Abstract
Pancreatic ductal adenocarcinoma (PDAC) shows remarkable propensity to metastasize. This predilection to escape from the primary tumor is driven by paracrine and autocrine mechanisms that guide cancer cells through a multi-step process concluding with colonization in distant tissues. Although cell-intrinsic features support the metastatic ability of cancer cells, permissive microenvironments within the primary organ and at sites of distant metastasis may be rate-limiting. Identification of cancer cell-extrinsic factors that regulate formation of these environments lend new therapeutic targets for intervening on the metastatic cascade. In addition, the bipolar, yet fundamental, role of the immune system in the metastatic process presents therapeutic opportunities. Herein, we review the current knowledge of the metastatic cascade in PDAC, and propose that genomically stable determinants of metastasis (e.g. the pro-metastatic niche and immune system) are actionable targets for preventing, containing, and treating metastasis in PDAC.
Keywords: Inflammation, Cancer, Pancreatic ductal adenocarcinoma, Immunotherapy, Immune evasion, Treatment paradigms, T cells, Macrophages, Neutrophils, Tumor microenvironment, Immunosurveillance, Vaccines, Therapeutic resistance, Metastasis, Clinical trials
Abbreviations: PDAC, pancreatic ductal adenocarcinoma; CAF, cancer-associated fibroblasts; CCR, C-C chemokine receptor; CSF, colony stimulating factor; CXCL, C-X-C motif chemokine ligand; CXCR, CXC chemokine receptor; DCC, disseminated cancer cell; DNA, deoxyribonucleic acid; EGF, epidermal growth factor; EMT, epithelial to mesenchymal transition; IL, interleukin; LIF, leukaemia inhibitory factor; LOX, lysyl oxidase; MIF, macrophage migration inhibitory factor; MET, mesenchymal-to-epithelial transition; MMP, matrix metalloproteinase; PanIN, pancreatic intraepithelial neoplasia; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; Prxx1, paired-related homeodomain transcription factor 1; PSC, pancreatic stellate cells; SAA, serum amyloid A; SIRP, signal regulatory protein; STAT3, signal transducer and activation of transcription; TGF, transforming growth factor; TIMP, tissue inhibitor matrix metalloproteinase; TLR, Toll-like receptor; TMEM, tumor microenvironments of metastasis; TNF, tumor necrosis factor; TRAIL-R, TNF-related apoptosis-inducing ligand receptor; Wnt1, Wnt family member 1; Zeb1, zinc finger E-box-binding homeobox 1
1. Introduction
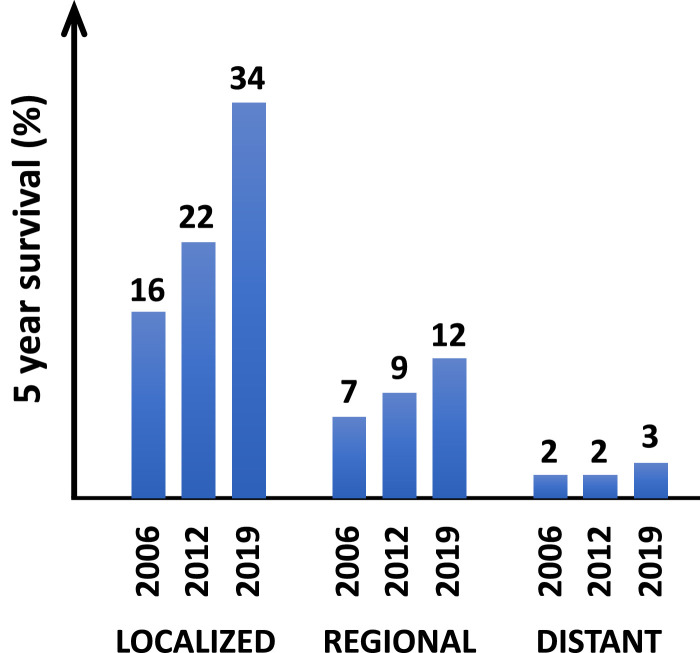
Pancreatic ductal adenocarcinoma (PDAC) is a lethal cancer with a 5-year overall survival of 3% for patients with metastatic disease [1]. This dismal outcome has remained largely unchanged for the past two decades (Fig. 1). This is in stark contrast to the promising progress being made for patients who present with localized PDAC (Fig. 1) and therefore, beckons for novel approaches capable of intervening on the metastatic process. PDAC is currently the 3rd most common cause of cancer deaths in the United States but is expected to become the 2nd leading cause within the next decade [2]. Mortality in PDAC is primarily the result of metastasis to the liver, lung, and peritoneal cavity [3]. Notably, at diagnosis, over 50% of patients with PDAC will present with metastatic disease [1]. Further, nearly 80% of patients who undergo surgery with curative intent ultimately relapse with two-thirds succumbing to distant recurrence [4]. As such, novel therapeutic strategies capable of disrupting, restraining, and reversing metastasis are needed to improve outcomes for patients.
Fig. 1.
Five-year survival for patients with pancreatic ductal adenocarcinoma diagnosed with localized, regional (i.e. lymph node involvement), or distant (i.e. metastatic to other organs) disease. Graph is based on mortality data from the National Center for Health Statistics reported for 2006 [90], 2012 [91], and 2019 [1] by the American Cancer Society. Numbers associated with histogram bars indicate percent of patients alive at ≥5 years after diagnosis.
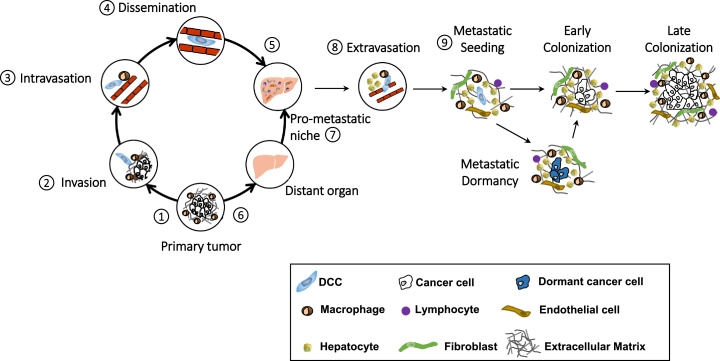
Metastasis is a multistep process (Fig. 2). Cancer cells must invade their local microenvironment to intravasate into the bloodstream and then lodge within distant organs before extravasating into the organ parenchyma to establish metastatic colonies. This highly regulated and inefficient process is dependent on cell-intrinsic properties that engender cancer cells with the ability to metastasize as well as on cancer cell-extrinsic features of the primary tumor and distant organ sites of metastasis that enable the metastastic process. Recent studies provide insight into the determinants that instruct pancreatic cancer cells with metastatic ability, guide the predilection of cancer cells for distinct organs, and govern the receptiveness of distant organs for metastasis. Understanding this biology is paramount to identifying strategies that may be capable of intervening on the metastatic process. In this review, we discuss paracrine and autocrine signals that educate and support pancreatic cancer cells through their metastatic journey. Our focus is on biology that instructs cancer cell dissemination away from the primary tumor and orchestrates the formation of a pro-metastatic niche that cultivates the seeding of disseminated cancer cells in distant organs.
Fig. 2.
Graphical representation of the metastatic cascade in pancreatic ductal adenocarcinoma. Metastasis is a multi-step process involving signals that support cancer cell dissemination (steps 1–5) and that condition distant organs (steps 6 and 7) for increased permissiveness of disseminated cancer cells (DCC). During metastasis, malignant epithelial cells detach from the basement membrane (step 1), invade into the surrounding stroma (step 2) and intravasate into the bloodstream (step 3) leading to dissemination (step 4) and subsequent, lodging in a distant organ (step 5), such as the liver. Concurrently, primary tumors release factors (step 6) that promote the development of a pro-metastatic niche environment in a distant organ (step 7). DCCs then extravasate (step 8) into the parenchyma of the distant organ where they seed (step 9). Thereafter, DCCs either lie dormant awaiting appropriate awakening signals or proceed to colonize the distant tissue.
2. Determinants regulating cancer cell dissemination
The multi-step process of metastasis (Fig. 2) is directed by a coordinated set of signals that converge to instruct cancer cells to detach from the basement membrane, invade into the surrounding tissue, and intravasate into the bloodstream where they emerge as disseminated cancer cells (DCCs). Concurrently, secreted signals instruct formation of a pro-metastatic niche in distant organs which then supports the journey of DCCs. During this process, DCCs must endure physical stress and evade immune elimination as they seek to extravasate into the parenchyma of other organs. After seeding, DCCs may initially lie dormant awaiting the necessary proliferative signals that trigger their awakening and subsequent outgrowth to form a metastatic lesion [5]. Overall, this process is highly inefficient such that the vast majority of cancer cells never successfully complete their metastatic journey [6]. In PDAC, cancer cells with high metastatic competency are predicted to be generated at a rate of approximately 1 in a million cells during tumor development [7]. This prediction suggests that cancer cell-extrinsic features may be critical in defining the fitness of cancer cells and their tropism for distant organs. To this end, a permissive tumor microenvironment and a receptive distant organ niche are fundamental in determining the capacity of cancer cells to escape from primary tissues and subsequently seed and colonize distant tissues. Here, we discuss key determinants, including paracrine signals produced by cancer cells (Table 1), that coordinate the metastatic process and address the role of non-malignant cells in facilitating cancer cell metastasis.
Table 1.
Cancer cell-derived paracrine signals involved in pancreatic cancer metastasis.
| Soluble factor | Targets | Proposed function |
|---|---|---|
| Sonic hedgehog (Shh) [8] | Fibroblasts | Myofibroblast proliferation |
| TGF-β [12] | Multiple cell types (including cancer cells, macrophages, T cells, fibroblasts) | EMT, CAF activation, immune suppression |
| MMP-7 [37] | Stromal matrix and protein components | Increased metastatic potential of cancer cells |
| Hyaluronic acid [14,16] | Cancer cells | Proliferation, increased cancer cell invasion |
| GM-CSF [63] | Myeloid cells | Recruitment and differentiation of myeloid cells, T cell exclusion |
| Colony stimulating factor 1 (CSF1) [40] | Macrophages | Support cancer cell invasion |
| Exosome-derived macrophage-inhibitory factor (MIF) [69] | Kupffer cells | Supports formation of a liver pro-metastatic niche |
| TIMP1 [70] | Hepatic stellate cells | Support formation of liver pro-metastatic niche |
| CXCL1 [62] | Granulocytes | Support formation of liver pro-metastatic niche; Immune suppression |
| CCL2 [45] | Myeloid cells | Cancer cell dissemination, myeloid cell recruitment, formation of liver pro-metastatic niche |
| Versican [74] | Macrophages | Promotes metastatic outgrowth |
2.1. Initiating signals that establish a microenvironment permissive of cancer cell invasion
The metastatic process begins with cancer cell detachment from the basement membrane and invasion into the surrounding stroma. These early metastatic steps rely on a permissive state of the microenvironment that surrounds cancer cells. For example, in the pancreas, pancreatic stellate cells (PSCs) are quiescent resident fibroblasts that in the setting of cancer, differentiate into activated cancer-associated fibroblasts (CAFs). CAFs accumulate during tumor development in response to a variety of cytokines that are involved in wound-healing and fibrosis. For instance, cancer cells produce survival factors for CAFs, such as sonic hedgehog (Shh) [8]. Within tumors, CAFs then establish sub-populations with functionally heterogenous and disparate roles which influence immune, vascular and cancer cells [9]. Reciprocal cell-cell interactions also shape the biology of CAFs within tumors. For example, transforming growth factor β (TGF-β) produced by both malignant and non-malignant cells promotes CAF activation and their formation of filopodia which in turn, facilitate CAF cell migration within tissues [10]. In essence, TGF-β triggers CAFs to walk toward cancer cells. Bi-directional communications between CAFs and cancer cells then confer a survival advantage to cancer cells that aspire to metastasize to distant organs. For instance, CAFs activated by TGF-β produce IL-11 and in doing so, trigger signal transducer and activation of transcription 3 (STAT3) signaling in cancer cells to support their metastatic potential [11]. Overall, CAFs instruct cancer cells to acquire an invasive and proliferative phenotype [12].
The extracellular matrix deposited within tumors influences the metastatic ability of cancer cells. Activated CAFs facilitate formation of this desmoplastic reaction through secretion of collagen, fibronectin, glycosaminoglycans and proteoglycans. Fibroblasts also produce lysyl oxidase (LOX) which acts to crosslink and stiffen collagen fibers in the tumor microenvironment and in doing so, increases integrin signaling supportive of cancer cell invasiveness [13]. The dense extracellular matrix in PDAC is also rich in hyaluronan, a large linear glycosaminoglycan produced by cancer cells and capable of generating remarkably high interstitial fluid pressures [14]. Notably, increased interstitial fluid pressure influences the transcriptome of malignant cells and in doing so, triggers cancer cell proliferation, invasion, and metastasis [15]. Hyaluronan also promotes cancer cell migration [16]. However, depletion of components of the extracellular matrix may paradoxically endow PDAC with increased metastatic ability [17]. This observation illustrates the remarkable complexity of determinants of the metastatic process and suggests that the stromal compartment may possess both pro- and anti-metastatic properties.
2.2. Signals that promote cancer cell invasion and migration
Cancer cells are engendered with metastatic potential during epithelial to mesenchymal transition (EMT). EMT is a process by which epithelial cells acquire migratory and invasive properties characteristic of mesenchymal cells. During this process, cancer cells lose their apical-basal cell polarity and detach from the basement membrane. Acquisition of mesenchymal properties is associated with downregulation of cell adhesion molecules (e.g. E-cadherin) and gain of a migratory phenotype [18]. In PDAC, EMT has been detected at the earliest stages of carcinogenesis [18,19]. A variety of signals produced within a cell's surrounding microenvironment instruct cancer cells to undergo EMT. For example, cytokines, including TGF-β and leukaemia inhibitory factor (LIF), that are produced by both PSCs and cancer cells, promote EMT [20]. In addition, EMT may be triggered via TGF-β independent mechanisms including hypoxia and pro-inflammatory cytokines (e.g. macrophage migration inhibitory factor, MIF) [21,22]. In vivo studies have shown that the EMT process is intricately regulated, yet fundamental to metastasis. For instance, whereas deletion of Twist or Snail, two principal transcription factors responsible for EMT, was found to be dispensable for PDAC metastasis [23], deletion of zinc finger E-box-binding homeobox 1 (Zeb1) not only impaired the EMT process but also reduced the metastatic ability of cancer cells [24]. Non-redundant subfunctions of transcription factors involved in EMT may explain this biology [24]. Nonetheless, these findings support a role for cellular plasticity in defining the metastatic ability of pancreatic cancer cells.
After detachment from the basement membrane, cancer cells invade and migrate through their surrounding stroma in search of endothelial and lymphatic vasculature. This process is facilitated by genetic drivers of pancreatic cancer including KRAS, p16INK4A, TP53, and SMAD4 [25]. For example, oncogenic Kras activation endows tumor necrosis factor (TNF)-related apoptosis-inducing ligand receptor (TRAIL-R) with the capacity to trigger activation of the Rac1/phosphoinositide 3-kinase (PI3K) signaling axis that then increases the migratory capacity and invasiveness of pancreatic cancer cells in a cell-autonomous manner [26]. Deletion of the Ink4a/ARF locus may then cooperate with Kras activation to promote metastasis [27]. In addition, mutations in Tp53 can induce the expression of platelet-derived growth factor receptor beta (PDGFRβ) on cancer cells through a cell-autonomous mechanism. Activation of PDFGRβ by PDGF enhances pancreatic cancer cell invasiveness [28]. Homozygous loss of Dpc4/Smad4 may also influence the metastatic ability of cancer cells. Loss of Dpc4 signaling triggers expression of the transcription factor Runx3 which slows proliferation, but also endows cancer cells with increased migratory capacity and the ability to produce matrix constituents supportive of metastasis [29]. Epigenomic modifications that arise during cancer cell evolution may also contribute in a cell-intrinsic manner to the metastatic ability of cancer cells [30]. Similarly, metastatic ability acquired during disease progression has been linked to alterations in the activity of enhancers, a class of regulatory DNA elements that regulate transcription over large genomic distances [31]. Together, these data implicate a role for genetic alterations in directing the metastatic ability of pancreatic cancer cells.
Driver gene mutations associated with metastasis show remarkable uniformity among different lesions in patients with PDAC [32,33]. This observation implies that the metastatic ability of cancer cells may be conferred by few genetic alterations. As such, multiple sub-clones derived from a primary tumor may undergo the metastatic process [34]. However, the remarkable inefficiency of metastasis predicts that additional factors are required for successful seeding of clones in distant organs. For example, mouse models suggest that cancer cell sub-clones may cooperate during metastasis [35]. As such, cancer cells may metastasize as cell clusters, a strategy that appears to enhance their metastatic colonization in distant tissues [34], [35], [36].
2.3. A permissive tumor microenvironment that supports metastasis
Inflammation is a hallmark of cancer and serves as a major cell-extrinsic determinate of cancer cell metastasis. For example, STAT3 is a key mediator of cancer inflammation and enforces cancer cell expression of matrix metalloproteinase-7 (MMP-7) which then supports cancer cell invasion [37]. Accordingly, induction of pancreatitis, which drives Stat3 activation in PDAC, increases pancreatic cancer cell intravasation into the bloodstream [19,38]. Within the tumor microenvironment, inflammatory cells contribute to this finding such that blocking inflammatory cell recruitment to tumors reduces the metastatic potential of PDAC. For instance, disruption of neutrophil recruitment to primary tumors by genetic ablation or inhibition of CXC chemokine receptor 2 (CXCR2) suppresses metastasis in mouse models of pancreatic cancer [39].
Within the tumor microenvironment, macrophages represent the dominant immune cell component. Tumor-infiltrating macrophages can be obligate partners for tumor cell invasion and as such, they migrate with cancer cells through the stroma in search of endothelium. A paracrine signaling loop between macrophages and malignant cells involving colony stimulating factor 1 (CSF1) produced by malignant cells and epidermal growth factor (EGF) produced by macrophages supports this co-migration [40]. In addition, macrophages produce cathepsins, proteases involved in the processing and activation of growth factors and transcription factors, that may then support the invasiveness of cancer cells [41]. Consistent with this, pharmacologic inhibition of macrophages decreases metastasis formation during spontaneous development of PDAC [42]. Thus, tumor-extrinsic signals may enable the invasive ability of cancer cells.
2.4. Signals that promote tumor cell intravasation into the bloodstream
For cancer cells that successfully traverse the stromal compartment and encounter tumor endothelium, additional coordinating signals are necessary for their intravasation into the bloodstream. Macrophages in the stroma may be instructors of this key step in metastasis. For example, macrophages cooperate with endothelial cells to orchestrate tumor microenvironments of metastasis (TMEMs), which is a triad of a macrophage, a cancer cell and an endothelial cell [43]. The formation of TMEMs is reliant on macrophage recruitment to the tumor by C-C chemokine receptor type 2 (CCR2) signaling [44]. CCL2 is a ligand for CCR2 and is produced by both cancer cells and stromal cells [45] Inhibition of CCR2 in mouse models of PDAC blocks monocyte recruitment to tumors and prevents liver metastasis [46,47]. Macrophages recruited to tumors are attracted to the perivascular space in a CXCR4-dependent manner through C-X-C motif chemokine ligand 12 (CXCL12) produced by perivascular fibroblasts [44,48]. In PDAC, CXCL12 may also attract cancer cells; support their survival; and enhance their invasiveness [49,50]. Cancer cells follow macrophages into the perivascular niche under the support of macrophage production of Wnt family member 1 (Wnt1), which disrupts E-Cadherin junctions [51]. In doing so, cancer cells associate with both endothelial cells and macrophages to form TMEMs, which promote vascular leakiness and facilitate cancer cell intravasation into the bloodstream.
Hematogenous dissemination of cancer cells is commonly considered a late event in cancer progression. For instance, this process was thought to not occur from precursors lesions of invasive carcinoma where cells remain attached to their basement membrane. However, accumulating evidence suggest that pancreas epithelial cells may undergo this process even at the earliest stages of cancer conception [19]. In a preclinical model of PDAC, epithelial cells originating from pre-cancerous pancreatic intraepithelial neoplasia (PanIN) lesions were found to detach from the basement membrane, undergo EMT, invade into the surrounding stroma, and intravasate into the bloodstream. This process was accentuated by inflammation induced by the secretagogue cerulean or following pancreatic duct ligation [19]. Remarkably, single epithelial cells were also detected in the liver suggesting that epithelial cells arising from precancerous lesions can successfully complete the metastatic journey even prior to progression to invasive carcinoma. Similar findings have been found in patients with precancerous cystic lesions of the pancreas who have no evidence of tumor or metastasis [52]. Although circulating pancreatic epithelial cells originating from PanIN lesions are not competent to form colonies [19], the ultimate fate of circulating pancreas epithelial cells harboring mutations in genetic drivers (e.g. Kras and Trp53) remains unclear. In patients with PDAC undergoing surgical resection, detection of circulating tumor cells in the blood is an independent predictor of tumor recurrence [53]. Accordingly, the evolution of pancreatic epithelial cells with invasive and metastatic ability has been proposed as a step-wise progression that spans many years [54,55]. However, tracking DNA copy number changes and their associated rearrangements suggests that for some tumors this process may proceed as a cataclysmic event [56]. For example, chromothripsis, a phenomenon by which many clustered chromosomal rearrangements occur in a single event, has been implicated as a mechanism involved in conferring PDAC with both invasive and metastatic ability. Thus, metastasis in PDAC may, at least in some cases, be an early rather than late event in cancer progression.
3. Immune evasion and the pro-metastatic niche of distant organs
Upon escaping from the primary tumor, cancer cells must endure a variety of stresses as they attempt to reach the promised pro-metastatic niche in a distant organ. During this process, DCCs must surmount mechanical stresses within the bloodstream. They must also evade immune recognition and elimination. Finally, they must become lodged within and navigate through a foreign microenvironment to seed and ultimately, colonize distant tissues. This process of Darwinian selection for DCCs with metastatic ability is highly regulated. Here, we discuss a role for cancer cell-extrinsic factors, including the immune system and the permissiveness of the distant organ niche, in defining the metastatic ability of DCCs.
3.1. Immune evasion is a prerequisite for metastasis
The exodus of cancer cells from the primary tumor exposes them to immune recognition and potential elimination. Thus, DCCs must possess strategies to evade the immune system [57]. To this end, DCCs which become lodged within distant organs must escape phagocytic clearance by macrophages, which may act as the first line infantry to protect distant organs from malignant cells. This evasion can be mediated through the upregulation of “don't eat me” signals on tumor cells, including CD47 and CD24 [58,59]. These molecules signal through receptors on macrophages, namely signal regulatory protein α (SIRPα) and Siglec-10 respectively, to inhibit phagocytosis. However, the role of these molecules in regulating PDAC metastasis remains ill-defined, although they could represent key targets for intervening on the metastatic process.
In addition to evading elements of the innate immune system, DCCs must also avoid elimination by tumor-specific T cells. In patients, with advanced PDAC, the presence of tumor-specific T cells correlates with improved outcomes [60,61]. The importance of T cells in preventing metastasis is also supported by preclinical models. For example, tumors infiltrated by T cells display decreased metastatic potential that can be reversed by T cell depletion [62]. Multiple factors may determine the capacity of T cells to infiltrate pancreatic cancer, including cancer cell-derived factors such as CXCL1 and GM-CSF which coordinate the recruitment of immunosuppressive myeloid cell populations [62,63]. Nonetheless, T cell infiltration associates with a decreased likelihood for metastatic or local recurrence in patients with surgically-resected PDAC [57]. Indeed, it is likely that T cells may intervene at multiple stages of the metastatic process. For instance, although T cells are commonly found trapped within the stromal compartment that surrounds PDAC [64], this may be advantageous to eliminating cancer cells as they attempt to invade their surrounding stroma and intravasate into the bloodstream. In addition, circulating T cells may limit the likelihood of initial seeding and colonization by DCCs [65]. Finally, T cells may slow tumor outgrowth of DCCs that successfully seed by directing micro-metastases into a state of cellular dormancy [66]. However, it currently remains unclear how to effectively leverage T cell immunosurveillance for the treatment of PDAC [57].
3.2. The pro-metastatic niche
Seeding and colonization by DCCs in a distant organ is the final stage of the metastatic process. During cancer development, host organs respond to inflammatory signals released in the setting of cancer development by triggering formation of a pro-metastatic niche environment. This niche acts as the “soil” for the seeding of DCCs. Formation of a pro-metastatic niche in distant organs is guided by specific paracrine signals which we discuss below. These mechanisms, which may differ between organs, ultimately converge on central themes that define fundamental features of the niche. For example, the pro-metastatic niche is characterized by an increased presence of myeloid cells, including macrophages as well as neutrophils, and is associated with increased deposition of matrix proteins (e.g. fibronectin and type I collagen), which together establish the “soil” [67]. The nature of the niche must then support DCC evasion of the immune system; provide the necessary factors to enable DCC seeding; and trigger DCC colonization and outgrowth. Thus, elements of the pro-metastatic niche that forms in distant organs may regulate the efficiency of the metastatic process.
The liver is the most common distant organ site of metastasis in PDAC. This propensity for DCCs to seed the liver cannot merely be explained by direct vascular drainage from the pancreas via the portal vein. To this end, elegant mouse and human studies support the concept that PDAC development triggers formation of a pro-metastatic niche in the liver. Hepatocytes, tissue inhibitor matrix metalloproteinase 1 (TIMP1), and tumor-derived exosomes have each been identified as proponents of niche formation [68], [69], [70]. As a result, it is likely that several paracrine mechanisms may trigger the development of a pro-metastatic niche and as result, offer PDAC with multiple strategies for increasing its likelihood for successful metastasis.
During PDAC development, non-malignant stromal cells produce IL-6 which is released into the portal vein and triggers hepatocyte activation [68]. Hepatocytes respond to IL-6 by activating the Stat3 signaling pathway and producing acute phase reactants, including serum amyloid A (SAA) proteins, which then facilitate both the release of myeloid chemoattractants (e.g. S100a8 and S100a9) to recruit myeloid cells to the tumor and the deposition of extracellular matrix proteins (e.g. fibronectin). In patients with PDAC, this signaling pathway is activated in the liver and correlates with metastasis and poor overall survival [68]. Disengaging any of the elements of the IL-6/STAT3/SAA signaling pathway prevents formation of a pro-metastatic niche in the liver in the setting of PDAC development. Interestingly, this pathway is not responsible for formation of a pro-metastatic niche environment in the lung, another common site of PDAC metastasis [68]. This sobering finding implicates a role for organ-specific programs that coordinate the pro-metastatic niche in distinct tissues.
Alternative pathways may also trigger formation of a pro-metastatic niche in the liver. For example, tumor-derived exosomes can prepare the pro-metastatic niche of distant organs by fusing with resident cells (e.g. lung fibroblasts and epithelial cells) and liver macrophages based on integrin expression patterns unique to the exosomes [71]. Integrin subtypes expressed on tumor-derived exosomes also predict the preferred site of metastasis [71]. For example, integrins α4β6 and α6β1 associate with lung metastasis and integrin αvβ5 correlates with liver metastasis. In PDAC, tumor-derived exosomes expressing MIF instruct liver macrophages (Kupffer cells) to release TGF-β to facilitate fibronectin production by hepatic stellate cells [69]. Fibronectin deposits then support the recruitment of inflammatory cells including bone marrow-derived macrophages and neutrophils. In doing so, exosomes trigger formation of a pro-metastatic niche in the liver. Notably, MIF levels were higher in exosomes collected from patients with PDAC who experienced disease progression after diagnosis compared to patients with no evidence of disease five years after diagnosis. Together, these data imply a role for exosomes in directing the metastatic potential of PDAC.
TIMP1 also demonstrates the capacity to coordinate orchestration of a pro-metastatic niche in the liver in a paracrine manner. TIMP1 is produced by cancer cells and is a known activation marker of hepatic stellate cells [70]. In genetic models of PDAC, hepatic stellate cells respond to TIMP1 binding to its receptor, CD63, which signals through PI3K and triggers release of cytokines (e.g. CXCL12) and growth factors that modulate the hepatic microenvironment and attract neutrophils. In doing so, TIMP-1 directs formation of a pro-metastatic niche which becomes detectable during the PanIN stage of cancer development but is significantly more pronounced with the emergence of invasive carcinoma. In patients with PDAC, TIMP1 correlates with a poor prognosis [72].
While the liver is the most common organ involved in PDAC, cancer cells can also spread to lymph nodes, lung, and peritoneum. In fact, although less common, metastasis has also been detected to the bones, brain, and skin illustrating the remarkable ability of DCCs in PDAC to adapt to a range of distant organ microenvironments. The niche environment that forms in distinct organs may be directed by organ-specific mechanisms. For example, blockade of IL-6 signaling inhibits formation of a pro-metastatic niche in the liver but is unable to prevent development of a pro-metastatic niche in the lung [68]. However, similar to the liver, the lung pro-metastatic niche is orchestrated by chemoattractants (e.g. S100A8 and S100A9) which lure myeloid cells into the lung parenchyma [73]. DCCs that become lodged within the lung vasculature recruit inflammatory monocytes to support their extravasation [45]. Cancer cells may also activate myeloid cells via release of versican, an extracellular matrix proteoglycan, which signals through Toll-like receptor 2 (TLR2) on macrophages, leading to TNF release which promotes lung metastasis [74]. Cancer cells also secrete myeloid chemoattractants, including CXCL1 and CCL2, which may contribute to orchestration of a niche environment supportive of cancer cell seeding and outgrowth [39,47]. However, it is likely that metastatic organotropism is not only defined by a receptive niche within a distant organ but also cancer cell predilection for that niche. For example, epithelial plasticity defined by p120 catenin can toggle cancer cell preference for the liver or lung [75]. Nonetheless, sustained inflammation within distant organs appears central to defining organ receptiveness to cancer cell metastasis.
3.3. The fate of DCCs in the distant organ niche and their awakening
DCCs that successfully seed a distant organ must decide whether to enter a state of dormancy or to attempt the process of colonization and outgrowth. This decision is regulated by both cancer cell-intrinsic and -extrinsic mechanisms. Whereas EMT supports invasion and intravasation of DCCs into the bloodstream, mesenchymal-to-epithelial transition (MET) promotes colonization by DCCs through stimulating proliferation and the expression of adhesive junctions that communicate with the surrounding niche [76]. In PDAC, DCCs that are locked into a mesenchymal state are capable of reaching a distant organ but fail to colonize [24]. From studies in genetic models of PDAC, two major isoforms of the paired-related homeodomain transcription factor 1 (Prrx1), Prxx1a and Prxx1b, have been implicated in reciprocal regulation of EMT and MET [77]. In addition, in the absence of a receptive niche in the liver, DCCs appear to initially enter a state of cellular dormancy as defined by decreased cellular proliferation [68]. Together, micro-metastatic dormancy may constitute a temporary barrier to metastatic colonization.
The cues that regulate cellular dormancy in PDAC remain ill-defined. Findings from other cancer histologies suggest that dormancy may be triggered in response to inhibitory signals originating from the parenchyma of the metastatic organ but may also reflect a lack of stimulatory signals that provoke their awakening. For example, neovascularization can support reactivation of dormant cells leading to metastatic outgrowth [78]. Further, chronic inflammation may stimulate neutrophil recruitment and in doing so, awaken dormant cancer cells by remodeling their surrounding extracellular matrix [5]. In contrast, immunosurveillance may lull DCCs into a state of cellular dormancy [66]. In a PDAC model of liver metastasis, age-associated changes in liver inflammation were found to support DCC colonization over cellular dormancy [79]. Together, these findings indicate a complex set of cellular communications that converge to inform the decision of a DCC to become dormant or to colonize.
Within a distant organ, metastatic colonization proceeds through a series of defined steps in which cancer cells assume an epithelial cell phenotype and begin to orchestrate a microenvironment that facilitates their outgrowth [80]. This process involves recruitment of haematopoietic (e.g. macrophages, neutrophils) and non-haematopoeitic (e.g. CAFs and endothelial cells) cells that support the formation of micro-metastases. Micro-metastases are rapidly infiltrated by macrophages which are recruited from the bone marrow and precede the accumulation of activated myofibroblasts [81]. Blockade of monocyte or neutrophil recruitment to the liver not only prevents the formation of a pro-metastatic niche but also impairs the outgrowth of micrometastases [39,47,82]. Macrophages that are recruited to metastatic foci in the liver secrete granulin to facilitate myofibroblast activation. In turn, myofibroblasts respond by releasing periostin which triggers formation of a fibrotic microenvironment that supports metastatic colonization [81]. In the absence of granulin production by macrophages, micro-metastases are coaxed into a state of cellular dormancy. Thus, the immune microenvironment that is recruited to DCCs may be fundamental to defining their ultimate cellular fate.
4. Outlook and perspective
Metastasis is a hallmark of cancer, not just pancreatic cancer. For the past 20 years, outcomes for patients with metastasis have remained relatively stable [83]. Notably, despite significant advancements in our understanding of metastasis, it is remarkable that there are no FDA approved drugs designed to specifically undermine the metastatic process. This failure to translate basic science to the clinic reflects multiple reasons including the diversity of ways that cancer cells may seek to metastasize, the complexity of the metastatic process, and challenges in demonstrating the success of a therapeutic that specifically derails metastasis. Certainly, targeting cancer cell-intrinsic features that define metastastic ability is daunting. This is due to the inherent genomic instability associated with cancer cells, and thus, their capacity to readily evolve resistance mechanisms to therapeutics targeting cell-intrinsic mechanisms of metastasis. However, manipulating cancer cell-extrinsic features, such as the pro-metastatic niche and the immune system, which are genomically stable elements of the metastatic process, introduces new therapeutic opportunities. Indeed, the immune system is central to nearly every step of metastasis.
The metastatic process is complicated and informed by many cues, some of which may even be redundant. As such, multiple factors may affect or contribute to the success or failure of mestastasis which lends to this complexity. In addition, metastatic outcomes may be confounded by many often unmeasured variables, including the microbiome, concomitant medications, lifestyle (i.e. stress, sleep, exercise), co-morbidities, underlying infections, and genetics. However, it is possible that many or all of these variables converge on central themes, such as distant organ biology and the immune system, that ultimately govern the metastatic ability of cancer. As a result, preventing metastasis may require a focus away from the cancer cell and toward the host. This reasoning is consistent with the assertion made by Sir William Osler that “The good physician treats the disease; the great physician treats the patient who has the disease.” To this end, the advent of immunotherapy is based on leveraging the capacity of the immune system to distinguish, with exquisite specificity, self from non-self [84]. This approach represents a leap of faith that strategies targeting factors extrinsic to neoplastic cells can intervene on cancer progression. Similarly, therapeutic measures to disrupt the formation of niche environments in common metastatic sites pose a diversion away from the cancer cell and a focus on cancer cell-extrinsic factors that are essential for metastasis.
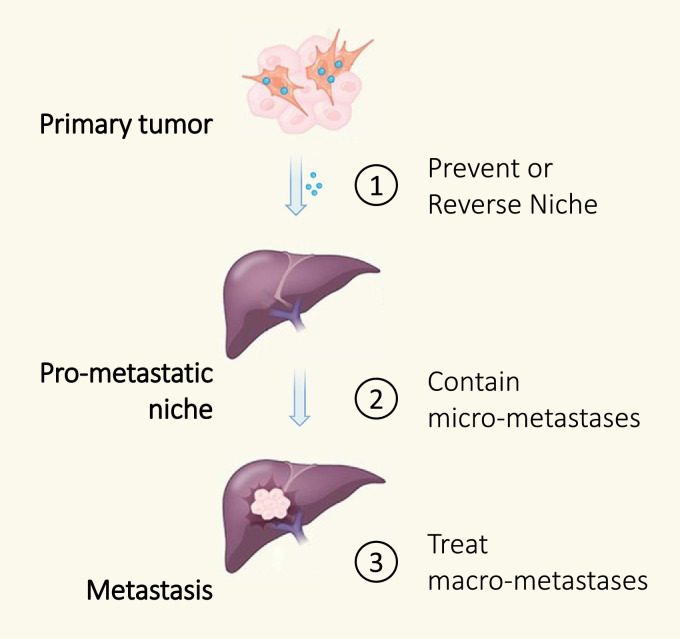
The objective of understanding the metastatic process in cancer is to inform biology that may guide the development of strategies to prevent, contain, and treat metastasis (Fig. 3). However, the approach to disrupting the metastatic process will also likely depend on the stage of disease. For example, the goal for patients with localized disease is to prevent metastasis and to contain any subclinical micro-metastases. In contrast, for patients with metastatic disease, the priority will be to contain and treat micro- and macro-metastatic lesions while inhibiting new metastases. As such, therapeutics to intervene on the metastatic process may be studied in distinct ways. For PDAC that is surgically-resectable, this might mean incorporating a short course of neoadjuvant immunotherapy prior to surgical resection, as has been done recently in lung cancer and melanoma [85,86], with the goal to contain the metastatic ability of residual cancer cells in the post-operative setting. Indeed, mouse models support a role for neoadjuvant immunotherapy for improved outcomes [87]. Reversing and preventing re-formation of the pro-metastatic niche in the post-operative setting may then reduce the likelihood of metastatic recurrence by eliminating the triggers that regulate the awakening of subclinical micro-metastatic lesions. For patients with locally-advanced and even metastatic disease, maintenance strategies designed to reverse pro-metastatic biology in distant organs and strategies that trigger productive T cell immunosurveillance may be needed to contain micro- and macro-metastases while inhibiting the seeding and colonization by a continuous pool of cancer cells attempting to undergo metastasis.
Fig. 3.
Approaches for intervening on the metastatic process. Shown is a schematic depicting the formation of a pro-metastatic niche in a distant organ (the liver is displayed as an example). This process can be facilitated by tumor-derived factors which initiate formation of the niche environment and in doing so, support seeding and colonization by disseminated cancer cells. The metastatic process can be derailed in several ways: Approach 1, prevent or reverse the niche formation in a distant organ; Approach 2, contain micro-metastases to prevent their outgrowth; and Approach 3, treat macro-metastases.
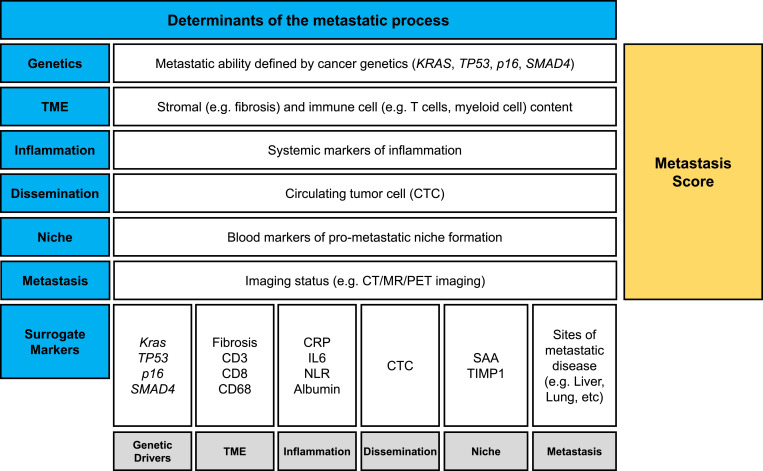
Distant recurrence after surgical resection of PDAC is common. However, metastasis is not universal and for some patients, long-term survival without recurrence can be seen. Moreover, the pattern of organ involvement (e.g. liver, lung, peritoneal) and the extent of metastatic spread (i.e. few or many lesions) can vary significantly between patients. We propose that assigning a relative risk for metastasis, a so-called metastasis score, based on disease and host characteristics might help to stratify patients who would most benefit from therapeutic strategies that target the metastatic cascade (Fig. 4). Similar scoring approaches, based on Kras mutation, tumor markers, and disease burden, have been proposed as a means to predict survival outcomes in patients with liver metastasis [88,89]. However, we envision the metastasis score as a way to also potentially inform each of the stages of the metastatic process so that it might even be used as a means for monitoring the success of interventions designed to resolve pro-metastatic elements and enforce anti-metastatic mechanisms. For example, a liver niche microenvironment supportive of cancer metastasis in PDAC is associated with increased production of SAA proteins [68]. Notably, SAA can be readily detected in the peripheral blood and therefore, might be a useful measure of the likelihood of a pro-metastatic niche in the liver.
Fig. 4.
Graphical representation of determinants of the metastatic process. Metastasis is determined by multiple cancer cell-intrinsic and -extrinsic properties that converge to establish the metastatic ability of pancreatic cancer cells. These determinants include cancer cell genetics, properties of the tumor microenvironment, systemic inflammation, evidence of cancer cell dissemination based on peripheral blood detection of circulating tumor material, the presence of pro-metastatic niche environments in distant organs, and detection of macro-metastatic lesions by computed tomography, magnetic resonance, and positron emission tomography imaging. Together, these surrogate markers combine to create a proposed metastasis score, or likelihood of distant spread of disease. Abbreviations: CRP, c-reactive protein; CT, computed tomography; CTC, circulating tumor cell; CTM, circulating tumor material; IL, interleukin; MR, magnetic resonance; NLR, neutrophil-to-lymphocyte ratio; PET, positron emission tomography; SAA, serum amyloid A; TIMP1, tissue inhibitor of metalloproteinase 1; TME, tumor microenvironment.
The time is ripe for translating strategies that specifically seek to target the metastatic cascade in PDAC. Metastasis portends a poor prognosis and thus, intervening on the metastatic cascade in patients with localized disease holds promise given the heightened risk for metastatic relapse in this patient population. Continued investigations into the determinants governing the formation and maintenance of pro-metastatic niche environments in distant organs will be integral to the design of novel therapeutic interventions. Moreover, validation of markers informative of the steps of the metastatic cascade may aid in the evaluation of these treatments. Certainly, disrupting the metastatic process in PDAC would dramatically impact patient outcomes.
4.1. Outstanding questions
In the realm of pancreatic cancer metastasis, many mechanisms still remain elusive. Some particularly sparse areas of the collective literature include mechanisms by which PDAC cells intravasate into blood vessels and traffic to distant organs. Moreover, the mechanisms that promote dormant tumor cell awakening remain ill-defined. In addition, the potential to shift tumors from an active to dormant state is unclear but could have dramatic therapeutic implications for patients. Sophisticated mouse models have provided new insights into the metastatic process in pancreatic cancer. Translation of this biology to the clinic, though, has lagged behind which reflects, at least in part, the complexity of human pancreatic cancer. However, with the goal of improving patient outcomes, ongoing questions naturally lean toward how past and future research findings will translate into rational therapeutic strategies. In many cases, clinical grade drugs exist that could be leveraged against individual steps within the metastatic cascade. To this end, future work in preclinical models focusing on the metastatic process could serve as a guide for clinical trial design.
Declaration of Competing Interest
G.L.B. is a consultant/advisory board member for Seattle Genetics, Aduro Biotech, AstraZeneca, Bristol-Myers Squibb, Incyte, Genmab, Takeda, Merck, and BiolineRx; reports receiving commercial research grants from Incyte, Bristol-Myers Squibb, Verastem, Halozyme, Biothera, Arcus, Newlink, Novartis, and Janssen; and is an inventor of intellectual property and recipient of royalties from Novartis and Advaxis, Inc. No potential conflicts of interest were disclosed by the other authors.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health grants R01 CA197916 (G.L.B.), R01 CA245323 (G.L.B.), T32 HL007439 (S.K.T.), and U01 CA224193 (G.L.B), the 2017 Stand Up to Cancer (SU2C) Innovative Research Grant SU2C-AACR-IRG 13-17 (G.L.B.) and grant support from the Robert L. Fine Cancer Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Robert L. Fine Cancer Research Foundation.
Search strategy and selection criteria
Data for this review were identified by searches of PubMed and references from relevant articles. Keyword search terms included “pancreatic cancer”, “metastasis”, “invasion”, “pre-metastatic”, “pro-metastatic”, “niche”, and “immune”. Articles published in English between 1997 and 2019 were included.
Footnotes
This is the second in a Series of four papers about pancreatic cancer (For the other papers in this Series see www.thelancet.com/series/pancreatic-cancer).
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Sperti C., Pasquali C., Piccoli A., Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21(2):195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 5.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409) doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen D.X., Bos P.D., Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 7.Haeno H., Gonen M., Davis M.B., Herman J.M., Iacobuzio-Donahue C.A., Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148(1–2):362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olive K.P., Jacobetz M.A., Davidson C.J., Gopinathan A., McIntyre D., Honess D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A.S., Ponz-Sarvise M. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collazo J., Zhu B., Larkin S., Martin S.K., Pu H., Horbinski C. Cofilin drives cell-invasive and metastatic responses to TGF-beta in prostate cancer. Cancer Res. 2014;74(8):2362–2373. doi: 10.1158/0008-5472.CAN-13-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calon A., Espinet E., Palomo-Ponce S., Tauriello D.V., Iglesias M., Cespedes M.V. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22(5):571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ligorio M., Sil S., Malagon-Lopez J., Nieman L.T., Misale S., Di Pilato M. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell. 2019;178(1):160–175. doi: 10.1016/j.cell.2019.05.012. e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldin C.H., Rubin K., Pietras K., Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 16.Cheng X.B., Kohi S., Koga A., Hirata K., Sato N. Hyaluronan stimulates pancreatic cancer cell motility. Oncotarget. 2016;7(4):4829–4840. doi: 10.18632/oncotarget.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aiello N.M., Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016–1026. doi: 10.1084/jem.20181827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhim A.D., Mirek E.T., Aiello N.M., Maitra A., Bailey J.M., McAllister F. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y., Gao W., Lytle N.K., Huang P., Yuan X., Dann A.M. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature. 2019;569(7754):131–135. doi: 10.1038/s41586-019-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M.H., Wu M.Z., Chiou S.H., Chen P.M., Chang S.Y., Liu C.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 22.Yang S., He P., Wang J., Schetter A., Tang W., Funamizu N. A novel mif signaling pathway drives the malignant character of pancreatic cancer by targeting NR3C2. Cancer Res. 2016;76(13):3838–3850. doi: 10.1158/0008-5472.CAN-15-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X., Carstens J.L., Kim J., Scheible M., Kaye J., Sugimoto H. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs A.M., Mitschke J., Lasierra Losada M., Schmalhofer O., Boerries M., Busch H. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 25.Jones S., Zhang X., Parsons D.W., Lin J.C., Leary R.J., Angenendt P. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Karstedt S., Conti A., Nobis M., Montinaro A., Hartwig T., Lemke J. Cancer cell-autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer Cell. 2015;27(4):561–573. doi: 10.1016/j.ccell.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguirre A.J., Bardeesy N., Sinha M., Lopez L., Tuveson D.A., Horner J. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17(24):3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissmueller S., Manchado E., Saborowski M., Morris J.P.t., Wagenblast E., Davis C.A. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell. 2014;157(2):382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittle M.C., Izeradjene K., Rani P.G., Feng L., Carlson M.A., DelGiorno K.E. RUNX3 controls a metastatic switch in pancreatic ductal adenocarcinoma. Cell. 2015;161(6):1345–1360. doi: 10.1016/j.cell.2015.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald O.G., Li X., Saunders T., Tryggvadottir R., Mentch S.J., Warmoes M.O. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet. 2017;49(3):367–376. doi: 10.1038/ng.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roe J.S., Hwang C.I., Somerville T.D.D., Milazzo J.P., Lee E.J., Da Silva B. Enhancer reprogramming promotes pancreatic cancer metastasis. Cell. 2017;170(5):875–888. doi: 10.1016/j.cell.2017.07.007. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makohon-Moore A.P., Zhang M., Reiter J.G., Bozic I., Allen B., Kundu D. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet. 2017;49(3):358–366. doi: 10.1038/ng.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiter J.G., Makohon-Moore A.P., Gerold J.M., Heyde A., Attiyeh M.A., Kohutek Z.A. Minimal functional driver gene heterogeneity among untreated metastases. Science. 2018;361(6406):1033–1037. doi: 10.1126/science.aat7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddipati R., Stanger B.Z. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 2015;5(10):1086–1097. doi: 10.1158/2159-8290.CD-15-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aceto N., Bardia A., Miyamoto D.T., Donaldson M.C., Wittner B.S., Spencer J.A. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung K.J., Gabrielson E., Werb Z., Ewald A.J. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155(7):1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuda A., Wang S.C., Morris J.P.t., Folias A.E., Liou A., Kim G.E. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19(4):441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesina M., Kurkowski M.U., Ludes K., Rose-John S., Treiber M., Kloppel G. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Steele C.W., Karim S.A., Leach J.D., Bailey P., Upstill-Goddard R., Rishi L. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Gocheva V., Wang H.W., Gadea B.B., Shree T., Hunter K.E., Garfall A.L. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24(3):241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griesmann H., Drexel C., Milosevic N., Sipos B., Rosendahl J., Gress T.M. Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut. 2016;66(7):1278–1285. doi: 10.1136/gutjnl-2015-310049. [DOI] [PubMed] [Google Scholar]
- 43.Robinson B.D., Sica G.L., Liu Y.F., Rohan T.E., Gertler F.B., Condeelis J.S. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15(7):2433–2441. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arwert E.N., Harney A.S., Entenberg D., Wang Y., Sahai E., Pollard J.W. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23(5):1239–1248. doi: 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchem J.B., Brennan D.J., Knolhoff B.L., Belt B.A., Zhu Y., Sanford D.E. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanford D.E., Belt B.A., Panni R.Z., Mayer A., Deshpande A.D., Carpenter D. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19(13):3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harney A.S., Arwert E.N., Entenberg D., Wang Y., Guo P., Qian B.Z. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived vegfa. Cancer Discov. 2015;5(9):932–943. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori T., Doi R., Koizumi M., Toyoda E., Ito D., Kami K. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3(1):29–37. [PubMed] [Google Scholar]
- 50.Marchesi F., Monti P., Leone B.E., Zerbi A., Vecchi A., Piemonti L. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64(22):8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 51.Linde N., Casanova-Acebes M., Sosa M.S., Mortha A., Rahman A., Farias E. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun. 2018;9(1):21. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhim A.D., Thege F.I., Santana S.M., Lannin T.B., Saha T.N., Tsai S. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146(3):647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poruk K.E., Blackford A.L., Weiss M.J., Cameron J.L., He J., Goggins M. Circulating tumor cells expressing markers of tumor-initiating cells predict poor survival and cancer recurrence in patients with pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23(11):2681–2690. doi: 10.1158/1078-0432.CCR-16-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makohon-Moore A.P., Matsukuma K., Zhang M., Reiter J.G., Gerold J.M., Jiao Y. Precancerous neoplastic cells can move through the pancreatic ductal system. Nature. 2018;561(7722):201–205. doi: 10.1038/s41586-018-0481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yachida S., Jones S., Bozic I., Antal T., Leary R., Fu B. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Notta F., Chan-Seng-Yue M., Lemire M., Li Y., Wilson G.W., Connor A.A. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature. 2016;538(7625):378–382. doi: 10.1038/nature19823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balachandran V.P., Beatty G.L., Dougan S.K. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 2019;156(7):2056–2072. doi: 10.1053/j.gastro.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barkal A.A., Brewer R.E., Markovic M., Kowarsky M., Barkal S.A., Zaro B.W. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu M., O'Connor R.S., Trefely S., Graham K., Snyder N.W., Beatty G.L. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated 'don't-eat-me' signal. Nat Immunol. 2019;20(3):265–275. doi: 10.1038/s41590-018-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balachandran V.P., Luksza M., Zhao J.N., Makarov V., Moral J.A., Remark R. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551(7681):512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lutz E.R., Wu A.A., Bigelow E., Sharma R., Mo G., Soares K. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2(7):616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J., Byrne K.T., Yan F., Yamazoe T., Chen Z., Baslan T. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity. 2018;49(1):178–193. doi: 10.1016/j.immuni.2018.06.006. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bayne L.J., Beatty G.L., Jhala N., Clark C.E., Rhim A.D., Stanger B.Z. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21(6):822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beatty G.L., Eghbali S., Kim R. Deploying immunotherapy in pancreatic cancer: defining mechanisms of response and resistance. Am Soc Clin Oncol Educ Book. 2017;37:267–278. doi: 10.1200/EDBK_175232. [DOI] [PubMed] [Google Scholar]
- 65.Kim V.M., Blair A.B., Lauer P., Foley K., Che X., Soares K. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J Immunother Cancer. 2019;7(1):132. doi: 10.1186/s40425-019-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eyles J., Puaux A.L., Wang X., Toh B., Prakash C., Hong M. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120(6):2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y., Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30(5):668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Lee J.W., Stone M.L., Porrett P.M., Thomas S.K., Komar C.A., Li J.H. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567(7747):249–252. doi: 10.1038/s41586-019-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grunwald B., Harant V., Schaten S., Fruhschutz M., Spallek R., Hochst B. Pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells via CD63 signaling to create a premetastatic niche in the liver. Gastroenterology. 2016;151(5):1011–1024. doi: 10.1053/j.gastro.2016.07.043. e7. [DOI] [PubMed] [Google Scholar]
- 71.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poruk K.E., Firpo M.A., Scaife C.L., Adler D.G., Emerson L.L., Boucher K.M. Serum osteopontin and tissue inhibitor of metalloproteinase 1 as diagnostic and prognostic biomarkers for pancreatic adenocarcinoma. Pancreas. 2013;42(2):193–197. doi: 10.1097/MPA.0b013e31825e354d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hiratsuka S., Watanabe A., Aburatani H., Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8(12):1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 74.Kim S., Takahashi H., Lin W.W., Descargues P., Grivennikov S., Kim Y. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reichert M., Bakir B., Moreira L., Pitarresi J.R., Feldmann K., Simon L. Regulation of epithelial plasticity determines metastatic organotropism in pancreatic cancer. Dev Cell. 2018;45(6):696–711. doi: 10.1016/j.devcel.2018.05.025. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao D., Dai C., Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9(12):1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 77.Takano S., Reichert M., Bakir B., Das K.K., Nishida T., Miyazaki M. Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Genes Dev. 2016;30(2):233–247. doi: 10.1101/gad.263327.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghajar C.M., Peinado H., Mori H., Matei I.R., Evason K.J., Brazier H. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lenk L., Pein M., Will O., Gomez B., Viol F., Hauser C. The hepatic microenvironment essentially determines tumor cell dormancy and metastatic outgrowth of pancreatic ductal adenocarcinoma. Oncoimmunology. 2018;7(1) doi: 10.1080/2162402X.2017.1368603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aiello N.M., Bajor D.L., Norgard R.J., Sahmoud A., Bhagwat N., Pham M.N. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat Commun. 2016;7:12819. doi: 10.1038/ncomms12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nielsen S.R., Quaranta V., Linford A., Emeagi P., Rainer C., Santos A. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18(5):549–560. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang H., Hegde S., Knolhoff B.L., Zhu Y., Herndon J.M., Meyer M.A. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22(8):851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steeg P.S. Targeting metastasis. Nat Rev Cancer. 2016;16(4):201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beatty G.L., Gladney W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21(4):687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang A.C., Orlowski R.J., Xu X., Mick R., George S.M., Yan P.K. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. doi: 10.1038/s41591-019-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forde P.M., Chaft J.E., Smith K.N., Anagnostou V., Cottrell T.R., Hellmann M.D. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J., Blake S.J., Yong M.C., Harjunpaa H., Ngiow S.F., Takeda K. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382–1399. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]
- 88.Fong Y., Fortner J., Sun R.L., Brennan M.F., Blumgart L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Margonis G.A., Sasaki K., Gholami S., Kim Y., Andreatos N., Rezaee N. Genetic and morphological evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. 2018;105(9):1210–1220. doi: 10.1002/bjs.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jemal A., Siegel R., Ward E., Murray T., Xu J., Smigal C. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 91.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]