Abstract
Background
Detecting pre-clinical bladder cancer (BC) using urinary biomarkers may provide a valuable opportunity for screening and management. Telomerase reverse transcriptase (TERT) promoter mutations detectable in urine have emerged as promising BC biomarkers.
Methods
We performed a nested case-control study within the population-based prospective Golestan Cohort Study (50,045 participants, followed up to 14 years) and assessed TERT promoter mutations in baseline urine samples from 38 asymptomatic individuals who subsequently developed primary BC and 152 matched controls using a Next-Generation Sequencing-based single-plex assay (UroMuTERT) and droplet digital PCR assays.
Findings
Results were obtained for 30 cases and 101 controls. TERT promoter mutations were detected in 14 pre-clinical cases (sensitivity 46·67%) and none of the controls (specificity 100·00%). At an estimated BC cumulative incidence of 0·09% in the cohort, the positive and negative predictive values were 100·00% and 99·95% respectively. The mutant allelic fractions decreased with the time interval from urine collection until BC diagnosis (p = 0·033) but the mutations were detectable up to 10 years prior to clinical diagnosis.
Interpretation
Our results provide the first evidence from a population-based prospective cohort study of the potential of urinary TERT promoter mutations as promising non-invasive biomarkers for early detection of BC. Further studies should validate this finding and assess their clinical utility in other longitudinal cohorts.
Funding
French Cancer League, World Cancer Research Fund International, Cancer Research UK, Tehran University of Medical Sciences, the International Agency for Research on Cancer, and the U.S. National Cancer Institute.
Keywords: Early detection, TERT promoter mutations, Urinary biomarker, Bladder cancer, Prospective cohort
Research in context.
Evidence before this study
The best hope for reducing bladder cancer (BC) mortality and morbidity remains early detection and subsequent surgical excision of non-muscle invasive bladder tumours. Despite the routine use of urine cytology and commercialization of several FDA-approved urine biomarkers for bladder cancer management, these methods do not offer the combined sensitivity and specificity needed to be clinically useful for bladder cancer detection, especially for early stage tumours. Telomerase reverse transcriptase (TERT) promoter mutations are the most frequent genetic alterations occurring in bladder cancer. They are equally distributed across different grades and stages of the disease and are reported to be early events in carcinogenesis. We and others have previously shown that TERT promoter mutations are detectable in urinary DNA from the exfoliated cells of bladder epithelium or in urinary cell-free DNA. We specifically have shown that TERT promoter mutations detected in urinary DNA by our simple, single-plex assay (UroMuTERT) have excellent sensitivity and specificity for the detection of all forms of urothelial cancer of the bladder, significantly outperforming that of urine cytology, especially for the detection of low-grade and/or early stage urothelial cancers.
The ability to detect these mutations in pre-diagnostic urine samples has enormous potential as a non-invasive tool for early detection and potentially cost-effective screening of high-risk individuals. We searched PubMed with terms “TERT promoter mutations”, “urine”, “blood”, “liquid biopsy”, “bladder cancer”, “urothelial cancer” “screening of bladder/urothelial cancer” and “urinary biomarker” for studies published between January 2006 and December 2018. No study has shown the detectability of a biomarker years prior diagnosis of bladder cancer in asymptomatic individuals.
Added value of this study
Building on our promising earlier studies of the non-invasive UroMuTERT assay, we investigated the potential of urinary TERT promoter mutations as an early detection biomarker for bladder cancer in asymptomatic individuals in a case-control study nested within a longitudinal population-based prospective cohort of 50,045 Iranian individuals. This is the first study showing that tumour-derived TERT promoter mutations could be detected in urine samples up to 10 years prior to the primary diagnosis of bladder cancer and were absent among matched controls who did not develop any cancer in the 10 years after sample collection. This pilot study also provides the first evaluation of a urinary biomarker in a population-based prospective cohort study and the first evidence of the ability of urinary TERT promoter mutations to detect pre-clinical bladder cancer.
Implications of all the available evidence
Our prospective pilot study demonstrates the potential of urinary TERT promoter mutations as early biomarkers for bladder cancer and brings them a step further in the validation phases of biomarker development for early detection. Both the high specificity of these biomarkers and their detection up to 10 years before clinical diagnosis of bladder cancer suggest great potential for clinical utility for early detection of pre-clinical tumours and possibly for surveillance of patients for disease recurrence. If the current findings are validated in other long-term population-based prospective cohorts, large prospective randomized controlled trials in high-risk cohorts should be designed to address the health and cost benefits of TERT promoter mutations screening on the global bladder cancer burden. As part of the integration of these biomarkers into BC screening and clinical management, test-positive individuals could be offered investigation with cystoscopy or urography for early clinical diagnosis and then triaged to treatment or a surveillance program, as appropriate.
Alt-text: Unlabelled box
1. Introduction
In 2018, approximately 549,000 individuals were diagnosed with bladder cancer (BC) across the world, and 200,000 died from this disease. In the USA as well as in Iran, BC is the 4th most common cancer diagnosed in men [1]. Histologically, BCs can be classified into urothelial carcinoma (UC), which is the most common sub-type (around 90%) and other rare non-urothelial tumours [2]. The absence of appropriate and reliable screening methods, the invasiveness of diagnostic modalities, and high recurrence rates (50–70%) after the initial treatments make BC one of the most challenging and expensive cancers to diagnose and treat [2,3]. Therefore, identifying accurate non-invasive biomarkers that can facilitate early detection and improve post-treatment monitoring in BC patients might significantly contribute to reducing the mortality, morbidity, and economic burdens of BC worldwide [4,5]. Owing to suboptimal performance and cost-effectiveness considerations, none of the commercially available urine biomarkers to date are recommended by urological societies for routine BC clinical management or for screening high-risk populations [6], [7], [8].
Two hotspot mutations in the promoter region of the Telomerase reverse transcriptase gene (TERT), called C228T and C250T, are frequently found in several tumour types [9], and they are detected at high frequency (60–85%) in all stages and grades of UCs [10], [11], [12], [13]. These mutations are considered as an early event in UC tumorigenesis and have been detected in the DNA from urine samples that were collected both at the time of primary clinical diagnosis of UC and during post-surgical follow-ups [10,11,[14], [15], [16]]. Recently, we developed a Next Generation Sequencing (NGS)-based assay (UroMuTERT) for the detection of low-abundance TERT promoter mutations. We tested it in urine samples from a case-control study and reached 87·1% sensitivity and 94·7% specificity for detecting primary or recurrent UCs [17]. When restricted to the detection of primary or early UC, our UroMuTERT assay demonstrated comparable performance to that of the recently developed more complex UroSEEK multiple gene assay that includes the screening of TERT promoter mutations and regions of interest in ten other somatically mutated genes (sensitivity of 86·7% versus 83%; Specificity of 94·7% versus 93%) [14].
Should these mutations also be detectable in urine at early or pre-clinical stages of tumour development, they would provide an unprecedented opportunity for developing a simple, non-invasive assay for the detection of UCs at any stages and grades, which therefore could be used for comprehensive screening and early detection purposes. At this step of the biomarker validation, expert groups recommend nested case-control study design within prospective cohorts in which samples collected at enrollment within the targeted population will be tested for the biomarker(s) in asymptomatic individuals who later developed cancer and those who did not [18,19].
In this pilot study, we report the detectability of urinary TERT-promoter mutations up to 10 years before BC diagnosis using urine samples of the Golestan Cohort Study [20] and evaluate their predictive values as non-invasive early biomarkers.
2. Materials and methods
2.1. Study design
We designed a case-control study nested within the Golestan Cohort Study, a population-based prospective cohort study of 50,045 individuals, aged 40–75 years, recruited from both urban and rural areas in the Golestan province of northeast Iran between 2004 and 2008 [20]. Informed consent was obtained from all participants. The study protocol and the informed consent used for this study were approved by the ethical review committees of Digestive Disease Research Center (DDRC), International Agency for Research on Cancer (IARC) and the National Cancer Institute (NCI). Upon enrollment, detailed questionnaires on participants’ demographics, lifestyle, diet, and various exposures were completed, and the participants were actively followed annually for vital status, incident cancer and cause of death. Blood, urine, hair and nail samples were collected from all participants at enrollment and stored for later studies. Demographics of all the cohort participants are summarized in the Supplementary Table 1.
Until 1st December 2018, the average duration of follow-up was 10·2 years and only <1% of the cohort participants had been lost to follow-up.
2.2. Cancer case ascertainment
When incident cancers or deaths were reported on the annual follow-up, a staff member was sent to the home of the patient or the deceased to complete a detailed questionnaire. This process was followed by sending a team to the appropriate medical centers to gather copies of all available and relevant medical reports. All information was then reviewed by 2-3 expert physicians to verify the diagnosis of cancer or cause of death. Further, cancer cases were blindly matched to the Golestan Population-based Cancer Registry database to avoid any possible misclassifications of cancer cases.
Forty participants developed primary urothelial carcinoma of the bladder, of whom 38 had provided a urine sample at enrollment. The cases were sub-categorized into muscle-invasive and non-muscle-invasive BCs, based on their documented stage of disease. For each of the 38 BC cases, we selected four incidence density matched controls (total n = 152). The controls were matched for date of birth (same calendar year), sex, date of urine collection (same half calendar year), and residence (urban/rural). In addition, the controls were selected to be free from any history of cancer at the last follow-up date.
2.3. Collection, processing and storage of blood and urine samples
The details of the collection and storage of biospecimens have been previously published [20]. Briefly, in the urban areas, all biological samples were immediately processed in the central cohort laboratory, while in the rural areas, blood and urine samples were first kept in refrigerators (+4 °C) and then transferred in cooling boxes to the central laboratory to be processed within 8h of collection. The blood samples were centrifuged and blood fractions (plasma, buffy coat, and red blood cells) were aliquoted in 500 µl straws and stored at −80 °C, while urine samples were stored at −20 °C. The samples were then shipped at regular intervals to the International Agency for Research on Cancer (IARC) and the National Cancer Institute (NCI) where they were stored at −20°C (blood) and at −80°C (urine).
2.4. DNA isolation and quantification
Because of the preciousness of samples collected in prospective cohorts, a maximum of 4·5 ml of frozen urine per subject was granted for use in the current study. DNA from the whole urine samples (median volume 2·9 mL; range 1·1–4·5 mL) was isolated using the QIAamp Circulating Nucleic Acid Kit (Qiagen) with the objective of collecting cell-free DNA and DNA from exfoliated cells within the same fraction. DNA from blood leukocytes was isolated using the Gentra Puregene blood kit (Qiagen). DNA quantification was performed using the Qubit assay (Invitrogen).
2.5. Detection of TERT promoter mutations
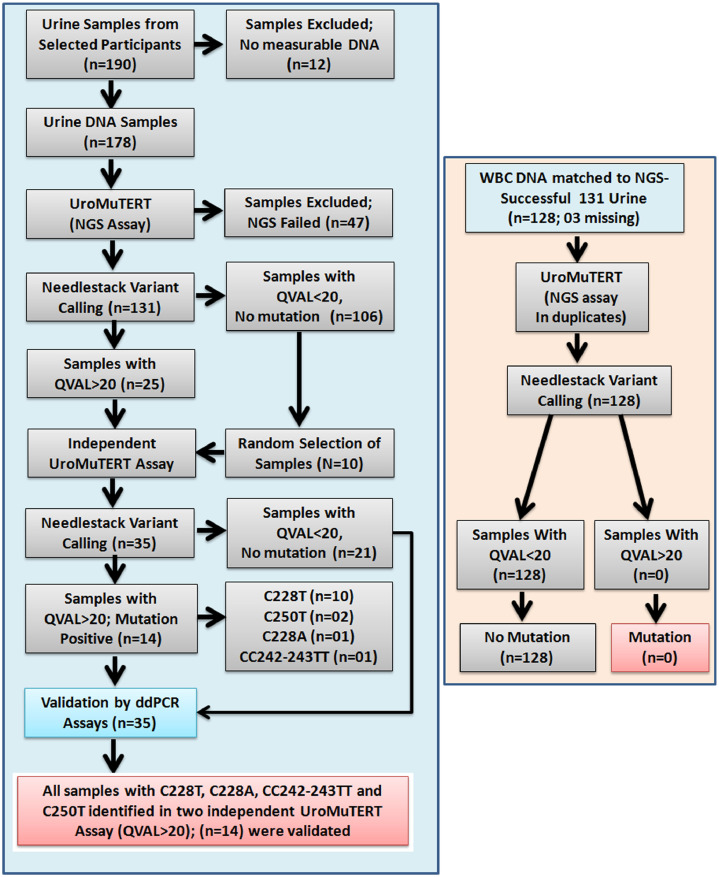
To ensure mutation calling accuracy and minimize fall positive results, we applied a stringent 2-step strategy using two independent sensitive methods to call TERT promoter mutations in the urinary and white blood cell DNA (Fig. 1).
Fig. 1.
A schematic presentation of the analytical workflow for the detection of TERT promoter mutations in urinary and white blood cell DNA samples of the bladder cancer cases and cancer-free controls selected from the Golestan Cohort Study.
2.5.1. UroMuTERT assay and mutation analysis
The single-plex Next-Generation Sequencing assay (UroMuTERT) was previously developed in our laboratory for the detection of low-abundance TERT promoter mutations [17]. Briefly, UroMuTERT utilizes a single amplicon of 147 bp that was designed to cover the genomic region of the TERT promoter that contain the 2 ‘hotspot’ C228T and C250T mutations. The detailed protocol for amplicon-based Ion Torrent Proton sequencing is provided in the Supplementary data. Variant calling was performed using our Needlestack algorithm based on negative binomial regression analysis and specifically designed for the detection of low-abundance mutations (https://github.com/IARCbioinfo/needlestack) [21]. A p-value for being a variant (outlier from the regression) was calculated for each sample and further transformed into q-values to account for multiple hypotheses testing (Supplementary Figure 1). A threshold of Phred scale q-values QVAL>20 was validated in the previous case-control study [17] and also applied in this study (Figure 1).
To control for potential low-allelic fraction amplification artefacts or sequencing errors, all DNA samples with evidence for a TERT mutation (QVAL>20) were re-amplified, re-sequenced using independent barcoded libraries and analysed with Needlestack algorithm together with sufficient wild-type samples at the targeted positions to ensure appropriate fit of the regression. Only samples with confirmed QVAL>20 were considered as positive after UroMuTERT analysis (Fig. 1).
2.5.2. Validation by ddPCR assay
We developed droplet digital PCR (ddPCR) assays for the two most common TERT promoter mutations: C228T and C250T as well as the two other rare ones C228A and CC242-243TT (Supplementary Table 2, Supplementary data). All samples positive after UroMuTERT analysis were run in duplicates using 10 ng of urinary DNA. Ten wild-type samples chosen at random were included as technical controls. Analysis of the ddPCR data was performed using the QuantaSoft™ Analysis Pro 1.0.596 software from Biorad (Supplementary Figure 2). The threshold representing the minimum number of positive droplets for calling a mutation was determined from the Poisson distribution of droplets in wild type and mutated cell lines and was set at 6 or 5 (Supplementary data). All laboratory analyses were conducted blindly to the case or control status of the samples.
2.6. Statistical analysis
Incidence density matching was performed using Stata statistical software. The distributions of the data were tested using the Shapiro–Wilk test. For normally distributed data, Pearson correlation coefficients and Student's t-tests were used, while for the non-normally distributed data, Spearman's rank correlation coefficients and Wilcoxon rank-sum tests were used to compare the differences between the continuous variables. Chi-squared tests were used to compare the dichotomous variables. Sensitivity, specificity and accuracy of the TERT promoter mutations were calculated and their confidence intervals computed using the Clopper-Pearson method [22]. The positive predictive values (PPVs) and negative predictive values (NPVs) were calculated based on an estimated cumulative incidence of BC (0·09%; see results section) in the Golestan cohort. Confidence intervals for the predictive values were defined as the standard logit confidence intervals and were calculated using the method of Mercaldo et al. 2007 [23]. All statistical analyses were performed using Stata statistical software version 14 (Stata Corporation, College Station, Texas, USA).
3. Results
3.1. Descriptive statistics
Of the initial 38 BC cases and their 152 matched controls, 10 were excluded due to the lack of measurable DNA in urine samples, 47 due to low-quality sequencing data after the UroMuTERT assay, and 2 controls due to unrecorded urine samples in the biorepository. The volume of urine aliquots used for DNA isolation (range: 1·1 - 4·5 mL) did not correlate with the DNA yield (range: 6 ng – 7 µg) (Supplementary Figure 3A). The 47 samples with low-quality sequencing data had a lower median DNA amount (Median: 15·1 ng) than the 131 samples with UroMUTERT data (Median: 51·8 ng) (p = 0·006) (Supplementary Figure 3B). No correlation between the quality of sequencing data and urine volume was observed (Supplementary Figure 3C). A significant proportion of the urine samples which failed the UroMuTERT assay (n = 57) were collected during the first years of enrollment (2004–2005), and hence had the longest storage duration (Supplementary Table 3).
Finally, 131 participants including 30 cases and 101 matched controls were retained for subsequent mutation analysis. The median age of participants at baseline was 57.8 years (range: 41.3 - 75.2 years). The median follow-up time from the date of urine collection until assignment as a case was 7·3 years (range: 0·4 – 10·3 years). There were no statistically significant differences in the demographics of the participants between cases and controls (Table 1).
Table 1.
Comparison of the baseline characteristics of the bladder cancer cases and the matched controls.
| Baseline characteristics | Bladder Cancer Cases (n = 30) N (%) |
Matched Controls (n = 101) N (%) |
P value |
|---|---|---|---|
| Age (years)a | 0·769 | ||
| 59·1 (42·2 – 75·1) | 57·7 (± 41·3 – 75·2) | ||
| Gender | 0·891 | ||
| • Male | 21 (70) | 72 (71·3) | |
| • Female | 9 (30) | 29 (28·7) | |
| Ethnicity | 0·322 | ||
| • Turkman | 16 (53·3) | 64 (63·4) | |
| • Non-Turkman | 14 (46·7) | 37 (36·6) | |
| Residence | 0·237 | ||
| • Rural | 21 (70·0) | 81 (80·2) | |
| • Urban | 9 (30·0) | 20 (19·8) | |
| Socioeconomic statusb | 0·665 | ||
| • Low | 8 (26·7) | 35 (34·7) | |
| • Middle | 13 (43·3) | 36 (35·6) | |
| • High | 9 (30·0) | 30 (29·7) | |
| Regular tobacco consumption | 0·039 | ||
| • Never | 13 (43·3) | 65 (64·4) | |
| • Ever | 17 (56·7) | 36 (35·6) | |
| Regular opium consumption | 0·037 | ||
| • Never | 17 (56·7) | 77 (76·2) | |
| • Ever | 13 (43·3) | 24 (23·8) | |
| Regular alcohol drinking | 0·181 | ||
| • Never | 26 (86·7) | 95 (94·1) | |
| • Ever | 4 (13·3) | 6 (5·9) | |
| Time from sample collection until diagnosis of BC (years)a | |||
| Bladder cancer categories | – | ||
| • Non-muscle-invasive | 9 (30·0) | – | |
| • Muscle-invasive | 16 (53·3) | – | |
| • Un-known | 5 (16·7) | ||
Displayed as Median (Range).
N: Number; BC: Bladder Cancer.
Socioeconomic status was determined using a wealth score that was created using multiple correspondence analysis on the ownership of house, vehicle, and some home appliances.
The participants with subsequent BC diagnosis had significantly higher rates of tobacco and opium consumption compared to their matched controls (Table 1). Of the 30 analysed BC cases, 9 (30·0%) had non-muscle-invasive carcinoma, 16 (53·3%) had muscle invasive carcinoma, and the invasiveness into the muscle was unknown for the remaining 5 (16·7%) cases (Table 1). The three-year overall survival rate was 40% among cases (66% survival rate for cases diagnosed with non-muscle-invasive tumours and 16% for cases with muscle-invasive carcinomas).
3.2. Detection of TERT promoter mutations in pre-diagnostic urine samples
Fourteen out of 30 cases (46·67%) tested positive for TERT promoter mutations in pre-diagnostic urinary DNA samples after UroMuTERT analysis [C228T (n = 10), C228A (n = 1), CC242-243TT (n = 1), and C250T (n = 2)] and were all validated by ddPCR assays. The most frequent TERT mutations reported in BC (C228T and C250T) were identified in 12 urine samples (85·71% of the detected mutations). None of the controls carried urinary TERT promoter mutations. We did not observe any correlation between the mutant allelic fractions (MAF) of TERT promoter mutations detected by either ddPCR or UroMuTERT assays and the urine volume or the urinary DNA concentration (Supplementary Figure 4). The MAFs detected using the UroMuTERT and ddPCR assays were highly correlated (r2 = 0·96), (Supplementary Figure 5). In contrast to our initial expectations, we found the median MAF to be high (UroMuTERT MAF 20·4%; range 0·7%−73·3%). To account for a potential rare occurrence of germ-line or mosaic TERT promoter mutations, as observed in our previous study [17], we applied the same mutation screening strategy to DNA isolated from white blood cells (WBCs) of 128 subjects with available WBCs (30 cases and 98 controls). No TERT promoter mutations were detected in the WBCs, confirming the somatic origin of the mutations in the urine samples. The sensitivity and specificity of detecting somatic TERT promoter mutations in pre-clinical urine samples of individuals who subsequently developed BC were 46·67% (95%CI: 28·34 – 65·67), and 100·00% (95%CI: 96·41 – 100·00) respectively (Table 2). Cases were categorized into two groups based on the median time to BC diagnosis. The sensitivity estimate was higher for urine samples collected less than 7 years before diagnosis (57·14%) than for older samples (37·50%), but the difference was not significant (p = 0·46) (Table 2). This indicates that urinary TERT promoter mutations can be detected early in the carcinogenesis process. Considering that 101 of 152 controls (66·5%) had conclusive results, we extrapolated that the size of the Golestan cohort with analysable TERT promoter mutation tests would be 33,254 participants. In this sample set, 30 cases had a conclusive test, leading to a cumulative incidence of 0·09%. Using this figure as the prevalence, the PPV and NPV of the biomarkers were 100·00% and 99·95% (99·93%–99·97%), respectively (Table 2). Taking into account the confidence intervals of the sensitivity and specificity, projected PPVs could vary from 0·70% to 100·00% and NPVs from 99·93% to 99·97%.
Table 2.
Performance of detecting TERT promotor mutation in the pre-diagnostic urine samples as an early detection biomarker for bladder cancer.
| Statistics | Urinary DNA (N = 131) | 95% CI |
|---|---|---|
| All Pre-diagnostic samples | ||
| True Positive (n) | 14 | – |
| True Negative (n) | 101 | – |
| False positive (n) | 0 | – |
| False negative (n) | 16 | – |
| Sensitivity (%) | 46·67 | 28·34 – 65·67 |
| Specificity (%) | 100·00 | 96·41 – 100·00 |
| Positive likelihood ratio (%) | – | – |
| Negative likelihood ratio (%) | 0·53 | 0·38 – 0·75 |
| Positive predictive value (%)a | 100·00 | |
| Negative predictive value (%)a | 99·95 | 99·93 – 99·97 |
| Accuracy (%) | 99·96 | 99·94 – 99·97 |
| < 7 years prior diagnosisb | ||
| True Positive (n) | 08 | – |
| False Negative (n) | 06 | – |
| Sensitivity (%) | 57·14 | 28·86 – 82·34 |
| > 7 years prior diagnosisb | ||
| True Positive (n) | 06 | – |
| False Negative (n) | 10 | – |
| Sensitivity (%) | 37·50 | 15·20 – 64·57 |
| Non-Muscle Invasive Bladder Cancer | ||
| True Positive (n) | 03 | – |
| False Negative (n) | 06 | – |
| Sensitivity (%) | 33·33 | 7·49 – 70·07 |
| Muscle Invasive Bladder Cancer | ||
| True Positive (n) | 10 | – |
| False Negative (n) | 06 | – |
| Sensitivity (%) | 62·50 | 35·43 – 84·80 |
CI: Confidence Interval.
Positive and negative predictive values were calculated using the prevalence of bladder cancer (0·09%) in the Golestan population based cohort.
Median time to BC diagnosis.
The sensitivity for detecting current or subsequent BC in pre-diagnostic urine samples was 33·33% for non-muscle invasive and 62·50% for muscle invasive carcinoma (p = 0·16) (Table 2). The detailed results of TERT promoter mutations and other demographic and clinical data of all cases are reported in Supplementary Table 4.
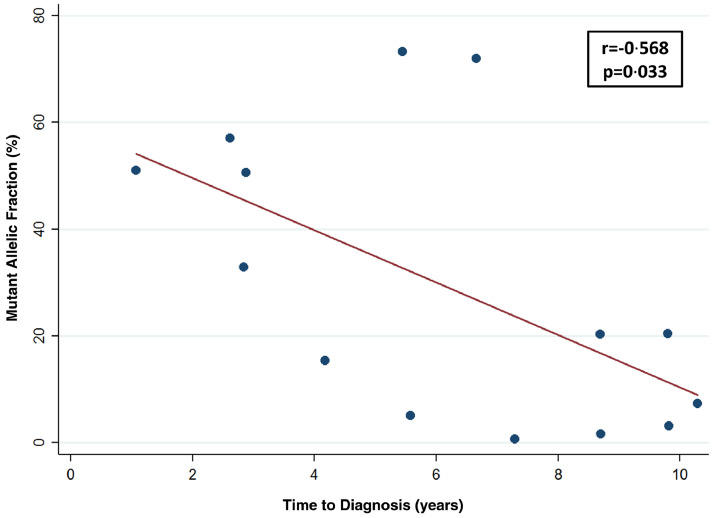
3.3. Relationship between MAFs and time to diagnosis of BC
There was a significant inverse correlation between MAF of TERT promoter mutations in the pre-diagnostic urine samples and the time interval from sample collection to BC diagnosis (r =−0·568, p = 0·033) (Fig. 2). The MAF was higher (median: 50·8%; range: 5·0% – 73·3%) in TERT positive BC cases diagnosed during the first seven years of urine collection compared to those diagnosed after seven years of urine collection (median: 5·2%; range: 0·7% – 20·4%), although the difference did not reach significance (p = 0·16).
Fig. 2.
Association of TERT promoter mutations Mutant Allelic Fractions (MAFs) with the time interval from urine collection until clinical diagnosis of bladder cancer. The MAFs in% of the mutations detected by the UroMuTERT assay (NGS-based assay) in the 14 urine samples of the asymptomatic subjects from the Golestan Cohort Study are plotted against the time in years from urine collection to diagnosis of bladder cancer.
4. Discussion
This pilot case-control study nested within the population-based prospective Golestan Cohort Study of more than 50 thousand participants showed that TERT promoter mutations could be detected in pre-diagnostic urine samples of asymptomatic individuals with 100% specificity, 46% sensitivity, and 100% projected PPV and 99·95% projected NPV, up to ten years before clinical diagnosis of primary BC.
TERT promoter mutations were previously reported in the urinary DNA from incident and early-stage BC cases, with high specificity (90%–99%) and good sensitivity (55%–62%) [10,11,14,15]. In a recent case-control study, we used the single-plex ultrasensitive UroMuTERT assay and showed a sensitivity of 86·7% and a specificity of 94·7% in detecting primary urothelial cancers of the bladder [17], indicating that with this very sensitive assay, evaluation of these urinary DNA markers could potentially have clinical utility for detecting urothelial cancers at the time of clinical diagnosis. In a recent screening study in clinical settings, Springer et al. evaluated a multigene panel assay (including C228T and C250T TERT promoter mutations) for detecting BC 0–18 months prior to clinical diagnosis in high-risk symptomatic patients, and they reported a sensitivity of 83% and a specificity of 93% for their panel, while TERT promoter mutations were detected in 57% of the cases [14]. Although this study documented promising potential for TERT promoter mutations as early detection biomarkers of BC, it evaluated only a limited period before clinical BC diagnosis and it provided no data on the predictive values of these assays in healthy asymptomatic individuals.
To overcome these limitations, we tested urinary TERT promoter mutations as early BC detection biomarkers over a longer time period in a well-defined nested case-control study of initially healthy adults within the population-based prospective Golestan Cohort Study [20]. To our knowledge, this study represents the first nested case-control study within a population-based prospective cohort of initially healthy adults which has assessed the predictive value of non-invasive biomarkers for early detection of BC years before the clinical diagnosis. Our pilot study provides the first evidence of the ability of detecting tumour-derived alterations in the urine of asymptomatic individuals who subsequently developed BC. We also showed that the detection of TERT promoter mutations was possible in pre-diagnostic low-volume urine samples (1·1–4·5 mL) that were processed within 8 h from collection and stored for years before molecular analysis, confirming the feasibility of replicating our findings in other longitudinal cohorts, where the volume of retrospectively collected urine samples is often limited. However, we can not exclude the possibility that low urine volume, long-term storage and pre-analytical processing steps might affect the detectability of these mutations. While a significant fraction of urine samples (31%) were excluded from this analysis because of the absence of measurable DNA or low quality data, we previously showed in our recent case-control study that prospectively collected urine samples of sufficient volume (9–44 ml), processed and stored at −80°C within the 2 h of collection and stored <6 months before DNA isolation gave 100% TERT mutation results for either urinary cell DNA or cell-free DNA [17]. This indicates that screening studies with freshly collected urine samples of sufficient volume might overcome some of the issues causing failure in the current study. In line with this, Zuiverloon and colleagues reported in a trial where urine samples from the same patients were pooled during a 24 h period that the sensitivity of their urine-based FGFR3 mutation assay increased with urine volume [24].
The most striking findings in this study were the detection of TERT promoter mutations in urine collected up to 10 years before clinical BC diagnosis and the lack of false positive results in any of the controls. This suggests a very slow tumorigenic process in at least some TERT-mutated BCs, which could provide a window of opportunity for early molecular detection and intervention. It also indicates that longitudinal studies with insufficient follow-up durations may not be able to evaluate the true specificity of these biomarkers, since individuals who are test-positive and have no current evidence of BC might still be diagnosed with clinical BC after a longer follow-up time.
The 100% observed specificity of the TERT mutation biomarkers is clearly a promising result, but it should be interpreted with caution as only 101 controls were tested, and individuals with a history of any cancer (including those that have been reported to have TERT promoter C228T or C250T mutations) were excluded from evaluation as controls [17]. Thus, although the true specificity of these biomarkers for BC may be very high, and high enough to be clinically useful for BC screening, the specificity of the biomarkers for BC detection should further be assessed in a large series of controls unselected for other cancer diagnosis.
The long follow-up time in this study allowed for comparisons of results by the time interval between urine collection and clinical BC diagnosis. The sensitivity of the TERT mutation assay was higher (57·1%) in patients diagnosed within 7 years of urine collection than in those with a longer urine-to-clinical diagnosis time interval (37·5%), and we also observed an inverse correlation between the TERT mutation load and the time from urine collection to clinical diagnosis. Altogether, these results suggest more pronounced shedding of mutated tumour cells and mutated cell-free DNA in the urine of individuals as they get closer to the time of clinical diagnosis, which could reflect increased tumour size. The association between urine-based mutation assay sensitivity and increased bladder tumour size was previously shown [24]. However, because of the limited number of cases in our study (n = 30), these findings should be validated in larger series of pre-diagnostic urine samples of individuals who later developed bladder cancer .
The dearth of longitudinal cohorts with an appropriate duration of follow-up and high quality biospecimens is a significant concern for the prospective validation of promising biomarkers, and only a few studies have assessed the utility of urine BC biomarkers in such prospective studies. The FDA-approved NMP22 and UroVysion biomarkers were tested in a combined panel in the UroScreen study, a large BC screening program that included workers who were exposed to aromatic amines and were followed for two years [25]. Given the low specificity of NMP22, and the high costs of UroVysion, the combined panel was found unsuitable for implementation in the screening programs targeting asymptomatic workers [26,27].
The high rates of muscle invasive BC cases and the observed poor overall survival rates in this study probably suggest a certain degree of delayed diagnosis [28] and/or an enrichment of primary muscle invasive carcinoma in the Golestan Cohort Study. There is growing molecular evidence that muscle invasive and non-muscle invasive BCs might represent neoplasms with different cell lineage and genetic features rather than subsequent stages of the same neoplasm [29]. Regardless of the above, the detectability of the studied biomarkers years prior to clinical diagnosis of BC offers a significant window of opportunity for intervention and highlights the importance of developing simple and non-invasive screening and early detection methods for reducing the burden of BC in resource-limited settings. In addition, by design, our study could not evaluate the proportion of asymptomatic individuals with tert-positive assays who would have carried clinically diagnosable BC at the time of the baseline urine collection. Screening studies are therefore required to evaluate whether a clinical diagnosis can be made through cystoscopy or urography in asymptomatic patients with a positive urinary TERT promoter mutations assay or whether it indicates undetectable pre-malignant stages in patients who would benefit from regular urinary TERT promoter mutations screening until clinical detection of early-stages tumours is possible. Since previous studies have shown the benefit of BC screening in survival improvement [5,30], the clinical utility of urinary TERT promoter mutations for BC survival should be assessed in properly designed randomized control trials.
Finally, studies have shown that screening high-risk populations using urinary biomarkers followed by cystoscopy could be cost-effective [5,30]. Notably, Lotan et al. indicated that cost-effectiveness could be reached if BC incidence is >1·6% in the targeted high-risk population, tumour marker costs is <$126, and the screening biomarker yields a sensitivity >26% and specificity> 54% [4]. Detecting TERT promoter mutations in urine may therefore be an interesting candidate for cost-effective screening program where the identification of appropriate high-risk populations seems to appear as the most critical parameter.
The strengths of this pilot study include its prospective design, nested in a large population-based cohort of asymptomatic adults; its long follow-up period; and the low cost and rapid throughput of the biomarker assay. The limitations include the relatively small number of BC cases and controls evaluated; the fact that 21% of the original cases and 34% of the original controls had to be excluded from analysis due to lack of measurable DNA in the urine samples or low-quality DNA sequencing data; and the exclusion of individuals with non-bladder cancers (some of which might have been TERT promoter mutation positive) from the control pool.
In conclusion, our results provide evidence from a population-based prospective cohort study that TERT promoter mutations are detectable in urine samples up to 10 years prior to BC diagnosis, with 100% specificity and 46·7% sensitivity. This highlights that urinary TERT promoter mutations may have the potential to be used as simple and inexpensive non-invasive biomarkers for early detection of BC. Further studies should assess the clinical utility of these biomarkers in other longitudinal cohorts.
Funding sources
The work reported in this paper was undertaken during the tenure of MIH's postdoctoral fellowship from the International Agency for Research on Cancer. MS was supported by the World Cancer Research Fund International (grant number: WCRF 2016/1633). The research project was funded by the French Cancer League, and the Golestan cohort study by the Cancer Research UK (grant number: C20/A5860), Tehran University of Medical Sciences (grant number: 81/15), the International Agency for Research on Cancer, and the Intramural Research Program of the U.S. National Cancer Institute. The funders had no role in study design, collection, analysis, and interpretation of data, in manuscript writing or in submission process for publication.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We thank all participants of the Golestan Cohort study for agreeing to be enrolled in the study and providing biological samples and follow-up data over many years. We also thank all the professionals including medical doctors, nurses, epidemiologists and nutritionists involved in the recruitment of participants and collection and storage of biospecimens for their precious expertise.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102643.
Contributor Information
Reza Malekzadeh, Email: malek@tums.ac.ir.
Florence Le Calvez-Kelm, Email: lecalvezf@iarc.fr.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kamat A.M., Hahn N.M., Efstathiou J.A., Lerner S.P., Malmström P.-.U., Choi W. Bladder cancer. The Lancet. 2016;388(10061):2796–2810. doi: 10.1016/S0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 3.Mossanen M., Gore J.L. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol. 2014;24(5):487–491. doi: 10.1097/MOU.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 4.Lotan Y., Svatek R.S., Sagalowsky A.I. Should we screen for bladder cancer in a high-risk population?: A cost per life-year saved analysis. Cancer. 2006;107(5):982–990. doi: 10.1002/cncr.22084. [DOI] [PubMed] [Google Scholar]
- 5.Zlotta A.R., Roumeguere T., Kuk C., Alkhateeb S., Rorive S., Lemy A. Select screening in a specific high-risk population of patients suggests a stage migration toward detection of non-muscle-invasive bladder cancer. Eur Urol. 2011;59(6):1026–1031. doi: 10.1016/j.eururo.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz-Drager B.J., Droller M., Lokeshwar V.B., Lotan Y., Hudson M.A., van Rhijn B.W. Molecular markers for bladder cancer screening, early diagnosis, and surveillance: the WHO/ICUD consensus. Urol Int. 2015;94(1):1–24. doi: 10.1159/000369357. [DOI] [PubMed] [Google Scholar]
- 7.Xylinas E., Kluth L.A., Rieken M., Karakiewicz P.I., Lotan Y., Shariat S.F. Urine markers for detection and surveillance of bladder cancer. Urol Oncol. 2014;32(3):222–229. doi: 10.1016/j.urolonc.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Larre S., Catto J.W., Cookson M.S., Messing E.M., Shariat S.F., Soloway M.S. Screening for bladder cancer: rationale, limitations, whom to target, and perspectives. Eur Urol. 2013;63(6):1049–1058. doi: 10.1016/j.eururo.2012.12.062. [DOI] [PubMed] [Google Scholar]
- 9.Killela P.J., Reitman Z.J., Jiao Y., Bettegowda C., Agrawal N., Diaz L.A., Jr. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinde I., Munari E., Faraj S.F., Hruban R.H., Schoenberg M., Bivalacqua T. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013;73(24):7162–7167. doi: 10.1158/0008-5472.CAN-13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allory Y., Beukers W., Sagrera A., Flandez M., Marques M., Marquez M. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65(2):360–366. doi: 10.1016/j.eururo.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Eich M.L., Rodriguez Pena M.D.C., Springer S.U., Taheri D., Tregnago A.C., Salles D.C. Incidence and distribution of UroSEEK gene panel in a multi-institutional cohort of bladder urothelial carcinoma. Mod Pathol. 2019;32(10):1544–1550. doi: 10.1038/s41379-019-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietzak E.J., Bagrodia A., Cha E.K., Drill E.N., Iyer G., Isharwal S. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur Urol. 2017;72(6):952–959. doi: 10.1016/j.eururo.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer S.U., Chen C.H., Rodriguez Pena M.D.C., Li L., Douville C., Wang Y. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife. 2018:7. doi: 10.7554/eLife.32143. pii: e32143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurst C.D., Platt F.M., Knowles M.A. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol. 2014;65(2):367–369. doi: 10.1016/j.eururo.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 16.Ward D.G., Baxter L., Gordon N.S., Ott S., Savage R.S., Beggs A.D. Multiplex PCR and next generation sequencing for the non-invasive detection of bladder cancer. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0149756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avogbe P.H., Manel A., Vian E., Durand G., Forey N., Voegele C. Urinary TERT promoter mutations as non-invasive biomarkers for the comprehensive detection of urothelial cancer. EBioMedicine. 2019;44:431–438. doi: 10.1016/j.ebiom.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepe M.S., Etzioni R., Feng Z., Potter J.D., Thompson M.L., Thornquist M. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93(14):1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 19.Pesch B., Bruning T., Johnen G., Casjens S., Bonberg N., Taeger D. Biomarker research with prospective study designs for the early detection of cancer. Biochim Biophys Acta. 2014;1844(5):874–883. doi: 10.1016/j.bbapap.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Pourshams A., Khademi H., Malekshah A.F., Islami F., Nouraei M., Sadjadi A.R. Cohort profile: The Golestan Cohort Study–a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol. 2010;39(1):52–59. doi: 10.1093/ije/dyp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delhomme TM, Avogbe PH, Gabriel A, Alcala N, Leblay N, Voegele C, et al. Needlestack: an ultra-sensitive variant caller for multi-sample next generation sequencing data. bioRxiv.2019:639377. doi: 10.1101/639377. [DOI] [PMC free article] [PubMed]
- 22.Zieliński W. The shortest Clopper–Pearson confidence interval for binomial probability. Commun Stat. 2009;39(1):188–193. [Google Scholar]
- 23.Mercaldo N.D., Lau K.F., Zhou X.H. Confidence intervals for predictive values with an emphasis to case-control studies. Stat Med. 2007;26(10):2170–2183. doi: 10.1002/sim.2677. [DOI] [PubMed] [Google Scholar]
- 24.Zuiverloon T.C., Tjin S.S., Busstra M., Bangma C.H., Boeve E.R., Zwarthoff E.C. Optimization of nonmuscle invasive bladder cancer recurrence detection using a urine based FGFR3 mutation assay. J Urol. 2011;186(2):707–712. doi: 10.1016/j.juro.2011.03.141. [DOI] [PubMed] [Google Scholar]
- 25.Pesch B., Taeger D., Johnen G., Gawrych K., Bonberg N., Schwentner C. Screening for bladder cancer with urinary tumor markers in chemical workers with exposure to aromatic amines. Int Arch Occup Environ Health. 2014;87(7):715–724. doi: 10.1007/s00420-013-0916-3. [DOI] [PubMed] [Google Scholar]
- 26.Huber S., Schwentner C., Taeger D., Pesch B., Nasterlack M., Leng G. Nuclear matrix protein-22: a prospective evaluation in a population at risk for bladder cancer. Results from the UroScreen study. BJU Int. 2012;110(5):699–708. doi: 10.1111/j.1464-410X.2011.10883.x. [DOI] [PubMed] [Google Scholar]
- 27.Schlomer B.J., Ho R., Sagalowsky A., Ashfaq R., Lotan Y. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol. 2010;183(1):62–67. doi: 10.1016/j.juro.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 28.Hollenbeck B.K., Dunn R.L., Ye Z., Hollingsworth J.M., Skolarus T.A., Kim S.P. Delays in diagnosis and bladder cancer mortality. Cancer. 2010;116(22):5235–5242. doi: 10.1002/cncr.25310. [DOI] [PubMed] [Google Scholar]
- 29.Van Batavia J., Yamany T., Molotkov A., Dan H., Mansukhani M., Batourina E. Bladder cancers arise from distinct urothelial sub-populations. Nat Cell Biol. 2014;16(10):982–991. doi: 10.1038/ncb3038. 1-5. [DOI] [PubMed] [Google Scholar]
- 30.Messing E.M., Madeb R., Young T., Gilchrist K.W., Bram L., Greenberg E.B. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107(9):2173–2179. doi: 10.1002/cncr.22224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.