Highlights
-
•
Ground glass opacities in subpleural regions are the main CT features of COVID-19.
-
•
Pulmonary consolidation mostly occurs in critical patient.
-
•
Imaging features vary according to disease severity.
-
•
CT imaging can evaluate the severity and treatment outcome.
Keywords: Coronavirus disease-2019, Pneumonia, Computer tomography, X-ray, Diagnosis
Abstract
Purpose
To report CT features of coronavirus disease-2019 (COVID-19) in patients with various disease severity.
Methods
The CT manifestations and clinical data of 73 patients with COVID-19 were retrospectively collected in 6 hospitals from Jan 21 to Feb 3, 2020. We analyzed the initial and follow-up CT features of patients with disease severity, according to the Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia.
Results
Six patients (8%) were diagnosed as mild type pneumonia; these patients had no obvious abnormal CT findings or manifested mild changes of lung infection. All 43 patients (59 %) with common type presented unique or multiple ground-glass opacities (GGO) in the periphery of the lungs, with or without interlobular septal thickening. In the 21 patients (29 %) with severe type, extensive GGO and pulmonary consolidation were found in 16 cases (16/21, 76 %) and 5 cases (24 %), respectively. An extensive "white lung", with atelectasis and pleural effusion were found in critical type patients (3, 4%). On the resolutive phase of the disease, CT abnormalities showed complete resolution, or demonstrated residual linear opacities.
Conclusions
Different CT features are seen according to disease severity, which can help COVID-19 stratification.
1. Introduction
Coronavirus Disease-2019 (COVID-19) is an acute infectious disease mainly involving the respiratory system, which was recently found in humans [1]. The first patient was found in Wuhan, Hubei Province, China on December 12, 2019. The symptoms of patients were fever, fatigue, dry-cough and the patients gradually developed severe dyspnea. The majority of them had a good prognosis [2,3], while a mortality rate of 2.1 % has been recently reported [4]. The average incubation period of the disease was found to be 6.4 days [5]. At present, the diagnosis relies on reverse transcription-polymerase chain reaction (RT-PCR) or gene sequencing of sputum, throat swab or lower respiratory tract secretion [6]. However, these methods are time-consuming and do not allow assessing the disease severity. Chest CT scanning can provide rapid screening and assess the severity. In this study, we report the CT characteristics of patients with COVID-19 of various severity to provide a more comprehensive overview of the disease, in order to help the clinical diagnosis and management.
2. Materials and methods
2.1. Study population
This retrospective study was approved by our Institutional Ethics Committee. The data of patients were collected from 6 hospitals in Anhui province, China from Jan 21 to Feb 3, 2020. The electronic medical records were reviewed and analyzed. Seventy-three patients with proven COVID-19 were enrolled in this study, including 41 males and 32 females; aged 5–86 years, with mean age of (41.6 ± 14.5) years. The diagnosis of COVID-19 was made in accordance with the Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia (fifth edition) formulated by the National Health Commission of the People's Republic of China [7]. The CT images and clinical data of all patients were collected. Among the 73 patients, 54 (74 %) had the travel history to or from Wuhan, 16 (22 %) had a history of close contact with the local COVID-19 patients before the illness onset, and the remaining 3 patients denied any contact or travel history. All patients were administered with anti-viral and supportive treatment, and prevention of complications based on their clinical condition.
2.2. CT scanning
A high-resolution CT scan was performed in all patients with 64-slice multi-detector row CT scanners (Toshiba Aquilion-64, Philips Brilliance-64, GE LightSpeed-64, Siemens Sensation-64, and Neusoft Viz-64). Patients were scanned in the supine position, during breath hold, from the lung apices down to the costophrenic angles. The acquisition parameters were as follows: tube voltage 100−120 kV, tube current 110−280 mA, or intelligent milliampere second (50–300 mA s), pitch 1.375, FOV 350−400 mm. The 1.25 mm or 2.5 mm thick images were reconstructed using a high-frequency reconstruction algorithm, and lung windowing and stored in the PACS system.
2.3. CT images analysis
All images were independently read by 3 senior radiological specialists. The location, shape, number and size of the abnormalities on chest CT were carefully observed and recorded. In case of discordant reading, consensus was reached during another reading session.
2.4. Clinical typing
The severity of the disease was classified into 4 categories according to the Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia (fifth edition) [7]: ① mild type: patients with mild clinical symptoms and no pulmonary changes on CT imaging; ② common type: patients with symptoms of fever and signs of respiratory infection, and having pneumonia changes on CT imaging; ③ severe type: patients presenting with any one item of the following: a. respiratory distress, respiratory rate ≥ 30/min; b. oxygen saturation of finger ≤ 93 % in resting condition; c. arterial partial pressure of oxygen (PaO2) /oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa); ④ critical type: patients meeting any one of the following criteria: a. respiratory failure requiring mechanical ventilation; b. shock; c. requiring ICU admission requirement due to multiple organ failure.
2.5. Statistical analysis
SPSS statistical software (version 22.0; SPSS Inc., Chicago, Illinois, United States) was used for analysis. The prevalence of imaging findings was estimated as the percentage of patients showing each abnormality. The measured data were expressed as mean ± standard deviation (± s), and one-way analysis of variance was used. P value < 0.05 was considered for statistical significance.
3. Results
3.1. Clinical features
The delay from contact with infected individual(s) to onset of disease was within 3 days in 28 patients (38 %), 3–7 days in 42 cases (58 %) and more than 7 days in 3 cases (4%). According to severity classification, patients with mild, common, severe and critical type represented 6 (8%), 43 (59 %), 21 (29 %) and 3 (4%) cases, respectively. The main clinical manifestations were fever in 68 cases (93 %), cough in 60 cases (82 %), fatigue in 55 cases (75 %), sputum production in 39 cases (53 %), anorexia in 20 cases (27 %), whereas 3 patients (4%) were symptom-free. The baseline characteristics of patients were listed in Table 1 .
Table 1.
Demographics and baseline characteristics of patients infected with coronavirus disease-2019 [n (%)].
| mild type (n = 6) |
common type (n = 43) |
severe type (n = 21) |
critical type (n = 3) |
|
|---|---|---|---|---|
| Gender | ||||
| Men | 3 (50 %) | 28 (65 %) | 10 (48 %) | 0 |
| Women | 3 (50 %) | 15 (35 %) | 11 (52 %) | 3 (100 %) |
| Age (years) | 29.2 ± 10.9 | 33.4 ± 12.2 | 44.2 ± 12.0 | 63.0 ± 21.2 |
| Exposure History | ||||
| Recent travel to or from Wuhan | 4 (67 %) | 29 (67 %) | 18 (85 %) | 3 (100 %) |
| Exposure to Infected Patients | 2 (33 %) | 12 (28 %) | 2 (10 %) | 0 |
| Unknown Exposure | 0 | 2 (5%) | 1 (5%) | 0 |
| Symptoms | ||||
| Fever | 5 (83 %) | 40 (93 %) | 20 (95 %) | 3 (100 %) |
| Cough | 4 (67 %) | 36 (84 %) | 18 (86 %) | 2 (67 %) |
| Fatigue | 4 (67 %) | 31 (72 %) | 17 (81 %) | 3 (100 %) |
| Sputum Production | 1 (17 %) | 22 (51 %) | 14 (67 %) | 2 (67 %) |
| Poor Appetite | 0 | 8 (19 %) | 9 (43 %) | 3 (100 %) |
| Days from Symptoms Onset to CT Scan | ||||
| Within 1 day | 2 (33 %) | 3 (7%) | 0 | 0 |
| 1 to 3 days | 3 (50 %) | 13 (30 %) | 4 (19 %) | 0 |
| >3 days | 1 (17 %) | 27 (63 %) | 17 (81 %) | 3 (100 %) |
| Days from PCR Testing to CT Scan | ||||
| Within 1 Day before PCR | 3 (50 %) | 28 (65 %) | 15 (71 %) | 3 (100 %) |
| ≤2 days after PCR | 2 (33 %) | 9 (21 %) | 4 (19 %) | 0 |
| >2 days after PCR | 1 (17 %) | 6 (14 %) | 2 (10 %) | 0 |
| Days of Follow-up Scans | 5.8 ± 1.2 | 4.9 ± 1.4 | 4.5 ± 1.0 | 3.7 ± 1.1 |
Note- PCR: polymerase chain reaction.
3.2. CT manifestations
3.2.1. CT manifestations by severity of disease
⑴Of the six patients with mild type pneumonia, three patients had no obvious abnormal changes in both lungs, and three cases were found the enlargement of lung hilus and thickening of lung texture (Fig. 1 A). ⑵ All 43 common type patients showed unique or multiple ground-glass opacities (GGO) in the periphery of both lungs (Fig. 1B). Twelve cases showed GGO as the unique manifestation (28 %, 12/43), 15 cases (35 %, 15/43) accompanied with paving stone sign, with(27 %, 4/15)or without (73 %, 11/15) inter and intralobular septal thickening; some of them (7%, 3/43) presenting air-bronchogram. The opacities had a fan-shaped distribution in 3 cases (7%, 3/43), mainly seen at the dorsal field of the lungs. ⑶ In the 21 patients with severe type, extensive GGO and pulmonary consolidation were found in 16 (76 %, 16/21) and 5 cases (24 %), respectively (Fig. 1C). The GGO were irregular, fan-shaped distribution, with ill-defined borders. Some patients presented peribronchial thickening (14/21, 67 %). Air bronchogram was a rare finding. ⑷ CT manifestations of critical type included confluent lesions and involved multiple lobes, pulmonary fibrosis and "white lung" formation (Fig. 1D). Atelectasis and pleural effusion were found in 1 and 3 patients, respectively. The location and morphology of pulmonary lesions in different types were summarized in Table 2 .
Fig. 1.
CT features of coronavirus disease-2019 by disease severity. A. An axial CT image in a 33-year-female mild type patient (2 days from symptom onset to CT scan) shows thickening of lung texture. B. An axial CT image in a 37-year-male common type patient (6 days from symptom onset to CT scan) shows multiple ground-glass opacities in both lungs. C. An axial CT image in a 56-year-female severe type patient shows extensive ground-glass opacities and pulmonary consolidation, enlargement of bronchi and vessels. D. An axial CT image in a 47-year-female critical type patient (9 days from symptom onset to CT scan) shows extensive ground-glass opacities in multiple lobes, formatting "white lung".
Table 2.
The location and morphology of pulmonary lesions in different types in Coronavirus Disease-19 patients [n (%)].
| mild type (n = 6) |
common type (n = 43) |
severe type (n = 21) |
critical type (n = 3) |
|
|---|---|---|---|---|
| Unilateral Lung Involvement | 0 | 15 (35 %) | 0 | 0 |
| Bilateral Lungs Involvement | 3 (50 %) | 28 (65 %) | 21(100 %) | 3 (100 %) |
| Unique Ground-glass Opacities | 0 | 12 (28 %) | 0 | 3 (100 %) |
| Multiple Ground-glass Opacities | 0 | 31 (72 %) | 16 (76 %) | 3 (100 %) |
| Paving Stone Sign | 0 | 15 (35 %) | 10 (48 %) | 3 (100 %) |
| Consolidation | 0 | 0 | 5 (24 %) | 3 (100 %) |
| Bronchial Wall Thickening | 0 | 2 (5%) | 14 (67 %) | 3 (100 %) |
| Pleural Effusion | 0 | 0 | 0 | 3 (100 %) |
| Thickening of Lung Texture | 3 (50 %) | 40 (93 %) | 19 (90 %) | 3 (100 %) |
| No Lung Abnormality | 3 (50 %) | 0 | 0 | 0 |
3.2.2. CT manifestations of disease improvement
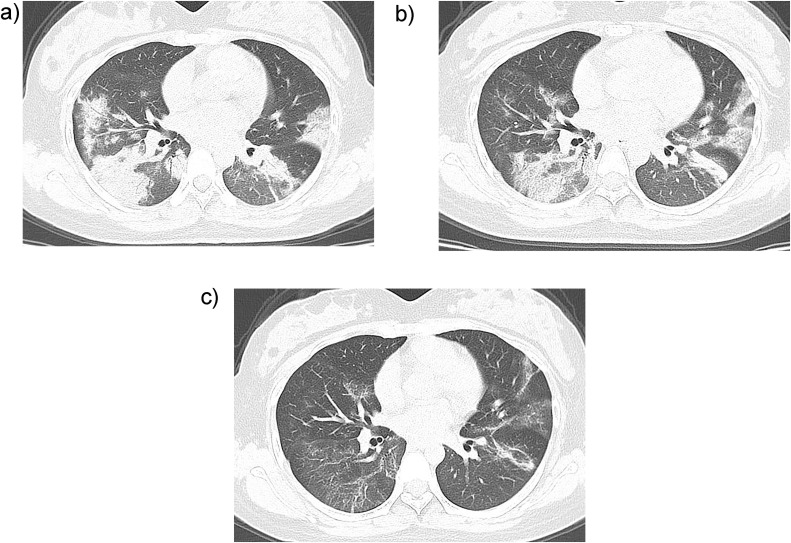
During follow-up, twelve patients (16 %) dramatically improved. A new CT showed that lesions had decreased in size by more than half in 8 cases (67 %, 8/12) after the patients had received antiviral and supportive treatment after one week of hospital admission. Among them, 3 patients showed remarkable absorption (Fig. 2 ). Four other patients showed residual interstitial abnormalities with persisting septal lines.
Fig. 2.
CT features of the disease amelioration in patient with coronavirus disease-2019. A 27-year-old female patient has fever for 10 days and pharyngalgia for 3 days, with the medical history of contacting with the infected patient. The detection of new coronavirus nucleic acid was positive. A. On admission day, CT scan shows multiple ground-glass opacities and pulmonary consolidation in both lungs. B. Three days after admission, CT scan shows that the density of the foci is lighter and the size is smaller than that at admission day. C. Seven days after treatment, re-examination of CT shows that the lesions in both lungs are further absorbed.
4. Discussion
Currently, RT-PCR of sputum, throat swab and lower respiratory tract secretion or sequencing of virus gene represents the gold standard technique for the diagnosis of COVID-19 [8,9]. However, the testing requires at least several hours, and has a false negative rate of more than 5%; the latter is even more time-consuming. CT imaging can demonstrate typical features making the diagnosis of COVID-19 quite likely, which can help to rapidly screen patients, and to stratify the patients' severity to quickly develop effective treatment strategies.
In the present study, the patients with mild type pneumonia had no obvious changes on CT images. Ground-glass opacities were the most common manifestation, in either the common or severe type patients. Most of the lesions were distributed along the bronchovascular bundle or the dorsolateral and subpleural part of the lungs and were seen with or without interlobular septal thickening. These changes might reflect fluid exudation in the alveolar lumen, secondary to dilation and congestion of alveolar septal capillary, and interstitial edema in the interlobular septa.
Pulmonary consolidation is mainly found in severe and critical types patients, which can coexist with ground glass and fibrotic changes. The pathological bases of these changes are not clear currently. We speculate that the changes of pulmonary interstitium may be due to inflammatory cell infiltration, edema, and interstitial thickening, whereas pulmonary parenchyma changes could reflect alveolar hemorrhage, edema, cell exudation and hyaline membrane formation. Of course, these hypotheses have not been confirmed by pathological examination.
Atelectasis and pleural effusion are rare findings on CT, and were only seen in 1 and 3 cases, respectively in the present study. All of them are found in critical stages patients, suggesting that patients may have a poorer prognosis when these signs occur.
Until the end of observation, 12 patients had recovered from this disease, the ground-glass opacities and consolidation resolved in most of them. The interlobular septum and bronchial wall thickening, band opacities and scattered patchy consolidation may remain in a minority of patients. These changes resemble the features of common viral pneumonia.
The CT features of COVID-19 need to be differentiated from those due to adenovirus pneumonia, influenza A (H1N1), and severe acute respiratory syndrome (SARS). Adenovirus pneumonia mostly occurs in children and mainly involves the middle and inner part of both lungs. The lung hila are widened. Pleural effusion, pneumothorax, mediastinal emphysema and subcutaneous emphysema occur frequently [10]. The CT manifestations of H1N1 pneumonia also combine ground glass opacities and consolidations, with a peribronchovascular predominance [11]. The lung manifestations of SARS are characterized by large pulmonary consolidation, often with obvious air bronchogram [12].
Bernheim et al. [13] review the CT findings of 121 symptomatic patients infected with COVID-19 in relationship to the time between symptom onset and the initial CT scan and find that the early patients (0–2 days) have far fewer frequency of GGO and consolidation and lower severity score of pneumonia as compared with the intermediate (3–5 days) and late (6–12 days) patients. The authors’ study is of significance for recognizing imaging patterns based on infection time course. However, in many patients, the disease severity is often not consistent with the course of disease. Our study analyses the chest CT characteristics of COVID-19 based on the staging of disease severity, which likely more accurately reflects the relationship between CT features and disease severity compared with the previous study.
This study has some limitations. Firstly, this is a retrospective study, the time of CT examination of patients was uneven, which bias CT features description. Secondly, no pathological study was performed in the present study, which makes it impossible to evaluate the relationship between CT features and pathological changes. Finally, it is not possible to exclude the possibility of superinfection in some of the patients.
In conclusion, CT imaging can play an important role in the early diagnosis and disease stratification of COVID-19.Patchy ground-glass opacities and large consolidation located in the peripheral part of both lungs are the typical CT manifestations. The size and type of CT abnormalities are related to disease severity.
CRediT authorship contribution statement
Kai-Cai Liu: Data curation, Writing - original draft. Ping Xu: Data curation, Conceptualization, Methodology. Wei-Fu Lv: Conceptualization, Methodology, Writing - review & editing, Supervision. Xiao-Hui Qiu: Data curation, Investigation. Jin-Long Yao: Data curation, Investigation. Jin-Feng Gu: Data curation, Investigation. Wei Wei: Methodology, Supervision.
Declaration of Competing Interest
The authors report no conflicts of interest.
Acknowledgement
This study is supported by the Fundamental Research Funds for the Central Universities of China (WK9110000061).
Contributor Information
Ping Xu, Email: xuping1027@163.com.
Wei-Fu Lv, Email: weifulv@ustc.edu.cn.
References
- 1.WHO . 2020. Novel Coronavirus – China.http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ Jan 12. (Accessed Jan 19, 2020) [Google Scholar]
- 2.Huang C.L., Wang Y.M., Li X.W., Ren L.L., Zhao J.P., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N.S., Zhou M., Dong X., Qu J.M., Gong F.Y., Han Y., Qiu Y., Wang J.L., Liu Y., Wei Y., Xia J.A., Yu T., Zhang X.X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel A., Jernigan D.B., 2019-nCoV CDC Response Team Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak - United States, December 31, 2019-February 4, 2020. Am. J. Transplant. 2020;20(3):889–895. doi: 10.1111/ajt.15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backer J.A., Klinkenberg D., Wallinga J. The incubation period of 2019-nCoV infections among travelers from Wuhan, China. Euro Surveill. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G., Fan Y.H., Lai Y.N., Han T.T., Li Z.H., Zhou P.W., Pan P., Wang W.B., Hu D.W., Liu X.H., Zhang Q.W., Wu J.G. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Health Commission of the people’s Republic of China . fifth edition. 2020. The Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia.www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml [Google Scholar]
- 8.Zhang N.R., Wang L.L., Deng X.Q., Liang R.Y., Su M., He C., Hu L.F., Su Y.D., Ren J., Yu F., Du L.Y., Jiang S.B. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020;92(4):408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu R.J., Zhao X., Li J., Niu P.H., Yang B., Wu H.L., Wang W.L., Song H., Huang B.Y., Zhu N., Bi Y.H., Ma X.J., Zhan F.X., Wang L., Hu T., Zhou H., Hu Z.H., Zhou W.M., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J.Y., Xie Z.H., Ma J.M., Liu William J., Wang D.J., Xu W.B., Homes Edward C., Gao George F., Wu G.Z., Chen W.J., Shi W.F., Tan W.J. Genomic characterisation and epidemiology of 2019 novel coronavirus implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan D.Y., Zhu H.D., Fu Y.Y., Tong F., Yao D.Q., Walline J., Xu J., Yu X.Z. Severe community-acquired pneumonia caused by human adenovirus in immunocompetent adults: a multicenter case series. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Zhang J.F., Xia X.D., Su D.J., Liu B.L., Zhao D.L., Liu Y., Zhao D.H. Serial evaluation of high-resolution CT findings in patients with pneumonia in novel swine-origin influenza A (H1N1) virus infection. Br. J. Radiol. 2014;85(1014):729–735. doi: 10.1259/bjr/85580974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J.T., Sheng W.H., Fang C.T., Chen Y.C., Wang J.L., Yu C.J., Chang S.C., Yang P.C. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg. Infect. Dis. 2004;10(5):818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K., Li S., Shan H., Jacobi A., Chung M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;(February):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]