Abstract
Introduction: In recent years, laser irradiation in the near-infrared ray (NIR) area has been reported to promote bone healing. There are also reports that laser irradiation accelerates orthodontic tooth movement. In this study, we investigated the effect of NIR laser irradiation and mechanical stimulation on osteoblasts.
Methods: We seeded osteoblast-like cells and laser irradiation was performed 24 hours after cell seeding. In addition, a control group not receiving anything, a group receiving only Nd: YAG (neodymium-doped yttrium aluminum garnet) laser irradiation, a group receiving only centrifugal loading, and a group receiving both Nd: YAG laser irradiation and centrifugal force loading were set, and after 24 hours and after 48 hours, cells were collected and quantitative real-time polymerase chain reaction (PCR) was performed.
Results: 24 hours after laser irradiation, the gene expression of alkaline phosphatase (ALP), the receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) was significantly higher in the 2.0 W group than in the control group. In addition, the RANKL/OPG ratio was higher in the 2.0 W group than in the control group. Also, in the group using laser irradiation and centrifugal loading in combination, 24 hours after laser irradiation, ALP and OPG showed significantly higher values than those in the centrifugal load only group. Furthermore, the RANKL/OPG ratio also showed high values.
Conclusion: These results suggest that osteoblast-like cells activate genes related to bone metabolism by combining mechanical stimulation and laser irradiation. This helps to elucidate the influence of laser irradiation during tooth movement.
Keywords: Nd: YAG laser, Bone metabolism, Osteoblast, Mechanical force
Introduction
Laser therapy is developing in the medical field. In clinical practice in the field of dentistry, lasers have wide applications. Currently, lasers used in the dental field are surface absorption type (CO2 laser and erbium-doped yttrium aluminium garnet [YAG] laser) and tissue penetrating type lasers (He: Ne laser, neodymium-doped yttrium aluminum garnet [Nd: YAG] laser, and diode laser). In cases where tissue incision and hemostasis are required, it is advantageous to use surface absorbing lasers at high power. In contrast, using tissue penetrating lasers at low power allows treatment using cell activation. In recent years, laser therapy in the near-infrared region (NIR) has attracted attention. These are used for periodontal therapy,1,2 sensory hypersensitivity treatment,3,4 and treatment of temporomandibular disorders.5 On another study using a rat fracture model, there were reports that Nd: YAG laser and diode laser irradiation promoted bone healing6,7 and that diode laser irradiation promoted bone grafting in rats and in vitro experiments.8,9 Also, another study reports that Nd: YAG laser irradiation promoted osteoblast differentiation,10 red laser irradiation caused fibroblast proliferation,11 and diode laser irradiation caused osteoblast proliferation.12 In the field of orthodontic dentistry, pain relief13,14 during tooth movement by laser irradiation and the acceleration of tooth movement have been reported.15-17 However, it is currently the case that there are many things the mechanisms of which are not known yet. In previous experiments, the irradiation effect of the Nd: YAG laser during orthodontic tooth movement was investigated using rats and it was proved that there was a change in bone metabolism and tooth movement.18 In addition, in a previous study from the authors, a wound healing test was performed by irradiating osteoblast-like cells with a diode laser and confirmed that the cells proliferated.12
There are many researches using the Nd: YAG laser in in vitro experiments, but there is no cell experiment model considering tooth movement. There are several reports that are used as cell experimental models of tooth movement by applying a mechanical stimulus to cells.19 Therefore, it was decided to investigate the effect of the irradiation of the Nd: YAG laser on the condition that the osteoblast-like cells were loaded with mechanical stimulation by centrifugal force.
Methods
Cell Culture
Cells of the human-derived osteoblast-like cells line Saos-2 (Riken, Tukuba, Japan ) were cultured in 100-mm (Corning, NY, USA) tissue culture dishes with the Dulbecco’s Modified Eagle Medium (DMEM; Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Bioserum, Melbourne, Victoria, Australia) and 50 mg/mL kanamycin (Meiji Pharmaceutical, Tokyo, Japan). Incubation was done at 37°C under a humidified atmosphere of 95% air and 5% CO2. The exchange of the culture medium was done every 3 days. The passage was carried out when the confluence reached 80%.
Preliminary Experiment for Determining Conditions
5.0 × 104 cells were seeded in a 24-well plate and the Nd: YAG laser was irradiated under the conditions of 0.6 W, 2.0 W, 4.0 W, and 6.0 W after 24 hours. The cells were collected using PBS (Mitsubishi Chemical Mediation, Tokyo, Japan) containing 0.2% trypsin (Nakarai Tesque, Kyoto, Japan) and 0.1% EDTA (Wako, Tokyo, Japan) 24 hours after Nd: YAG laser irradiation, with the number of viable cells counted using a 0.4 w/ v% trypan blue solution (Wako). As a result, it was found that the cells died when the output was 4.0 W or more. Therefore, it was decided to experiment with control, 0.6 W, and 2.0 W conditions (Figure 1).
Figure 1.
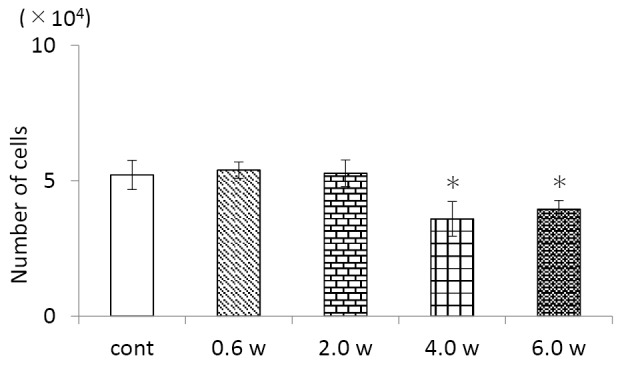
Number of Cells After Nd:YAG Laser Irradiation for 24 Hours.
cont: Control group not irradiated with the Nd: YAG laser.
0.6 W, 2.0 W, 4.0 W, 6.0 W: Experimental groups irradiated with the Nd: YAG laser at each output. *P < 0.05 vs. control, n=3.
Human-derived osteoblast-like cells were irradiated with the Nd: YAG laser and the number of viable cells was determined 24 hours later.
When the cells were irradiated with the Nd: YAG laser at an output of 4.0 W or higher, a decrease in the number of viable cells was observed.
Laser Irradiation
For this study, the Nd: YAG laser (Inpulse, Incisive, Richmond, Ca, USA) with a wavelength of 1064 nm was used. The guide light was a diode laser with a wavelength of 630 to 680 nm. A 0.32-mm optical fiber was used as the tip of the Nd: YAG laser. The handpiece was set using a stand so that the distance from the tip to the bottom of the culture dish was 2.4 cm. Laser irradiation was performed for 15 seconds under each condition. Energy density was 5.17 J/cm2 (0.6 W), 17.23 J/cm2 (2.0 W), 34.47 J/cm2 (4.0 W), and 51.7 J/cm2 (6.0 W) in total. Also, the non-irradiated cultured cells were used as the control. The specifications of laser irradiation are shown in Table 1.
Table 1. The Specifications of the Nd: YAG Laser Used in This Study .
| Wavelength (nm) | 1064 |
| Operating mode | Pulsed |
| Frequency (Hz) | 20–30 |
| Pulse duration (µs) | 100 |
| Duty cycle | 0.001 |
| Power | 30–200 mJ/100 µs |
| Average power (W) | 0.6–6.0 |
Quantitative Real-Time PCR Analysis
2.0 × 105 cells were seeded in a 24-well plate and the Nd: YAG laser irradiated each condition after 24 hours. 24 hours after laser irradiation, the cells were collected using PBS containing 0.2% trypsin and 0.1% EDTA, homogenized using a syringe and a 20 G needle (both Terumo, Tokyo, Japan), and the RNeasy Mini Kit (RNeasy Mini Spin Colum, QIAGEN, Venlo, Netherlands) RNA was extracted by the column method used (Figure 2).
Figure 2.
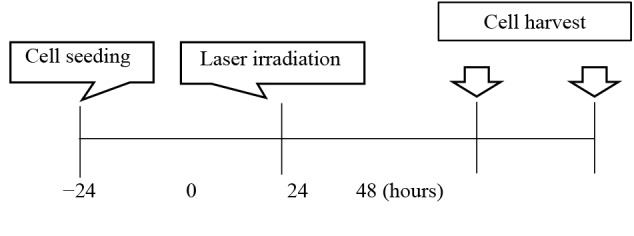
The Time Course of This Experiment.
The total RNA was quantified by reading the absorbance at a wavelength of 260 nm by a spectrophotometer (BioSpecnano, Shimadzu, Kyoto, Japan). The cDNA was synthesized from 1 mg total RNA using a qPCR reverse transcription kit (ReverTra Ace-α, Toyobo, Osaka, Japan) and a random primer (Toyobo). The cDNA was obtained from total RNA using a Simpli Amp Thermal Cycler (Applied Biosystems, Foster City, CA, USA). Gene expression was monitored using the SYBR Green Real-time PCR Master Mix (Toyobo, Osaka, Japan) and a Light Cycler 480 II system (Roche Diagnostics, Basel, Switzerland) with the prepared cDNA as the template. Table 2 shows the PCR primer sequences for alkaline phosphatase (ALP), Receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The reaction conditions were as follows: 45 cycles of denaturation at 95°C for 15 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 10 seconds. Gene expression values were normalized to the control values. The fold-increase in gene expression in human-derived osteoblast-like cells cultured alone was also monitored, and the mRNA level was normalized to that of GAPDH.
Table 2. PCR Primer Sequences .
| Gene | Sequence |
| GAPDH | Forward 5′-TGGTATCGTGGAAGGACTCA-3′ |
| Reverse 5′-GCCATCACGCCACAGTT-3′ | |
| ALP | Forward 5′-TACAAGGTGGGCGGTGA-3′ |
| Reverse 5′-ACAGCAGACTGCGCCTGGTAGTT-3′ | |
| RANKL | Forward 5′-ATATCGTTGGATCACAGCAC-3′ |
| Reverse 5′-CAAGAGGACAGACTCACTTTATG-3′ | |
| OPG | Forward 5′-CTGCTGAAGTTATGGAAACAT-3′ |
| Reverse 5′-AGGTTAGCATGTCCAATGTG-3′ |
Mechanical Stress
Centrifugal force was applied for 20 minutes at 489 rpm using RPM LCO 6-SP (Tommy Seiko, Tokyo, Japan), following the method described by Redlich et al.19 After centrifugal force loading, the laser was irradiated, cell recovery was performed after 24 and 48 hours, and the RT-PCR experiment was performed using the same method. For the cells subjected to both centrifugal loading and laser irradiation, a laser irradiation setting of 2.0 W, which was the most effective, was selected.
Statistical Analysis
The mean of the three experiments was calculated and was considered to represent the values of each experimental parameter. The data from the three independent experiments are presented as the mean and standard deviation. One-way analysis of variance was used for group comparisons. The differences between the control group and each experimental group were analyzed by the Dunnett test. P values of < 0.05 were considered statistically significant.
Results
Compared with the control group, the expression of the RANKL, OPG, and ALP genes 24 hours after irradiation was not significantly different in the 0.6 W laser irradiation group but significantly higher in the 2.0 W laser irradiation group. Furthermore, the RANKL/OPG ratio did not change significantly in the 0.6 W laser irradiation group compared to the control group but was higher in the 2.0 W laser irradiation group compared with the control group (Figure 3).
Figure 3.
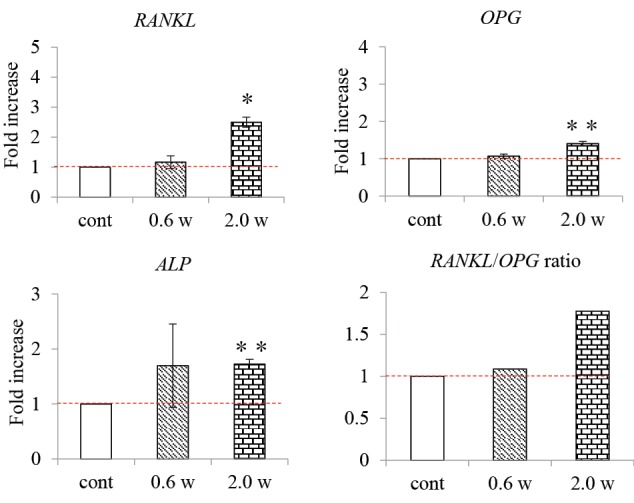
RANKL, OPG, and ALP Gene Expression Levels 24 Hours After Nd:YAG Laser Irradiation.
cont: Control group not irradiated with the Nd: YAG laser.
0.6 W, 2.0 W: Experiment groups irradiated with the Nd: YAG laser at each output. **P < 0.01, * P < 0.05 vs. control; n=3.
The graphs show the fold-increase in the expression of the RANKL, OPG, and ALP genes 24 hours after Nd: YAG laser irradiation.
The expression of the RANKL, OPG, and ALP genes and the RANKL/OPG ratio 24 hours after irradiation were higher in the 2.0 W laser irradiation group than in the control group.
Compared with the control group, there was no significant difference in RANKL, OPG, and ALP gene expression 48 hours after the start of the experiment in the 0.6 W and 2.0 W laser irradiation groups. In addition, there was no difference in the RANKL/OPG ratio between the 0.6 W and 2.0 W laser irradiation groups compared to the control group (Figure 4).
Figure 4.
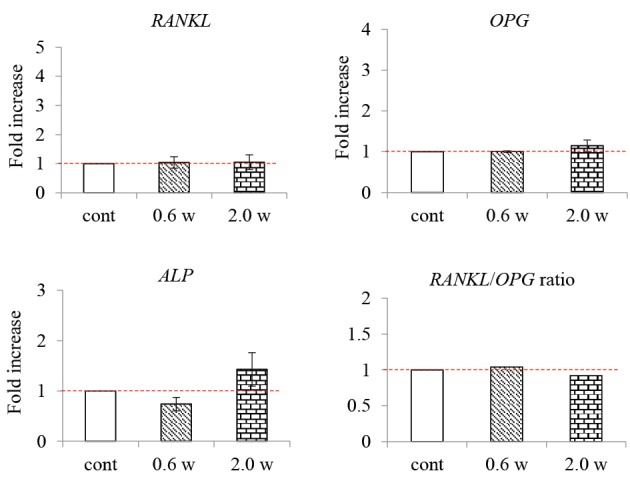
RANKL, OPG, and ALP Gene Expression Levels 48 Hours After Nd:YAG Laser Irradiation.
cont: Control group not irradiated with the Nd: YAG laser.
0.6 W, 2.0 W: Experiment groups irradiated with an Nd: YAG laser at each output. **P < 0.01, *P < 0.05 vs. control; n=3.
The graphs show the fold-increase in the expression of the RANKL, OPG, and ALP genes 48 hours after irradiation with an Nd: YAG laser. 48 hours after the start of the experiment, there was no significant difference in gene expression between the control and experimental groups.
The gene expressions of OPG, RANKL and ALP were significantly higher in the Nd: YAG laser irradiation group and the centrifugal force load group than in the control group 24 hours after the experiment. In addition, the gene expression of RANKL and ALP was significantly higher in the laser centrifugal load group than the centrifugal load group, and OPG gene expression was significantly lower.
The RANKL/OPG ratio was higher in all experimental groups compared to the control group. In particular, the laser irradiation/centrifugal loading group exhibited the highest ratio (Figure 5).
Figure 5.
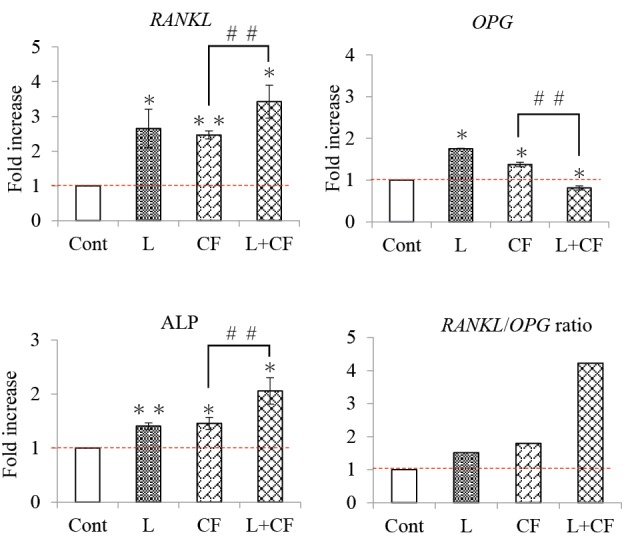
RANKL, OPG, and ALP Gene Expression Levels 24 Hours After Nd:YAG Laser Irradiation and Centrifugal Force.
Cont: Control group not treated with Nd: YAG laser irradiation and centrifugal force loading.
L: Experiment group irradiated with an Nd: YAG laser at an output of 2.0 W. CF: Experimental group subjected to centrifugal force as a mechanical stress. L+CF: Experimental group in which centrifugal force was applied as a mechanical stress and Nd: YAG laser irradiation was performed at an output of 2.0 W.
**P < 0.01, *P < 0.05 vs. control; n=3. ##P < 0.01 vs. CF; n=3.
The graphs show the fold-increase in the expression of the RANKL, OPG, and ALP genes 24 hours after Nd: YAG laser irradiation and centrifugal force loading.
The RANKL/OPG ratio was higher in the laser irradiation/centrifugal force loading group compared to the other groups.
The expression of the ALP gene 48 hours after the experiment was significantly higher in the Nd: YAG laser irradiation and the centrifugal load group than in the control group. There were no significant differences compared to the other groups (Figure 6).
Figure 6.
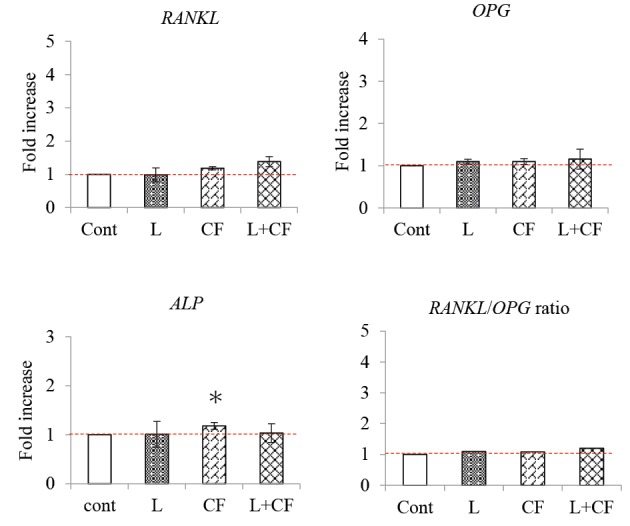
RANKL, OPG, and ALP Gene Expression Levels 24 Hours After Nd:YAG Laser Irradiation and Centrifugal Force.
Cont: Control group not treated with Nd: YAG laser irradiation and centrifugal force loading.
L: Experiment group treated with Nd: YAG laser irradiation at an output of 2.0 W. CF: Experimental group treated with centrifugal force as a mechanical stress. L+CF: Experimental group in which centrifugal force was applied as a mechanical stress and Nd: YAG laser irradiation was performed at an output of 2.0 W.
*P < 0.05 vs. control; n=3.
The graphs show the fold-increase in the expression of the RANKL, OPG, and ALP genes 48 hours after treatment with Nd: YAG laser irradiation and centrifugal force loading.
The RANKL/OPG ratio, 48 hours after the experiment was initiated, tended to be higher in the experimental groups than in the control group, but there was no significant difference in RANKL and OPG expression.
Discussion
In the setting of this study, preliminary experiments were performed by irradiating the cells with an Nd: YAG laser. Compared to the control group, there was no significant difference in the 0.6 W and 2.0 W groups, but the number of human-derived osteoblast-like cells was significantly reduced in both the 4.0 W and 6.0 W groups. According to the Arndt-Schulz’s law, weak stimuli promote the reaction both biologically and physiologically, and the strong stimulus suppresses this reaction and is said to be harmful.20,21 The results of the preliminary experiment indicated that a harmful reaction to irradiation occurred in the cells in the 4.0 and 6.0 W groups. Our previous experiments demonstrated that human-derived osteoblast-like cells irradiated with an Nd: YAG laser were induced to proliferate.22 In the present study, no proliferation of live cells was observed, potentially because the cells were not damaged by scratches and therefore the response may be different. When combined with the defense responses of the living body, the laser effect may be more likely. In another study, cell proliferation was confirmed by NIR laser irradiation of osteoblast-like cells (MC3T3-E1).12 However, the cells did not proliferate in the present study. This could be related to the use of a different cell type and differences in experimental methods for confirming cell growth. Another potential factor is the laser; although the output was similar, the use of a different type of laser could have affected the results.
Several reports on the effects of Nd: YAG laser irradiation on cells have been published. It was reported that an increase in intracellular Ca2+ in osteoblast-like cells was observed in the Nd: YAG laser-irradiated group via the activation of the TRPC1 ion channel compared to the control, reported by Chellini et al.10 Ninomiya et al reported that bone formation was promoted by irradiating the damaged rat femur with the Nd: YAG laser compared to the control group.6 Thus, laser irradiation is thought to have a beneficial effect on bone formation. It has been reported that low-power laser irradiation in a rat tooth movement model resulted in increased OPG expression compared to the control group.23 Oliveira et al reported that ALP activity increased in cells irradiated with a laser at the wavelengths of 660 nm and 780 nm.24
In the present study, the expression of the OPG and ALP genes was significantly higher in the 2.0 W group 24 hours after Nd: YAG laser irradiation. In the previous reports, it was confirmed that laser irradiation had a positive effect on bone formation. It was also reported that RANKL expression in a rat tooth movement model increased compared to the control group following low-power laser irradiation.25 The results of the present study were similar, with RANKL gene expression significantly higher in the 2.0 W group 24 hours after Nd: YAG laser irradiation. Our experiments also demonstrated that the RANKL/OPG ratio was higher than that of the control group 24 hours after laser irradiation. This appears to be related to having a negative effect on bone formation. However, the sequence of reactions between tooth movement and laser irradiation is complex and cannot be explained at a single point. Tanaka et al26 reported that enhanced bone resorption was associated with an increase in the RANKL/OPG ratio. In addition, Oliveira et al24 reported increased ALP activity in cells irradiated with a laser at the wavelengths of 660 nm and 780 nm. Similar results were obtained in the present study, suggesting that both the bone metabolism and resorption systems may be activated. Usumez et al27 reported that Nd: YAG laser irradiation in a rat gingival wound model resulted in increased expression of the TGF-β, PDGF, and bFGF genes compared to the control group. Likewise, in the present study, the activation of the cells by laser irradiation may have affected gene expression.
Naito et al reported that centrifugal loading significantly increased ALP gene expression on rat bone marrow-derived osteoblasts.28 Also, Okamoto et al29 reported that RANKL gene expression increased due to centrifugal loading on MC3T3-E1 cells, and Naruse et al30 reported the increased expression of the OPG gene by centrifugal loading on cultured periodontal ligament cells. Similarly, in this study, the gene expression of OPG, RANKL and ALP was significantly higher in the load group than in the control group. Furthermore, when Nd: YAG laser irradiation was used in combination, the gene expression of ALP and RANKL was higher in the laser load group after 24 hours than in the load group. In addition, the gene expression of OPG showed significantly lower values in the laser load group than in the load group after 24 hours, resulting in a high RANKL/OPG ratio.
These results suggest that combining mechanical stimulation with Nd: YAG laser irradiation may increase ALP gene expression and the RANKL/OPG ratio in human osteoblast-like cells. Furthermore, under the present research conditions, the effect of Nd: YAG laser irradiation on osteoblast-like cells did not last for 48 hours, suggesting that the effect of laser irradiation decreases with time.
It was found that Nd: YAG Laser irradiation acted on osteoblast-like cells and activated the genes related to the bone metabolism system. Through further research, it is expected that it will have a positive impact on bone metabolism during tooth movement in orthodontic treatment.
Conclusion
These results suggest that osteoblast-like cells activate the genes related to bone metabolism by combining mechanical stimulation and laser irradiation. This helps to elucidate the influence of laser irradiation during tooth movement. The mechanics of laser irradiation and tooth movement are very complex. In addition, these reactions occur simultaneously, increasing complexity. Further detailed research is needed.
Ethical Considerations
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by grants from JSPS KAKENHI (numbers 16K11790, 16K20644, 25862016 and 18K17256). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Please cite this article as follows: Tsuka Y, Kunimatsu R, Gunji H, Abe T, Medina CC, Nakajima K, et al. Examination of the effect of the combined use of Nd: YAG laser irradiation and mechanical force loading on bone metabolism using cultured human osteoblasts. J Lasers Med Sci. 2020;11(2):138-143. doi:10.34172/jlms.2020.24.
References
- 1.Alzoman HA, Diab HM. Effect of gallium aluminium arsenide diode laser therapy on Porphyromonas gingivalis in chronic periodontitis: a randomized controlled trial. Int J Dent Hyg. 2016;14(4):261–266. doi: 10.1111/idh.12169. [DOI] [PubMed] [Google Scholar]
- 2.Gündoğar H, Şenyurt SZ, Erciyas K, Yalım M, Üstün K. The effect of low-level laser therapy on non-surgical periodontal treatment: a randomized controlled, single-blind, split-mouth clinical trial. Lasers Med Sci. 2016;31(9):1767–1773. doi: 10.1007/s10103-016-2047-z. [DOI] [PubMed] [Google Scholar]
- 3.García-Delaney C, Abad-Sánchez D, Arnabat-Domínguez J, Valmaseda-Castellón E, Gay-Escoda C. Evaluation of the effectiveness of the photobiomodulation in the treatment of dentin hypersensitivity after basic therapy A randomized clinical trial. J Clin Exp Dent. 2017;9(5):e694–e702. doi: 10.4317/jced.53635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Femiano F, Femiano R, Lanza A, Lanza M, Perillo L. Effectiveness on oral pain of 808-nm diode laser used prior to composite restoration for symptomatic non-carious cervical lesions unresponsive to desensitizing agents. Lasers Med Sci. 2017;32(1):67–71. doi: 10.1007/s10103-016-2087-4. [DOI] [PubMed] [Google Scholar]
- 5.Sayed N, Murugavel C, Gnanam A. Management of temporomandibular disorders with low level laser therapy. J Maxillofac Oral Surg. 2014;13(4):444–50. doi: 10.1007/s12663-013-0544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ninomiya T, Miyamoto Y, Ito T, Yamashita A, Wakita M, Nishisaka T. High-intensity pulsed laser irradiation accelerates bone formation in metaphyseal trabecular bone in rat femur. J Bone Miner Metab. 2003;21(2):67–73. doi: 10.1007/s007740300011. [DOI] [PubMed] [Google Scholar]
- 7.Weber JB, Pinheiro AL, de Oliveira MG, Oliveira FA, Ramalho LM. Laser therapy improves healing of bone defects submitted to autologous bone graft. Photomed Laser Surg. 2006;24(1):38–44. doi: 10.1089/pho.2006.24.38. [DOI] [PubMed] [Google Scholar]
- 8.Son J, Kim YB, Ge Z, Choi SH, Kim G. Bone healing effects of diode laser (808 nm) on a rat tibial fracture model. In Vivo. 2012;26(4):703–9. [PubMed] [Google Scholar]
- 9.Pinheiro AL, Soares LG, Marques AM, Cangussú MC, Pacheco MT, Silveira L Jr. Biochemical changes on the repair of surgical bone defects grafted with biphasic synthetic micro-granular HA + β-tricalcium phosphate induced by laser and LED phototherapies and assessed by Raman spectroscopy. Lasers Med Sci. 2017;32(3):663–72. doi: 10.1007/s10103-017-2165-2. [DOI] [PubMed] [Google Scholar]
- 10.Chellini F, Sassoli C, Nosi D, Deledda C, Tonelli P, Zecchi-Orlandini S. et al. Low pulse energy Nd:YAG laser irradiation exerts a biostimulative effect on different cells of the oral microenvironment: “an in vitro study”. Lasers Surg Med. 2010;42(6):527–39. doi: 10.1002/lsm.20861. [DOI] [PubMed] [Google Scholar]
- 11.Naderi MS, Razzaghi M, Esmaeeli Djavid G, Hajebrahimi Z. A comparative study of 660 nm low-level laser and light emitted diode in proliferative effects of fibroblast cells. J Lasers Med Sci. 2017;8(suppl 1):S46–S50. doi: 10.15171/jlms.2017.s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunimatsu R, Gunji H, Tsuka Y, Yoshimi Y, Awada T, Sumi K. et al. Effects of high-frequency near-infrared diode laser irradiation on the proliferation and migration of mouse calvarial osteoblasts. Lasers Med Sci. 2018;33(5):959–966. doi: 10.1007/s10103-017-2426-0. [DOI] [PubMed] [Google Scholar]
- 13.Doshi-Mehta G, Bhad-Patil WA. Efficacy of low-intensity laser therapy in reducing treatment time and orthodontic pain: a clinical investigation. Am J Orthod Dentofacial Orthop. 2012;141(3):289–297. doi: 10.1016/j.ajodo.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Qamruddin I, Alam MK, Mahroof V, Fida M, Khamis MF, Husein A. Effects of low-level laser irradiation on the rate of orthodontic tooth movement and associated pain with self-ligating brackets. Am J Orthod Dentofacial Orthop. 2017;152(5):622–630. doi: 10.1016/j.ajodo.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26(3):282–91. doi: 10.1002/(sici)1096-9101(2000)26:3<282::aid-lsm6>3.0.co;2-x.. [DOI] [PubMed] [Google Scholar]
- 16.Cruz DR, Kohara EK, Ribeiro MS, Wetter NU. Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Lasers Surg Med. 2004;35(2):117–120. doi: 10.1002/lsm.20076. [DOI] [PubMed] [Google Scholar]
- 17.Genc G, Kocadereli I, Tasar F, Kilinc K, El S, Sarkarati B. Effect of low-level laser therapy (LLLT) on orthodontic tooth movement. Lasers Med Sci. 2013;28(1):41–7. doi: 10.1007/s10103-012-1059-6. [DOI] [PubMed] [Google Scholar]
- 18.Tsuka Y, Fujita T, Shirakura M, Kunimatsu R, Su SC, Fujii E. et al. Effects of Neodymium-Doped Yttrium Aluminium Garnet (Nd:YAG) Laser Irradiation on Bone Metabolism During Tooth Movement. J Lasers Med Sci. 2016;7(1):40–4. doi: 10.15171/jlms.2016.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redlich M, Asher Roos H, Reichenberg E, Zaks B, Mussig D, Baumert U. et al. Expression of tropoelastin in human periodontal ligament fibroblasts after simulation of orthodontic force. Arch Oral Biol. 2004;49(2):119–24. doi: 10.1016/j.archoralbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 20. Tunér J, Hode L . The New Laser Therapy Handbook: A guide for research scientists, doctors, dentists, veterinarians and other interested parties within the medical field. Grängesberg: Prima Books; 2010.
- 21. Hamblin MR, De Sousa M, Agrawal T, eds. Handbook of Low-Level Laser Therapy. Singapore: Pan Stanford Publishing Pte Ltd; 2017.
- 22.Tsuka Y, Kunimatsu R, Gunji H, Nakajima K, Kimura A, Hiraki T. et al. Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: in vitro study. Lasers Med Sci. 2019;34(1):55–60. doi: 10.1007/s10103-018-2586-6. [DOI] [PubMed] [Google Scholar]
- 23.de Melo Conti C, Suzuki H, Garcez AS, Suzuki SS. Effects of Photobiomodulation on Root Resorption Induced by Orthodontic Tooth Movement and RANKL/OPG Expression in Rats. Photochem Photobiol. 2019;95(5):1249–57. doi: 10.1111/php.13107. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira FA, Matos AA, Matsuda SS, Buzalaf MA, Bagnato VS, Machado MA. et al. Low level laser therapy modulates viability, alkaline phosphatase and matrix metalloproteinase-2 activities of osteoblasts. J Photochem Photobiol B. 2017;169:35–40. doi: 10.1016/j.jphotobiol.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Alazzawi MMJ, Husein A, Alam MK, Hassan R, Shaari R, Azlina A. et al. Effect of low level laser and low intensity pulsed ultrasound therapy on bone remodeling during orthodontic tooth movement in rats. Prog Orthod. 2018;19(1):10. doi: 10.1186/s40510-018-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka H, Mine T, Ogasa H, Taguchi T, Liang CT. Expression of RANKL/OPG during bone remodeling in Vivo. Biochem Biophys Res Commun. 2011;411(4):690–4. doi: 10.1016/j.bbrc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Usumez A, Cengiz B, Oztuzcu S, Demir T, Aras MH, Gutknecht N. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci. 2014;29(6):1807–13. doi: 10.1007/s10103-013-1336-z. [DOI] [PubMed] [Google Scholar]
- 28.Naito K, Matsuzaka K, Ishigami K, Inoue T. Mechanical force promotes proliferation and early differentiation of bone marrow derived osteoblast-like cells in Vitro. Oral Med Pathol. 2009;13(4):143–9. doi: 10.3353/omp.13.143. [DOI] [Google Scholar]
- 29.Okamoto A, Ohnishi T, Bandow K, Kakimoto K, Chiba N, Maeda A. et al. Reduction of orthodontic tooth movement by experimentally induced periodontal inflammation in mice. Eur J Oral Sci. 2009;117(3):238–47. doi: 10.1111/j.1600-0722.2009.00625.x. [DOI] [PubMed] [Google Scholar]
- 30.Naruse S, Matsuzaka K, Kokubu E. Expressions of RANKL and OPG mRNA on Rat Periodontal Ligament Cells Following Heavy Mechnical Stress. Journal of Japan Association of Dental Traumatology. 2009;5(1):10–17. [Japanese]. [Google Scholar]


