, mycoplasma airsacculitis/pneumonia or Mycobacterium avium infection was concerned. Further, aspergillosis, which is the highly observed in penguins, was considered as the primary differential diagnosis. Moreover, aspergillosis affects humans [4]; thus, these findings provided a safety precaution for pathologists to wear a mask and goggles at a clean bench during the autopsy. Fungus was observed in the air sac, and all CT findings were further confirmed by pathological dissection. Cultures of nodule samples from the air sac showed fungal growth. The characteristic conidial head of Aspergillus spp. was confirmed (Fig. 3). DNA extraction and amplification of ITS and D1/D2 regions were performed as previously described [5]. The ITS and D1/D2 regions of the fungal DNA obtained from the cranial air sac tissues were amplified. The D1/D2 sequence of the Aspergillus spp. was 100% identical to that of Aspergillus fumigatus, whereas the ITS sequence of the Aspergillus spp. showed the highest bit score against that of A. fumigatus on BLAST results.
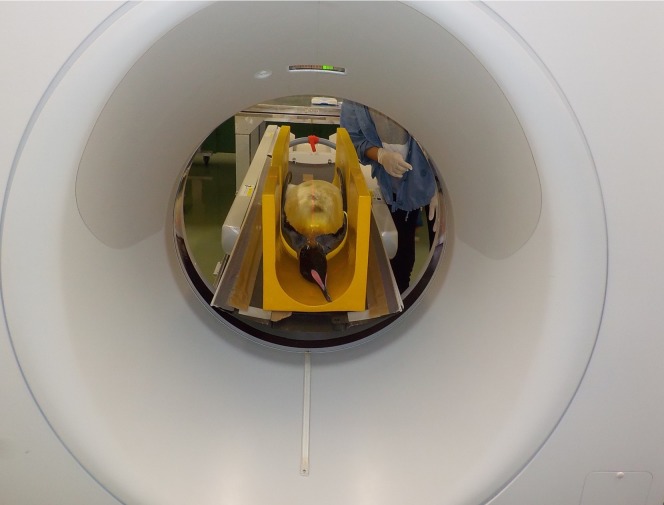
Fig. 1.
A photograph of autopsy imaging–computed tomography (Ai-CT) of the dead body of the King Penguin.
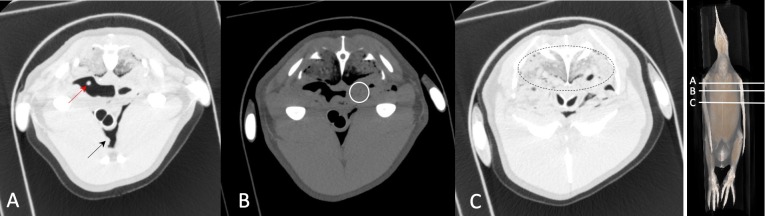
Fig. 2.
Autopsy imaging–computed tomography (Ai-CT) images of the dead body of the King Penguin. Thickening of the membrane (A, red arrow, which is the part of thickening, another part is not visible because of partial volume effect), multiple nodules (A, black arrow) in the cranial air sac, suspected abscess (CT number of the mass is 34 H.U.) (B, circle), lung infiltration, and air sac contraction (C) can be observed.
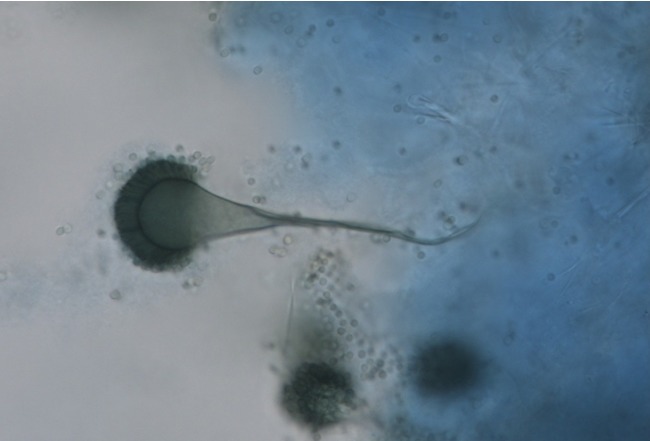
Fig. 3.
In a sample scratching the air sac surface, the characteristic conidial head of Aspergillus spp. was observed.
Based on the imaging findings, pathological dissection findings, fungal culture, and DNA analysis, the cause of death was comprehensively determined to be respiratory failure due to aspergillosis. In response to this, the aquarium, associated rooms, and yards were cleaned as a countermeasure to prevent aspergillosis in other penguins. These measures were taken in response to determining the cause of death to reduce economic losses caused by the death of valuable zoo animals.
The results of an antemortem blood test including anemia, poor nutrition, and inflammation were nonspecific to aspergillosis [1], and Aspergillus antigen and antibody were both negative. Therefore, we considered that the sensitivity of blood tests for aspergillosis was low [9]. In addition to antemortem clinical findings, it is necessary to cultivate the fungus and perform DNA analysis for the definitive diagnosis of aspergillosis.
Before the dissection, thickening of the membrane, suspected abscess, lung infiltration, and air sac contraction were determined. These findings facilitated the dissection workflow [12]. A. fumigatus abundantly produced spores on the air sac surface. Spores of filamentous fungi have a risk of being spread in the surrounding environment. Particularly, A. fumigatus is easily dispersed in the air because the spores are hydrophobic. More importantly, the finding of suspected aspergillosis alerted the pathologist regarding any potential hazards during the autopsy in terms of the unexpected inspiration of fungal spores [7]. This is the first report of Ai for aspergillosis in King Penguin. In conclusion, Ai is useful not only for determining the cause of death but also for readiness and safe pathological dissection.
Acknowledgments
This work was supported by the JSPS Grant-in-Aid for Scientific Research (C) Number JP18K05981 and JP17K08095. The authors thank Dr. Yoshiyasu Kobayashi and Dr. Noriyuki Horiuch for pathological dissection.
REFERENCES
- 1.Beernaert L. A., Pasmans F., Van Waeyenberghe L., Haesebrouck F., Martel A.2010. Aspergillus infections in birds: a review. Avian Pathol. 39: 325–331. doi: 10.1080/03079457.2010.506210 [DOI] [PubMed] [Google Scholar]
- 2.Franckenberg S.2015. Fatal gunshot to a fox: the Virtopsy approach in a forensic veterinary case. Forensic Radiol. Imaging. 3: 72–75. doi: 10.1016/j.jofri.2014.11.001 [DOI] [Google Scholar]
- 3.Grabherr S., Grimm J., Dominguez A., Vanhaebost J., Mangin P.2014. Advances in post-mortem CT-angiography. Br. J. Radiol. 87: 20130488. doi: 10.1259/bjr.20130488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godet C., Laurent F., Bergeron A., Ingrand P., Beigelman-Aubry C., Camara B., Cottin V., Germaud P., Philippe B., Pison C., Toper C., Carette M. F., Frat J. P., Béraud G., Roblot F., Cadranel J., ACHROSCAN Study Group. 2016. CT imaging assessment of response to treatment in chronic pulmonary aspergillosis. Chest 150: 139–147. doi: 10.1016/j.chest.2016.02.640 [DOI] [PubMed] [Google Scholar]
- 5.Kwon-Chung K. J., Sugui J. A.2013. Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 9: e1003743. doi: 10.1371/journal.ppat.1003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K. J., Sasaki M., Miyauchi A., Kishimoto M., Shimizu J., Iwasaki T., Miyake Y., Yamada K.2011. Virtopsy in a red kangaroo with oral osteomyelitis. J. Zoo Wildl. Med. 42: 128–130. doi: 10.1638/2010-0015.1 [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin S., Kind K., Thomson L., Bouhaidar R.2016. Unexpected active tuberculosis on Post Mortem CT: A case report and review of the literature. Forensic Sci. Int. 266: e64–e67. doi: 10.1016/j.forsciint.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 8.Okuda T., Shiotani S., Sakamoto N., Kobayashi T.2013. Background and current status of postmortem imaging in Japan: short history of “Autopsy imaging (Ai)”. Forensic Sci. Int. 225: 3–8. doi: 10.1016/j.forsciint.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 9.Tell L. A.2005. Aspergillosis in mammals and birds: impact on veterinary medicine. Med. Mycol. 43 Suppl 1: S71–S73. doi: 10.1080/13693780400020089 [DOI] [PubMed] [Google Scholar]
- 10.Thali M. J., Kneubuehl B. P., Bolliger S. A., Christe A., Koenigsdorfer U., Ozdoba C., Spielvogel E., Dirnhofer R.2007. Forensic veterinary radiology: ballistic-radiological 3D computertomographic reconstruction of an illegal lynx shooting in Switzerland. Forensic Sci. Int. 171: 63–66. doi: 10.1016/j.forsciint.2006.05.044 [DOI] [PubMed] [Google Scholar]
- 11.Villouta G., Hargreaves R., Rtveros V.1997. Haematological and clinical biochemistry findings in captive Humboldt penguins (Spheniscus humboldti). Avian Pathol. 26: 851–858. doi: 10.1080/03079459708419258 [DOI] [PubMed] [Google Scholar]
- 12.Watson E., Heng H. G.2017. Forensic radiology and imaging for veterinary radiologists. Vet. Radiol. Ultrasound 58: 245–258. doi: 10.1111/vru.12484 [DOI] [PubMed] [Google Scholar]
- 13.Yamada K., Sato F., Horiuchi N., Higuchi T., Kobayashi Y., Sasaki N., Nambo Y.2016. Autopsy imaging for cardiac tamponade in a Thoroughbred foal. J. Equine Sci. 27: 115–118. doi: 10.1294/jes.27.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen K., Lövblad K. O., Scheurer E., Ozdoba C., Thali M. J., Aghayev E., Jackowski C., Anon J., Frickey N., Zwygart K., Weis J., Dirnhofer R.2007. Post-mortem forensic neuroimaging: correlation of MSCT and MRI findings with autopsy results. Forensic Sci. Int. 173: 21–35. doi: 10.1016/j.forsciint.2007.01.027 [DOI] [PubMed] [Google Scholar]