Highlights
-
•
COVID-19 in the elderly patients was severe and highly fatal.
-
•
COVID-19 progressed rapidly in patients who died.
-
•
Cardiovascular disease, COPD, dyspnea, lymphocytopenia and ARDS predict mortality.
-
•
The elderly patients need close monitoring and timely treatment.
Keywords: Coronavirus infections, SARS-CoV-2, Pneumonia, Prognosis
Abstract
Objective
To investigate the characteristics and prognostic factors in the elderly patients with COVID-19.
Methods
Consecutive cases over 60 years old with COVID-19 in Renmin Hospital of Wuhan University from Jan 1 to Feb 6, 2020 were included. The primary outcomes were death and survival till March 5. Data of demographics, clinical features, comorbidities, laboratory tests and complications were collected and compared for different outcomes. Cox regression was performed for prognostic factors.
Results
339 patients with COVID-19 (aged 71±8 years,173 females (51%)) were enrolled, including 80 (23.6%) critical, 159 severe (46.9%) and 100 moderate (29.5%) cases. Common comorbidities were hypertension (40.8%), diabetes (16.0%) and cardiovascular disease (15.7%). Common symptoms included fever (92.0%), cough (53.0%), dyspnea (40.8%) and fatigue (39.9%). Lymphocytopenia was a common laboratory finding (63.2%). Common complications included bacterial infection (42.8%), liver enzyme abnormalities (28.7%) and acute respiratory distress syndrome (21.0%). Till Mar 5, 2020, 91 cases were discharged (26.8%), 183 cases stayed in hospital (54.0%) and 65 cases (19.2%) were dead. Shorter length of stay was found for the dead compared with the survivors (5 (3–8) vs. 28 (26–29), P < 0.001). Symptoms of dyspnea (HR 2.35, P = 0.001), comorbidities including cardiovascular disease (HR 1.86, P = 0.031) and chronic obstructive pulmonary disease (HR 2.24, P = 0.023), and acute respiratory distress syndrome (HR 29.33, P < 0.001) were strong predictors of death. And a high level of lymphocytes was predictive of better outcome (HR 0.10, P < 0.001).
Conclusions
High proportion of severe to critical cases and high fatality rate were observed in the elderly COVID-19 patients. Rapid disease progress was noted in the dead with a median survival time of 5 days after admission. Dyspnea, lymphocytopenia, comorbidities including cardiovascular disease and chronic obstructive pulmonary disease, and acute respiratory distress syndrome were predictive of poor outcome. Close monitoring and timely treatment should be performed for the elderly patients at high risk.
Introduction
Corona Virus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections occurred in Wuhan in December 2019.1 Till March 5, 2020, the SARS-CoV-2 infected 80,565 patients and caused 3015 deaths in China. Based on early statistical data of china, the case-fatality rate (CRF) in the patients over 60 years old is much higher than overall CRF, 14.8% CRF in patients over 80 years, 8.0% CRF in patients aged 70–79 years and 3.6% CRF in patients aged 60–69 years.2 The proportion of deaths over 60 years old accounts for 81% of the total deaths in the national wide, which implicates aged people are more vulnerable to the SARS-CoV-2.
Till present there are rare reports in literature focusing on the clinical characteristics of the elderly patients with COVID-19, and the risk factors for poor outcomes remains to be elucidated. The present study aims to describe the clinical characteristics and to investigate the prognostic factors of the elderly patients with COVID-19, which might provide evidence for the risk stratification and help to improve the clinical practice and reduce fatality.
Patients and methods
Study design and participants
The study complied with the edicts of the Declaration of Helsinki and was approved by the Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2020-K032). Written informed consent was waived by the same committee. In this retrospective, single-center study, we included all confirmed cases of COVID-19 over 60 years old admitted from Jan 1 to Feb 6 at isolation ward of Renmin Hospital of Wuhan University in Wuhan, China. This is a designated hospital capable of receiving severe COVID-19 patients. All enrolled patients were diagnosed according to Interim guidance for novel coronavirus pneumonia published by National Health Commission of the People's Republic of China.3 According to the report of the WHO—China Joint Mission on COVID-19,4 patients with COVID-19 were divided into mild (laboratory confirmed, without pneumonia), moderate (laboratory confirmed and with pneumonia), severe (dyspnea, respiratory frequency ≥30/minute, blood oxygen saturation ≤93%, PaO2/FiO2 ratio <300, and/or lung infiltrates >50% of the lung field within 24–48 h) and critical (respiratory failure requiring mechanical ventilation, shock or other organ failure that requires intensive care).
Procedures
SARS-CoV-2 nucleic acid was detected in all patients by real-time PCR to confirm the virus infection. Chest X-ray and/or computed tomography were performed for the diagnosis of pneumonia. Patients’ medical records were carefully reviewed and analyzed by three trained physicians. Patient data including demographics, comorbidities, signs and symptoms, laboratory results and complications were obtained. Patient history were collected for comorbidities including hypertension, diabetes, cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic liver disease, chronic obstructive pulmonary disease (COPD), malignancy and autoimmune diseases.
The occurrence of complications was confirmed by three physicians according to the following criteria. Acute kidney injury was identified according to the Kidney Disease: Improving Global Outcomes of Definition.5 Cardiac injury was defined if the serum level of cardiac troponin I (cTnI) was above the 99th percentile upper reference limit. ARDS was defined according to the Berlin definition.6 Arrhythmia was defined as emerging premature beat, tachycardia, atrial fibrillation, ventricular fibrillation and clinically significant bradycardia according to electrocardiography or medical records, transient sinus tachycardia associated with fever was excluded. Cardiac insufficiency was defined when the serum level of NT-pro BNP exceeded the normal range and the presence of associated symptoms, such as dyspnea, orthopnea and edema of lower extremity. The interval from symptom onset to admission and the length of stay were also recorded.
Outcomes
The primary outcomes were survival and death till March 5, 4 weeks from the last admission. Patients cured were discharged when the symptoms improved significantly, with no fever for at least three days, obvious absorption of inflammation in pulmonary imaging, and negative results for at least two consecutive tests of SARS-CoV-2 nucleic acid. Patients who didn't meet the discharge standard continued hospitalization for treatment and observation. The patients discharged or stayed in hospital till the end date of follow-up were classified into survival group.
Statistical analysis
Basic statistical analyses were performed using SPSS version 17.0. The normality of continuous variables was tested using the Kolmogorov-Smirnov test. Hypothesis testing was performed for comparing continuous and categorical variables in different outcome groups using Mann-Whitney U test and Chi-squared test respectively. The clinical and laboratory variables, which differed significantly under comparative statistics, were included in a univariate Cox analysis (proportional hazards regression) using R language v3.5.2 with packages “survival” and “survminer”. The models used in the univariate analysis were tested by Likelihood ratio test, Wald test and Score (log-rank) test. Multivariate Cox regressions were subsequently performed for comorbidities and complications, in which the “Age” factor was added to correct the models and only variables with statistical significance in univariate analysis were included. All significance levels were computed for 2-tailed testing and the cutoff of significance was set at P < 0.05.
Results
Demographics and baseline clinical characteristics
Of 339 patients identified, the mean age was 71 years, and 173 were female (51%). As presented in Table 1 , 60.7% of the patients had comorbidities, while 23.9% had two or more comorbidities. Common comorbidities included hypertension (138 cases, 40.8%), diabetes (54 cases, 16.0%) and cardiovascular disease (48 cases, 14.2%). The most common symptom was fever, which occurred in 311 (92%) patients. Symptoms such as cough (179 cases, 53.0%), dyspnea (138 cases, 40.8%) and fatigue (135 cases, 39.9%) were also prevalent in those patients. Till Mar 5, 2020, 91 cases were discharged (26.8%), 183 cases (54.0%) stayed in hospital and 65 cases (19.2%) were dead. The median duration of hospital stays for the discharged was 21 days (interquartile range, 15–26). The patients discharged or stayed in hospital till the end date of follow-up were classified into survival group. Compared with the survival subjects, there were fewer women in the dead group and the dead patients were significantly older. Comorbidities including hypertension, cardiovascular disease, cerebrovascular disease, and COPD were more prevalent in the dead group. It should be noted that there were more patients complaining of dyspnea in the dead group (38/65 vs. 100/274, p < 0.001). In addition, fever and headache were less common in patients who died. Considering the vital signs, significant higher respiration rates were found in the dead group, in comparison to the survived.
Table 1.
Demographic data and baseline clinical characteristics.
| Characteristics | Total (n = 339) | Survival (n = 274) | Dead (n = 65) | P value (Dead vs. Survival) |
|---|---|---|---|---|
| Age, median (IQR), y | 69 (65–76) | 68 (64–74) | 76 (70–83) | <0.001 |
| Female gender | 173 (51.0) | 147 (53.6) | 26 (40.0) | 0.048 |
| Comorbidities | ||||
| Hypertension | 138 (40.8) | 106 (38.8) | 32 (50.0) | 0.031 |
| Diabetes | 54 (16.0) | 43 (15.8) | 11 (17.2) | 0.116 |
| Cardiovascular disease | 53 (15.7) | 32 (11.7) | 21 (32.8) | <0.001 |
| Cerebrovascular disease | 21 (6.2) | 11 (4.0) | 10 (15.6) | <0.001 |
| Chronic kidney disease | 13 (3.8) | 9 (3.3) | 4 (6.3) | 0.066 |
| Chronic liver disease | 2 (0.6) | 1 (0.4) | 1 (1.6) | 0.065 |
| COPD | 21 (6.2) | 10 (3.7) | 11 (17.2) | <0.001 |
| Malignancy | 15 (4.4) | 12 (4.4) | 3 (4.7) | 0.12 |
| Autoimmune disease | 5 (1.5) | 4 (1.5) | 1 (1.6) | 0.121 |
| Symptoms | ||||
| Fever | 311 (92.0) | 255 (93.4) | 56 (87.5) | 0.041 |
| Dry cough | 179 (53.0) | 149 (54.6) | 30 (46.9) | 0.068 |
| Expectoration | 93 (27.5) | 76 (27.8) | 17 (26.6) | 0.119 |
| Fatigue | 135 (39.9) | 109 (39.9) | 26 (40.6) | 0.12 |
| Anorexia | 94 (27.8) | 79 (28.9) | 15 (23.4) | 0.083 |
| Myalgia | 16 (4.7) | 15 (5.5) | 1 (1.6) | 0.05 |
| Dyspnea | 138 (40.8) | 100 (36.6) | 38 (59.4) | <0.001 |
| Pharyngalgia | 13 (3.9) | 10 (3.7) | 3 (4.7) | 0.112 |
| Diarrhea | 43 (12.7) | 35 (12.8) | 8 12.5) | 0.121 |
| Nausea | 13 (3.8) | 12 (4.4) | 1 (1.6) | 0.07 |
| Chest tightness | 88 (26.0) | 73 (26.7) | 15 (23.4) | 0.105 |
| Dizziness | 13 (3.8) | 11 (4.0) | 2 (3.1) | 0.114 |
| Headache | 12 (3.5) | 12 (4.4) | 0 (0.0) | 0.029 |
| Vital signs | ||||
| Heart rate, median (IQR), (bpm) | 82 (77–91) | 82 (76–90) | 84 (78–101) | 0.063 |
| SBP, median (IQR), (mmHg) | 130 (119–142) | 130 (120–140) | 130 (114–152) | 0.519 |
| DBP, median (IQR), (mmHg) | 76 (70–83) | 76 (70–82) | 76 (65–90) | 0.938 |
| Respiration rate, median (IQR), (times per min) | 20 (18–21) | 20 (18–20) | 21 (18–30) | <0.001 |
Continuous variables were expressed as median (interquartile range) and categorical variables were expressed as number (percentage). COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; IQR, interquartile range; SBP, systolic blood pressure.
Laboratory findings
Of the included 339 patients, lymphocytopenia was found in 211 cases (63.2%). The counts of CD4+ and CD8+ cells were all decreased compared with normal values. The comparisons of laboratory findings between the survival and dead group were shown in Table 2 . When compared with the survival group, the count of neutrophils was significantly increased, while the counts of lymphocyte, monocyte and platelet were decreased. The counts of CD4+ and CD8+ T cells, which play important role in antiviral immunity,7 , 8 were all significantly decreased in the dead group. The prothrombin time was significantly prolonged, and the concentration of d-dimer was evidently increased in the dead group. The alanine aminotransferase, an important indice for liver function, showed no evident difference between survival and death. At the same time, serum urea and creatinine levels were observed to be higher in the dead group. In addition, the markers for myocardial injury, inflammation, and bacterial infections were all increased significantly in the dead group.
Table 2.
Laboratory findings of patients with different outcomes.
| Laboratory tests | Normal Range | Total (N = 339) | Survival (n = 274) | Dead (n = 65) | P value (Dead vs. Survival) |
|---|---|---|---|---|---|
| Blood Routine | |||||
| White blood cell count, × 109/L | 3.5–9.5 | 5.74 (4.37–8.29) | 5.54 (4.29–7.64) | 8.61 (5.27–13.25) | <0.001 |
| Neutrophil count, × 109/L | 1.8–6.3 | 4.43 (2.76–6.62) | 4.01 (2.63–5.97) | 7.65 (4.35–11.74) | <0.001 |
| Lymphocyte count, × 109/L | 1.1–3.2 | 0.90 (0.59–1.29) | 0.97 (0.68–1.37) | 0.57 (0.39–0.84) | <0.001 |
| Monocyte count, × 109/L | 0.1–0.6 | 0.42 (0.28–0.59) | 0.43 (0.30–0.62) | 0.32 (0.22–0.49) | 0.007 |
| Hemoglobin concentration, g/L | 130–175 | 121 (109–130) | 121 (110–129) | 122 (106–137) | 0.434 |
| Platelet count, × 109/L | 125–350 | 205 (151–259) | 211 (159–268) | 172 (103–219) | <0.001 |
| Blood Coagulation | |||||
| Prothrombin time, s | 9–13 | 12.1 (11.6–12.7) | 12.0 (11.6–12.6) | 12.9 (11.9–14.1) | <0.001 |
| Activated partial thromboplastin time, s | 25–31.3 | 28.5 (26.2–31.3) | 28.3 (25.6–31.2) | 29.1 (27.4–31.8) | 0.072 |
| D-dimer, mg/L | 0–0.55 | 1.20 (0.62–3.25) | 1.08 (0.52–2.05) | 4.38 (1.32–17.01) | <0.001 |
| Blood Biochemistry | |||||
| Alanine aminotransferase, U/L | 9–50 | 27 (17–44) | 28 (17–43) | 24 (19–49) | 0.83 |
| Aspartate aminotransferase, U/L | 15–40 | 32 (23–46) | 29 (22–43) | 43 (30–68) | <0.001 |
| Urea, mmol/L | 3.6–9.5 | 5.5 (4.0–8.0) | 5.1 (3.9–7.0) | 8.9 (6.0–14.2) | <0.001 |
| Creatinine, μmol/L | 57–111 | 61 (50–76) | 60 (49–71) | 80 (55–114) | <0.001 |
| Creatine kinase, U/L | 50–300 | 63 (40–104) | 60 (40–97) | 84 (50–222) | 0.005 |
| Lactate dehydrogenase, U/L | 120–250 | 301 (224–429) | 286 (220–355) | 439 (360–643) | <0.001 |
| Myocardial Injury Markers | |||||
| Creatine kinase-MB, ng/mL | 0–5 | 1.26 (0.85–2.36) | 1.15 (0.81–1.91) | 2.95 (1.30–4.30) | <0.001 |
| Hypersensitive troponin I, ng/mL | 0–0.04 | 0.010 (0.006–0.030) | 0.007 (0.006–0.018) | 0.073 (0.023–0.336) | <0.001 |
| Inflammatory Markers | |||||
| C-reactive protein, mg/L | 0–10 | 49.6 (18.5–93.2) | 44.2 (13.5–82.2) | 102.0 (58.9–187.4) | <0.001 |
| Interleukin-6, pg/mL | <10 | 10.9 (5.2–25.4) | 10.5 (4.9–18.8) | 93.8 (35.9–182.3) | <0.001 |
| Cellular immunity | |||||
| CD4+ cell count, /µl | 404–1612 | 314 (190–484) | 349 (217–516) | 191 (107–282) | <0.001 |
| CD8+ cell count, /µl | 220–1129 | 179 (85–286) | 204 (97–298) | 73 (42–160) | <0.001 |
| Bacterial Infection Marker | |||||
| Procalcitonin, ng/mL | <0.1 | 0.08 (0.04–0.17) | 0.06 (0.04–0.13) | 0.22 (0.11–1.13) | <0.001 |
The variables were expressed as median (interquartile range) and compared using Mann-Whitney U test.
Spectrum of disease and complications
Renmin Hospital of Wuhan University is a designated hospital for severe COVID-2019 patients. As described in Table 3 , in the present study, 70.5% of the enrolled patients are classified as severe or critical. Almost all of the dead patients were critically ill (92.3%), only four severe patients died till the end of follow-up. Only one death was found in moderate cases, which was due to sudden cardiac death.
Table 3.
Spectrum of disease and complications.
| Total (n = 339) | Survival (n = 274) | Dead (n = 65) | P value (Dead vs. Survival) | |
|---|---|---|---|---|
| Spectrum of Disease | ||||
| Moderate | 100 (29.5) | 99 (36.1) | 1 (1.5) | <0.001 |
| Severe | 159 (46.9) | 155 (56.6) | 4 (6.2) | |
| Critical | 80 (23.6) | 20 (7.3) | 60 (92.3) | |
| Complications | ||||
| Bacterial Infection | 143 (42.8) | 94 (34.4) | 49 (81.7) | <0.001 |
| AKI | 27 (8.1) | 11 (4.0) | 17 (28.3) | <0.001 |
| ARDS | 71 (21.0) | 15 (5.5) | 56 (87.5) | <0.001 |
| Liver Enzyme Abnormalities | 96 (28.7) | 74 27.1) | 22 (36.7) | <0.001 |
| Acute cardiac injury | 70 (21.0) | 31 (11.4) | 39 (65.0) | <0.001 |
| Arrhythmia | 35 (10.4) | 22 (8.1) | 13 (20.6) | <0.001 |
| Cardiac insufficiency | 58 (17.4) | 33 (12.1) | 25 (42.4) | <0.001 |
| Shock | 8 (2.4) | 5 (1.8) | 3 (4.7) | 0.049 |
The variables were expressed as number (percentage) and compared with Chi-squared test. AKI, acute kidney injury; ARDS, acute respiratory distress syndrome.
During hospital stay, bacterial infection was found in 143 cases (42.8%), liver enzyme abnormalities were observed in 96 cases (28.7%), and acute respiratory distress syndrome (ARDS) occurred in 71 (21.0%) patients. 70 patients had acute cardiac injuries (21.0%). The incidences of all the complications were significantly higher in the dead group in comparison to the survivors.
The duration of onset-to-admission and hospital stays
The interval time from symptom onset to admission was recorded for each patient (Table 4 ). There was no significant difference between the survival and dead group. Given that the final length of hospital stays for the patients remained in hospital was unknown, the duration for those patients was calculated from admission to the end date of follow-up. The results showed that the length of hospital stay was dramatically shorter for the dead patients. The median duration of hospitalization was only 5 days in the dead group, which was significantly shorter than that in the survival group (5 (3–8) vs. 28 (26–29), P < 0.001).
Table 4.
Duration of disease onset to admission and hospital stays.
| Total (N = 339) | Survival (N = 274) | Dead (N = 65) | P value (Dead vs. Survival) | |
|---|---|---|---|---|
| Symptom onset to admission, median (IQR), days | 10 (7–14) | 10 (7–13) | 10 (7–14) | 0.532 |
| Hospital stay, median (IQR), days | 28 (15–28) | 28 (26–29) | 5 (3–8) | <0.001 |
The variables were expressed as median (IQR) and compared using Mann-Whitney U test. IQR, interquartile range.
Prognostic factors of the elderly COVID-19 patients
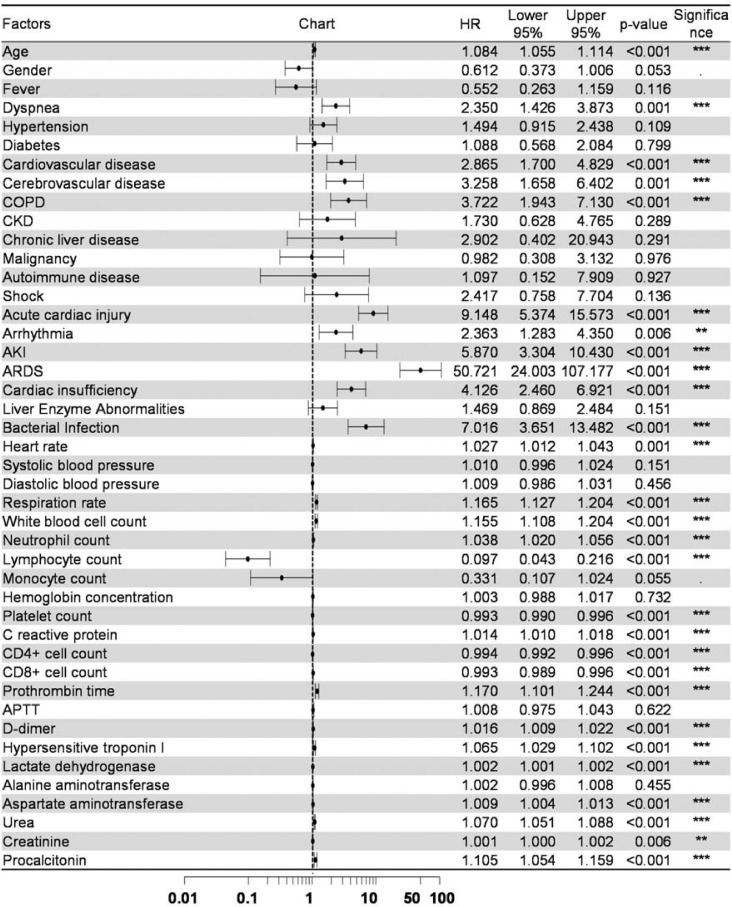
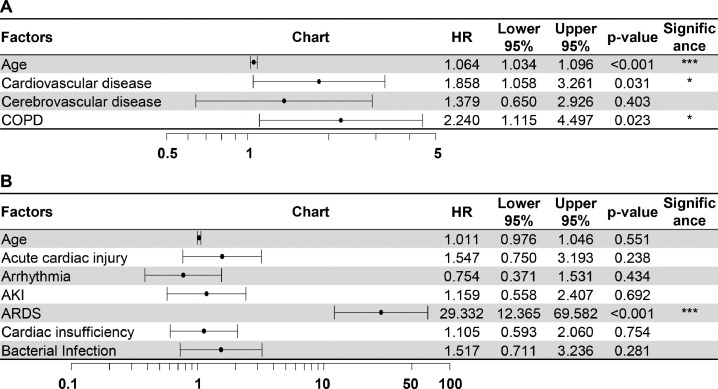
Univariate Cox regression was used to analyze the risk factors for fatal outcomes in the elderly patients with COVID-19 (Fig. 1 ), the hazard ratio (HR) and 95% confidence interval (CI) were shown in the columns right to the chart. Older age was shown to increase the likelihood of death even in elderly patients (HR 1.08, CI 1.06–1.11, P < 0.001). Symptoms of dyspnea (HR 2.35, CI 1.43–3.87, P = 0.001) and comorbidities including cardiovascular disease (HR 2.87, CI 1.70–4.83, P < 0.001), cerebrovascular disease (HR 3.26, CI 1.66–6.40, P = 0.001) and COPD (HR 3.72, CI 1.94–7.13, P < 0.001) were all predictive of fatal outcomes. Complications including acute cardiac injury, arrhythmia, acute kidney injury (AKI), ARDS, cardiac insufficiency and bacterial infection were all predictors of death. The models used in the univariate analysis were tested by Likelihood ratio test, Wald test and Score (log-rank) test and all the three methods supported the significance of the models. Then the “Age” factor was added to correct the models for the multi-factor regressions of comorbidities and complications (Fig. 2 ). In the multi-factor analysis, only cardiovascular disease (HR 1.86, CI 1.06–3.26, P = 0.031), COPD (HR 2.24, CI 1.12–4.50, P = 0.023) and ARDS (HR 29.33, CI 12.37–69.58, P < 0.001) remained to be the predictors for death when other factors in the model kept constant, while the contributions of other factors were no longer significant in the model. Among the vital signs, increased heart rate and respiration rate could increase the likelihood of fatal outcomes. For the laboratory tests, white blood cell count (HR 1.16, CI 1.14–1.20, P < 0.001) and prothrombin time (HR 1.17, CI 1.13–1.24, P < 0.001) were shown to increase the risk of death, and a low level of lymphocytes was a strong predictor of poor outcome (HR 0.10, CI 0.04–0.22, P < 0.001). Significances were also found in other factors such as procalcitonin and hypersensitive troponin I. However, for those factors the HR was around 1.0, which limited their values as predictors.
Fig. 1.
Univariate Cox regression for prognostic factors.
Univariate Cox regression analysis of risk factors associated with fatality. AKI, acute kidney injury; APTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; HR, hazard ratio. ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001.
Fig. 2.
Multivariate Cox regression for prognostic factors.
Multivariate Cox regressions were performed for comorbidities (A) and complications (B), in which the “Age” factor was added to correct the models. AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; HR, hazard ratio. *P < 0.05, ⁎⁎⁎P < 0.001.
Discussion
In the present study, we described the clinical characteristics of elderly COVID-19 patients who were faced with the highest risk of death after SARS-CoV-2 infection. And we investigated the prognostic factors of COVID-19 based on at least 4 weeks of follow-up. SARS-CoV-2 caused a much more severe pneumonia in the aged people than younger patients. Of the 339 elderly COVID-19 patients included, over 70% were severe or critical, and the case fatality rate was 19%. Fast progress of disease was found in the dead with a median survival time of 5 days after admission. Several factors were found to be predictors for poor outcomes, such as symptom of dyspnea, comorbidities like cardiovascular disease and COPD, and complications like ARDS. It should be noted that once ARDS occurred, the probability of death would increase dramatically. On the other side, increased lymphocyte count was predictive of better outcomes.
The distinct features of COVID-19 in these included elderly patients were evidently increased severity and fatality. The reported proportion of severe-to-critical patients in the whole population was 18.5% and the case-fatality rate was 2.3%,9 significantly lower than the present study. As Renmin Hospital of Wuhan University was a designated hospital for severe COVID-19 patients, the proportion of severe and critical patients and fatality rate might be different from other centers. In the 65 dead cases 92.3% were critically ill. The only one death in moderate cases was caused by sudden cardiac death. As indicated by the short length of stay (median, 5 days) for the dead, the disease progressed considerably fast for those patients. The most common symptoms at admission were fever, cough, dyspnea and fatigue, which was consistent with the general symptoms of viral infection and pneumonia. Dyspnea was more prevalent in the dead patients. In consistence, the respiration rate at admission was significantly higher in the dead group compared with survival group. The incidence of comorbidities was relatively higher in the elderly COVID-19 patients compared with the whole population.9 The most common comorbidities were hypertension, diabetes, cardiovascular disease and cerebrovascular disease. The median heart rate, blood pressure and respiration rate were in the normal range at admission both in survival and dead group, indicating that the vital signs of most patients were stable at admission. Common complications during hospitalization were bacterial infection, liver enzyme abnormalities, ARDS and acute cardiac injury.
Significant differences were found in laboratory findings between the dead and survivors. Generally, evident decrease of lymphocytes was observed in the dead group. Specifically, the count of CD4+ and CD8+ lymphocytes were both significantly reduced in the dead group, indicating the suppressed cellular immunity in these patients. At the same time, more severe inflammatory reactions might exist in the dead cases as represented by significant increase of inflammatory markers. In addition, more frequent coagulation disorders, myocardial injuries and bacterial infections were found in the dead group compared to the survival group, as revealed by the related markers. Compared with the survivors, serum concentrations of urea and creatinine were higher in the dead, indicating worse renal functions, although the median values were still within the normal range.
As found by the cox regression, there were several prognostic factors in the elderly COVID-19 patients. Dyspnea and high respiration rates were probably manifestations for impaired respiratory function and might reflect the severity of lung lesions caused by infection or inflammation. The impaired oxygenation caused by COVID-19 brings great challenge to the cardiopulmonary function, especially for the elderly. Comorbidities such as cardiovascular disease and COPD could greatly increase the vulnerability of elderly patients when faced with this disease. It is reasonable that these factors could predict poor outcomes. ARDS, the fundamental pathophysiology of severe viral pneumonia, could be a marker of rapid progress of the disease. Once ARDS occurred, the 28-day mortality would be near 50%.10 Consistent with that, in the present study, the occurrence of ARDS was an extremely strong predictor of fatal outcomes, which could increase the probability of death by a factor of 29.3. In laboratory tests, lymphocytopenia were found in around 60% of the overall patients and in 81.5% of the dead patients, which was similar with previous studies on SARS.11 It has been reported that lymphocytes could be killed by coronavirus due to the damage of the cytoplasmic components or apoptosis.12 , 13 The degree of lymphocytopenia might reveal either the severity of virus invasion or the condition of the antiviral immunity, thus could predict the outcome.
According to the above, patients’ conditions on admission including dyspnea, comorbidities of cardiovascular disease and COPD, lymphocytopenia, and ARDS during hospitalization, could predict the risk of death. These factors should be considered for risk stratification. We have found that COVID-19 progressed rapidly for some severe or critical patients. Therefore, for the elderly patients the risk factors should be taken into consideration. For those at high risk, close monitoring and timely treatment might be very important and could help to improve the outcome.
Limitations
This study has several limitations. As Renmin Hospital of Wuhan University was a designated hospital for severe COVID-19 patients, the proportion of severe and critical patients and fatality rate might be different from the whole infected population. In addition, as a retrospective study on a severe disease, some relevant data such as interleukin-6 were incomplete and could not be included in the risk factor analysis.
Conclusions
High proportion of severe to critical cases and high fatality rate were observed in the elderly patients with COVID-19. Rapid progress of disease was noted in the dead patients with a median survival time of 5 days after admission. Patients’ conditions on admission such as dyspnea, lymphocytopenia, cardiovascular disease and COPD, and the occurrence of ARDS during hospitalization were predictive of fatal outcome. Close monitoring and timely treatment should be performed for the elderly patients at high risk.
Declaration of Competing Interest
The authors declare that there is no conflict of interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China Grant numbers 81570450, 81900455.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.6th Ed. National Health Commision of the People's Republic of China; 2020. Interim guidance for novel coronavirus pneumonia.http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml Accessed February 27th, 2020. [Google Scholar]
- 4.Report of the WHO-China Joint Mission on Coronavirus Disease . 2019. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report Accessed Mar 5th, 2020. [Google Scholar]
- 5.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson N.D., Fan E., Camporota L. The berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 7.Jansen J.M., Gerlach T., Elbahesh H., Rimmelzwaan G.F., Saletti G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J Clin Virol. 2019;119:44–52. doi: 10.1016/j.jcv.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Whitmire J.K., Ahmed R. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) t cell responses. Curr==70== Opin Immunol. 2000;12(4):448–455. doi: 10.1016/s0952-7915(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 9.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Bellani G., Laffey J.G., Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 11.Zumla A., Hui D.S., Perlman S. Middle east respiratory syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of Sars. J Exp Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu H., Zhou J., Wong B.H. Middle east respiratory syndrome coronavirus efficiently infects human primary t lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213(6):904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]