Key Points
Question
How does topical dorzolamide-timolol affect eyes with neovascular age-related macular degeneration with persistent exudation following frequent fixed-interval intravitreal anti–vascular endothelial growth factor (anti-VEGF) injections?
Findings
In this randomized clinical trial, among 50 patients with neovascular age-related macular degeneration receiving frequent anti-VEGF injections, those randomized to use topical dorzolamide-timolol had no better vision compared with placebo but had a greater reduction in central subfield thickness and maximum pigment epithelial detachment height from baseline to approximately 3 months.
Meaning
These findings suggest adjuvant dorzolamide-timolol reduces macular edema in patients with persistent exudation following frequent anti-VEGF injections, although no differences in visual acuity were identified in this short-term trial.
Abstract
Importance
Some eyes with neovascular age-related macular degeneration (AMD) have persistent exudation despite frequent intravitreal anti–vascular endothelial growth factor (VEGF) injections. Adjuvant therapies that further reduce edema may improve vision outcomes.
Objective
To compare the short-term effect of topical dorzolamide-timolol vs placebo in eyes with neovascular AMD that have persistent exudation following intravitreal anti-VEGF injections.
Design, Setting, and Participants
Randomized placebo-controlled clinical trial with enrollment from March 1, 2017, through October 30, 2018. Multicenter trial at 4 clinical sites in the United States. Sixty-three patients with neovascular AMD who had persistent exudation despite intravitreal anti-VEGF injections at 4-week, 5-week, or 6-week intervals.
Interventions
Patients were randomized to use dorzolamide-timolol or artificial tears for the study duration. They continued to receive the same anti-VEGF drug at the same interval as the 2 visits before enrollment for 3 additional study visits.
Main Outcomes and Measures
The primary outcome measure was change in mean central subfield thickness on optical coherence tomography from baseline to visit 3 (approximately 3 months). Secondary measures included change in mean maximum subretinal fluid height, mean maximum pigment epithelial detachment height, and mean visual acuity (VA).
Results
This trial included 52 patients. All 27 patients (100%) assigned to dorzolamide-timolol and 23 of 25 (92%) assigned to placebo were analyzed for the primary outcome. Mean (SD) age was 78.4 (7) years, and 34 of 50 patients (68%) were women. Mean (SD) injections were 20.5 (14) (range, 4-58) before enrollment. Mean (SD) baseline logMAR VA was 0.361 (0.26) (approximate Snellen equivalent, 20/50). Comparing the dorzolamide-timolol with placebo group from baseline to visit 3, mean (SD) change in central subfield thickness (primary outcome) was −36.6 (54) μm vs 1.7 (52.3) μm (difference, 30.8; 95% CI, 0.3-61.3; P = .04); secondary outcomes: maximum PED height was −39.1 (65) μm vs 1.1 (16) μm (difference, 39.6; 95% CI, 9.6-69.6; P = .01) and change in VA from baseline to visit 3 was −2.3 (5) vs 0.3 (1) letters (difference, 2.6 letters; 95% CI, −1.9 to 7.1 letters; P = .78).
Conclusions and Relevance
These findings suggest use of dorzolamide-timolol in patients with neovascular AMD with persistent exudation resulted in anatomic but not visual acuity improvements compared with placebo at approximately 3 months. Additional clinical trials with longer follow-up and larger sample sizes presumably would be needed to determine the role, if any, of dorzolamide-timolol in neovascular AMD.
Trial Registration
ClinicalTrials.gov Identifier: NCT03034772
This randomized clinical trial compares the short-term effect of topical dorzolamide-timolol vs placebo in eyes with neovascular age-related macular degeneration that have persistent exudation following intravitreal anti–vascular endothelial growth factor injections.
Introduction
Intravitreal anti–vascular endothelial growth factor (VEGF) agents are currently the criterion standard for treating neovascular age-related macular degeneration (AMD).1,2,3 Various treatment regimens have been proposed, including monthly injections, pro re nata, and treat and extend.2,4,5 However, some patients have persistent exudation despite frequent injections and may be considered suboptimal responders to anti-VEGF therapy.4,5
Studies have suggested that outflow through the anterior chamber may play a role in clearance of intravitreal anti-VEGF drugs.6,7,8 By decreasing aqueous production, outflow of intraocular fluid may be reduced, which could slow drug clearance. A prior case series of patients with neovascular AMD who had persistent exudation following anti-VEGF suggested that adding topical dorzolamide-timolol (Cosopt; Akorn Inc) decreased exudation.9
As a result, dorzolamide-timolol may be a promising adjuvant treatment for these patients, especially if decreasing exudation is associated with better visual acuity outcomes. However, without a control group in the prior study, it is possible the decreased exudation resulted from continued anti-VEGF therapy rather than the drops, and it remains unclear whether the visual acuity might benefit from this treatment. Therefore, we performed a randomized placebo-controlled clinical trial to assess the effects of dorzolamide-timolol on anatomy (primary outcome) and visual acuity (one of several secondary outcomes) in this setting.
Methods
This multicenter, randomized, single-masked, parallel-group clinical trial was conducted at 4 sites (Ophthalmic Consultants of Boston, Boston, Massachusetts; Retinal Consultants of Houston, Houston, Texas; Retina Service of Wills Eye Hospital and Mid Atlantic Retina, Philadelphia, Pennsylvania; Associated Retinal Consultants, Royal Oak, Michigan) in accordance with the tenets of the Declaration of Helsinki and conformed to the Health Insurance Portability and Accountability Act. The protocol was approved by Wills Eye Hospital (Philadelphia site) or Western (all other sites) institutional review boards. Participants provided written informed consent and were not compensated for participation. The formal trial protocols can be found in Supplement 1.
Study Population
Eligible patients were 45 years or older with neovascular AMD, having received at least 4 injections of ranibizumab (Genentech) or aflibercept (Regeneron) in the 6 months prior to enrollment with persistent exudation, defined as documented intraretinal and/or subretinal fluid (SRF) at each visit on optical coherence tomography (OCT) using either Spectralis HRA+OCT (Heidelberg Engineering Inc) or Cirrus HD-OCT (Carl Zeiss Meditec). One eye per patient was eligible for enrollment. Each participant was imaged with the same OCT platform throughout the study. Eligible participants received the same anti-VEGF agent at each of the 2 visits preceding enrollment. The injection interval was required to be 5 (±1) weeks for at least 2 visits immediately preceding enrollment. Patients needed to have a baseline central subfield thickness (CST) of at least 270 μm on OCT retinal thickness map.
Exclusion criteria included (1) prior uveitis or current intraocular inflammation; (2) epiretinal membrane or macular hole causing distortion of macular anatomy; (3) vitreous hemorrhage; (4) prior ophthalmic surgery within 6 months; and (5) prior vitrectomy or glaucoma surgery. Patients using prescription eye drops including glaucoma, corticosteroid, or nonsteroidal antiinflammatory drops were excluded. Those with a sulfonamide allergy or contraindication to β-blockers, including bradycardia, hypotension, decompensated heart failure, asthma, or chronic obstructive pulmonary disease, were also excluded.
Randomization, Intervention, and Masking
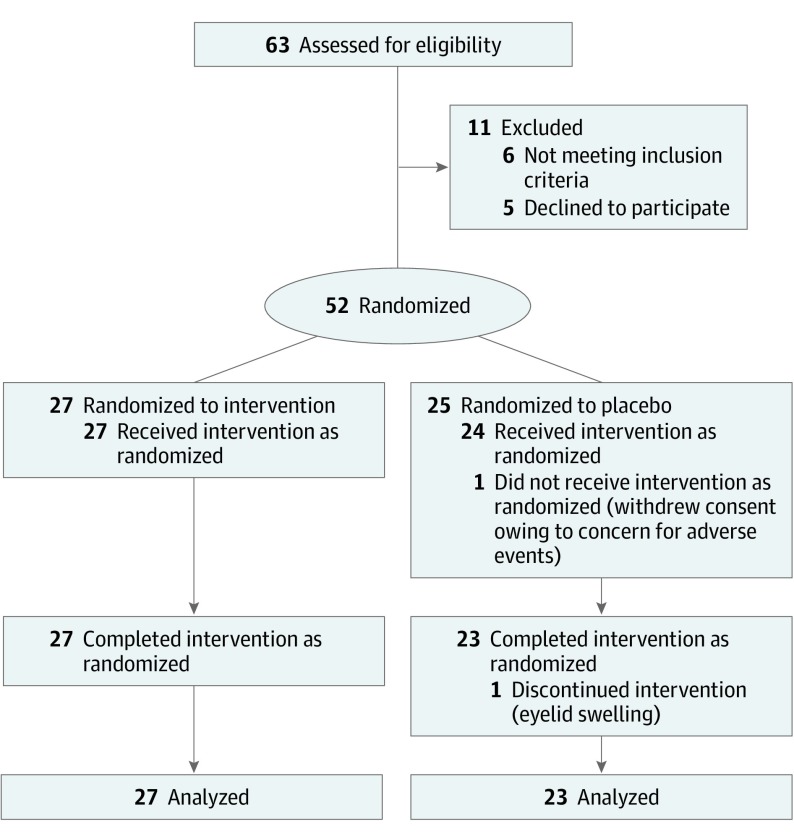
Patients were randomized 1:1 in parallel assignment to topical dorzolamide-timolol (Akorn Inc) or placebo (artificial tears, Jefferson Specialty Apothecary) twice daily for the study duration. The randomization list was computer-generated without blocking. Masked bottles were placed into sequentially numbered opaque envelopes based on the randomization scheme, which was concealed thereafter and not visible to the enrolling physician or staff. Physicians enrolled participants, and a research staff member provided the patient with an opaque envelope containing the drops in sequential order. The enrolling physician was unaware of which drop the patient would be assigned to receive at the time of enrollment. Patients continued to receive the same anti-VEGF drug at the same interval (SD, 1 week) as the 2 visits before enrollment for the study duration. Study coordinators testing visual acuity, intraocular pressure (IOP), and performing OCT tests were masked. The process of recruitment was documented in a Consolidated Standards of Reporting Trials flow diagram (Figure 1).
Figure 1. CONSORT Diagram Showing Randomization and Treatment of Study Patients.
Following enrollment, patients returned for 3 visits, which were defined as visit 1, visit 2, and visit 3. At each visit, best-available visual acuity (VA) based on testing with pinhole or habitual correction (not protocol defined, on Snellen charts, and without protocol refraction), IOP using a Reichert Tono-Pen XL (Reichert Inc) or applanation (if Tono-Pen IOP ≥25 mm Hg), OCT imaging, and intravitreal anti-VEGF injection were performed. Compliance with topical therapy was verified by patient reporting. Patients were masked to the treatment assignment.
Optical coherence tomography images from all study visits were analyzed. Automated CST measurements, defined as average macular thickness in the central 1-mm grid, were generated from the raster scan protocol using built-in software on the Heidelberg Spectralis or Zeiss Cirrus with tracking enabled to facilitate accurate follow-up measurements. If the subsequent scan was not well-aligned automatically, a masked investigator manually aligned them. Maximum SRF height was derived from the baseline OCT b-scans and measured using the built-in caliper tool from the outer retina to the hyperreflective line of the retinal pigment epithelium (RPE). This same point was measured on subsequent OCTs. Similarly, measurements were obtained of the maximum pigment epithelial detachment (PED) height (peak of the hyperreflective line of the RPE to the hyperreflective line of Bruch membrane), central foveal thickness (CFT, from the internal limiting membrane to the hyperreflective line of the RPE), and central subfoveal fluid (SFF) height (from the outer retina to the hyperreflective line of the RPE). A single masked examiner performed the measurements at each site.
Outcome Measures
The prespecified primary outcome measure was change in mean CST from baseline to visit 3. Prespecified secondary anatomic end points included change in mean maximum SRF height and mean maximum PED height from baseline to visit 3. Post hoc secondary anatomic end points included change in central foveal thickness (CFT) and central SFF height from baseline to visit 3. Additional prespecified secondary outcome measures included change in mean VA and IOP from baseline to visit 3.
Sample Size
The sample size was calculated based on change in mean CST from baseline to visit 3. Assuming a power of 0.9 and an α of .05, approximately 44 patients would be required to demonstrate a CST improvement from 400 to 350 μm, with a standard deviation of 50 μm. Estimating a 10% dropout rate, approximately 50 patients were targeted for enrollment.
Statistical Analysis
All data were analyzed using SPSS Statistics, version 25.0 (IBM). Statistical analyses were not prespecified. Snellen VA was converted to logMAR for analysis. For continuous variables, the mean difference and 95% confidence intervals between the 2 groups were derived using analysis of covariance, with the baseline value and type of anti-VEGF drug used as covariates. For binary outcomes, a logistic regression was used with the baseline value as a covariate. Missing data were excluded from analyses such that complete-case analysis was conducted and multiple secondary outcomes were evaluated without formal control for multiplicity, ie, without adjustment for multiple analyses. All P values were 2-sided. A P value less than .05 was considered statistically significant for the primary outcome, and a P value less than .001 was considered statistically significant for any other outcomes because there was no adjustment for multiple analyses.
Results
Sixty-three patients were assessed for eligibility, and 27 were randomized to the dorzolamide-timolol group and 25 to the placebo group. All 27 patients (100%) in the dorzolamide-timolol group and 23 patients (92%) in the placebo group completed the primary outcome visit 3 at a mean (SD) time of approximately 3 months (99.4 [15] days; range, 84-126 days). No obvious imbalances in baseline demographics, visual acuity, anatomic findings, or prior treatment were noted at baseline (Table 1). Mean age (SD) was 78.4 (7) years (range, 65-94 years). Eyes had received a mean (SD) of 20.5 (14) injections (range, 4-58) prior to enrollment.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Dorzolamide-timolol (n = 27) | Placebo (n = 23) | |
| Age, mean (SD) [range], y | 79.7 (7.5) [65-94] | 77.5 (6.3) [68-87] |
| Male | 9 (33) | 7 (30) |
| Right eyes | 14 (52) | 10 (43) |
| Intravitreal anti-VEGF agent used | ||
| Aflibercept | 13 (48) | 14 (61) |
| Ranibizumab | 14 (52) | 9 (39) |
| Bevacizumab | NA | NA |
| Prior injections with same anti-VEGF, mean (SD) [range] | 20.0 (15.6) [4-58] | 21.1 (11.6) [4-51] |
| Current injection interval | ||
| Every 4 wk | 17 (63) | 12 (52) |
| Every 5 wk | 3 (11) | 8 (35) |
| Every 6 wk | 7 (26) | 3 (13) |
| Baseline VA, mean (SD) | ||
| logMAR | 0.370 (0.31) | 0.348 (0.19) |
| Approximate Snellen equivalent (letters) | 20/50 (67) | 20/50 (67) |
| Baseline IOP, mean (SD), mm Hg | 14.37 (2.9) | 14.22 (3.1) |
| Time between baseline and visit, mean (SD), d | ||
| Visit 1 | 32.41 (5.7) | 32.43 (4.9) |
| Visit 2 | 66.04 (11.3) | 66.17 (9.4) |
| Visit 3, mean (SD) [range], d | 98.65 (16.0) [84-126] | 100.70 (13.1) [84-126] |
| Baseline OCT measurements | ||
| Presence of fluid | ||
| Subretinal | 23 (85) | 19 (83) |
| Intraretinal | 13 (48) | 7 (30) |
| Presence of pigment epithelial detachment | 25 (93) | 21 (91) |
| Central subfield thickness at baseline, mean (SD), μm | 348.3 (75.5) | 321.3 (80.5) |
| Pigment epithelial detachment height, mean (SD), μm | 230.2 (116.4) | 178.9 (111.0) |
| Maximum subretinal fluid height, mean (SD), μm | 103.15 (68.5) | 90.0 (70.5) |
| Central subfoveal, mean (SD), μm | ||
| Fluid height | 48.3 (63.4) | 62.6 (97.1) |
| Thickness | 261.9 (108.0) | 229.9 (77.2) |
Abbreviations: IOP, intraocular pressure; VA, visual acuity; VEGF, vascular endothelial growth factor.
Anatomic Outcomes
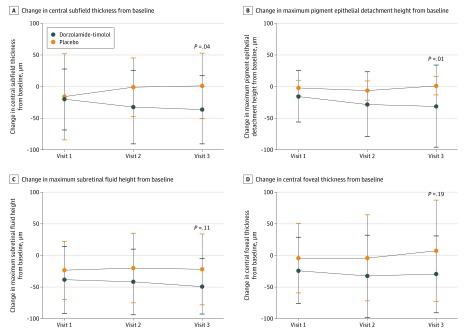
Mean (SD) change in CST from baseline to visit 3 was −36.6 (54) μm in the dorzolamide-timolol group compared with 1.7 (52.3) μm for placebo (adjusted difference 30.8; adjusted 95% CI, 0.3-61.3; P = .04). Mean (SD) change in maximum PED height from baseline to visit 3 was −39.1 (65) μm in the dorzolamide-timolol group compared with 1.1 (16) μm for placebo (adjusted difference 39.6; adjusted 95% CI, 9.6-69.6; P = .01) (Figure 2). Mean (SD) change in maximum SRF height from baseline to visit 3 was −49.4 (55) μm in the dorzolamide-timolol group compared with −22.2 (56) μm for placebo (adjusted difference 23.6; adjusted 95% CI, −5.7 to 53; P = .11). Mean (SD) change in CFT from baseline to visit 3 was −30.0 (62) μm in the dorzolamide-timolol group compared with 7.4 (90) μm for placebo (adjusted difference 26.2; adjusted 95% CI, −13.7 to 66.1; P = .19) (Figure 2). Mean (SD) change in central SFF height from baseline to visit 3 was −14.0 (31) μm in the dorzolamide-timolol group compared with −11.7 (66) μm for placebo (adjusted difference 2.3; adjusted 95% CI, −26.9 to 31.4; P = .51) (eFigure 1 in Supplement 2). Approximately 3 months after baseline, no differences were found between the dorzolamide-timolol and placebo groups in presence of SRF, intraretinal fluid, or PED (Table 2).
Figure 2. Distribution of Changes in Mean Optical Coherence Tomography Parameters Including Central Subfield Thickness (A), Maximum Pigment Epithelial Detachment Height (B), Maximum Subretinal Fluid Height (C), and Central Foveal Thickness (D) in Eyes With Neovascular Age-Related Macular Degeneration Receiving Intravitreal Anti–Vascular Endothelial Growth Factor With Adjuvant Topical Dorzolamide-Timolol vs Placebo.
P values were obtained using analysis of covariance, with adjustment for baseline optical coherence tomography parameter and type of anti–vascular endothelial growth factor therapy used. Error bars represent standard deviation.
Table 2. OCT Findings at Visit 3.
| OCT findings at visit 3 | No. (%) | Adjusted OR (95% CI) | P valuea | |
|---|---|---|---|---|
| Dorzolamide-timolol (n = 27) | Placebo (n = 23) | |||
| Presence of fluid | ||||
| Subretinal | 17 (63) | 18 (78) | 0.24 (0.02-2.3) | .22 |
| Intraretinal | 9 (33) | 7 (30) | 1.2 (0.4-4.3) | .68 |
| Presence of pigment epithelial detachment | 22 (81) | 20 (87) | 1.1 (0.06-18.8) | .95 |
Abbreviations: OCT, optical coherence tomography; OR, odds ratio.
P value by binary logistic regression with adjustment for baseline OCT parameter.
Visual Acuity, IOP, and Safety Outcomes
Visual acuity and IOP outcomes are reported in Table 3. Mean logMAR VA by visit for the dorzolamide-timolol and placebo groups is shown in eFigure 2 in Supplement 2 and mean change in VA by visit for the groups is shown in eFigure 3 in Supplement 2. At visit 3, mean (SD) logMAR VA was 0.420 (0.35) (approximate Snellen equivalent, 20/50) for the dorzolamide-timolol group compared with 0.367 (0.25) (approximate Snellen equivalent, 20/50) for placebo. Approximately 3 months after baseline, mean (SD) change in logMAR VA was 0.031 (0.15) or −2.3 (5) letters in the dorzolamide-timolol group compared with 0.018 (0.16) or 0.3 (1) letters for placebo (difference, 0.012; 95% CI, −0.08 to 0.10; P = .78 or 2.6 letters; 95% CI, −1.9 to 7.1 letters). At visit 3, mean (SD) IOP was 12.56 (3.3) for the dorzolamide-timolol group compared with 13.43 (3.0) for placebo. Approximately 3 months after baseline, mean (SD) change in IOP was −1.81 (3.8) in the dorzolamide-timolol group compared with −0.78 (2.8) for placebo (adjusted difference, 1.0; adjusted 95% CI, −0.7 to 2.7; P = .24). The eTable in Supplement 2 lists adverse events recorded during the study. No relevant differences between groups were noted.
Table 3. Mean Visual Acuity and IOP Outcomes.
| Outcome | Mean (SD) | Adjusted difference (95% CI) | P valuea | |
|---|---|---|---|---|
| Dorzolamide-timolol (n = 27) | Placebo (n = 23) | |||
| VA, logMAR | ||||
| Visit 1 | 0.375 (0.41) | 0.361 (0.24) | NA | NA |
| Visit 2 | 0.377 (0.35) | 0.348 (0.25) | NA | NA |
| Visit 3 | 0.420 (0.35) | 0.367 (0.25) | NA | NA |
| Change from baseline, logMAR | ||||
| Visit 1 | 0.004 (0.19) | 0.013 (0.12) | NA | NA |
| Visit 2 | 0.006 (0.14) | −0.0005 (0.14) | NA | NA |
| Visit 3 | 0.031 (0.15) | 0.018 (0.16) | 0.012 (−0.08 to 0.10) | .78 |
| IOP, mm Hg | ||||
| Visit 1 | 13.00 (3.2) | 14.15 (3.0) | NA | NA |
| Visit 2 | 12.96 (3.3) | 14.02 (3.1) | NA | NA |
| Visit 3 | 12.56 (3.3) | 13.43 (3.00) | NA | NA |
| Change in IOP from baseline, mm Hg | ||||
| Visit 1 | −1.37 (3.3) | −0.07 (3.1) | NA | NA |
| Visit 2 | −1.41 (3.7) | −0.20 (3.6) | NA | NA |
| Visit 3 | −1.81 (3.8) | −0.78 (2.8) | 1.0 (−0.7 to 2.7) | .24 |
Abbreviations: IOP, intraocular pressure; NA, not applicable. VA, visual acuity.
P value using analysis of covariance, with adjustment for baseline visual acuity and IOP, respectively.
Discussion
In our study, adjuvant topical dorzolamide-timolol in neovascular AMD eyes with persistent exudation resulted, on average, in decreased macular edema at approximately 3 months, but no differences in visual acuity accompanied this anatomic finding when comparing the 2 groups at the primary outcome. As a result, it is unclear whether the differences in anatomic outcomes noted in this trial are clinically relevant.
This randomized clinical trial confirms findings from prior nonrandomized studies on the anatomic effects of topical aqueous suppressants in eyes receiving anti-VEGF injections. Byeon et al10 first reported using dorzolamide-timolol in patients receiving bevacizumab for macular edema in retinal vein occlusion. Five weeks after injection, mean central retinal thickness was lower in the dorzolamide-timolol group compared with those receiving no drops. By 9 weeks there was no difference, suggesting the drop may have delayed drug clearance.10 A prospective, nonrandomized case series11 of 10 eyes with neovascular AMD and persistent exudation following anti-VEGF treatment found a decrease in mean CST from 419.7 μm at baseline to 334.1 μm at final visit after adding dorzolamide-timolol.11 Subsequently, a retrospective study with 15 patients replicated these findings, demonstrating reduced mean central macular thickness from 383.5 μm at baseline to 298.3 μm at final visit after adding dorzolamide-timolol.11 Finally, a secondary analysis of Comparison of Age-Related Macular Degeneration Treatments Trials (CATT)12 found that 19 patients who were receiving aqueous suppressants had a trend toward greater VA improvement and reduction in total OCT thickness compared with a control group not receiving these medications.12 The strength of our randomized clinical trial is that these anatomic changes were confirmed in a study that had concurrent controls with a placebo to remove bias from potential confounders such as changes in thickness with time or additional injections unrelated to the topical drops.
Analyzing the OCT data, the greater decrease in mean CST in the dorzolamide-timolol group was associated with a reduction in intraretinal fluid, not just SRF, because the results of this study did not show that the decrease in mean maximum SRF height was greater in the dorzolamide-timolol group. Studies have suggested that intraretinal fluid is harmful while SRF may not be deleterious in neovascular AMD.13,14,15 The CATT found that eyes with SRF in the absence of intraretinal edema appeared to have better VA compared with eyes with dry maculas.16 As a result, dorzolamide-timolol may be beneficial in preserving long-term vision by helping minimize intraretinal edema, although no differences in visual acuity outcomes accompanied the differences in anatomic outcomes when comparing groups in our trial. It also is unknown whether the anatomic improvement alone permits further extension of the injection intervals because injections at subsequent visits in this study were kept on a fixed schedule as mandated by protocol, regardless of the anatomic outcomes. Future studies looking at a treat-and-extend protocol with dorzolamide-timolol might help determine this.
As noted previously, the greater anatomic improvement in the dorzolamide-timolol group was not accompanied by greater VA improvement in this group. Chronic exudation prior to starting the drops may have led to irreversible retinal damage because the mean number of prior injections was 20.5. This short-term trial may not have allowed enough time for functional improvement owing to a lag between anatomic changes on OCT and VA.17 Alternatively, it may be that these anatomic improvements do not cause any relevant visual acuity improvements. In CATT, the association of greater improvement in VA seen in patients receiving aqueous suppressants from the start of anti-VEGF therapy suggests that initiating dorzolamide-timolol earlier may improve functional outcomes.15
Several theories exist as to how dorzolamide-timolol may be helping. As a potent aqueous suppressant, it decreases aqueous flow by approximately 50%.18 With decreased aqueous production, turnover of intraocular fluid may be slower, delaying drug clearance. Another explanation may be a direct effect of β-blockade from timolol. Mouse models demonstrated a role of the β-adrenergic pathway in VEGF upregulation.19,20 By blocking this pathway, studies found a reduction in VEGF and neovascularization.21 One study22 showed CNV attenuation after β-blockade in a mouse model. However, the effect of systemic β-blockade on patients with neovascular AMD is mixed. While 1 study23 suggested systemic β-blockade reduced injection frequency, another24 found no benefit. Higher dosages may be necessary to suppress VEGF, which could explain the dichotomous outcomes between animal and clinical studies. Perhaps topical delivery provides higher intraocular concentrations compared with systemic use in humans. Carbonic anhydrase inhibition from the dorzolamide component may also play a role. Multiple studies have confirmed a positive effect of dorzolamide for cystoid macular edema in inherited retinal degenerations.25,26,27 Also, systemic carbonic anhydrase inhibition may speed resolution of SRF in central serous retinopathy.28 Carbonic anhydrase inhibition may work via enhanced RPE pump function,29,30 increased retinal and choroidal perfusion,31,32 and increased retinal oxygenation.33
Limitations
The study has several limitations. A protocol refraction, a protocol visual acuity testing measurement using an Early Treatment Diabetic Retinopathy Study chart, and IOP testing protocol were not mandated, which may have affected accuracy. Several OCT parameters required manual measurements, potentially affecting precision. However, CST, the primary outcome, was obtained via an automated software algorithm. While patients verified drop compliance, some noncompliance is possible. For example, some patients may not have used the drop on the morning of each visit but were otherwise compliant. Nevertheless, the dorzolamide-timolol group still had improvements in exudation. Other limitations include short follow-up and a small sample size wherein the P value for the primary outcome was equal to .04, so there still is about a 1 in 25 chance that the differences noted were owing to chance alone. Also, although unlikely, it is unclear whether artificial tears may affect exudation.
Conclusions
In summary, this randomized placebo-controlled clinical trial demonstrated that an adjuvant dorzolamide-timolol group had a greater reduction in exudation compared with placebo among patients with neovascular AMD and persistent exudation following anti-VEGF injections. The results were short term and not accompanied by differences in visual acuity outcomes. Future studies initiating drops soon after first diagnosis and/or over a longer period would be needed to determine whether there is a visual benefit to this treatment.
Trial protocol.
eFigure 1. Distribution of changes in central subfoveal fluid height from the baseline visit
eFigure 2. Mean visual acuity at the baseline visit and subsequent three visits
eFigure 3. Mean change in visual acuity from the baseline visit
eTable. Adverse events summary
Data Sharing Statement.
References
- 1.Martin DF, Maguire MG, Fine SL, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-1398. doi: 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 3.Heier JS, Brown DM, Chong V, et al. ; VIEW 1 and VIEW 2 Study Groups . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. doi: 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 4.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. doi: 10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kertes PJ, Galic IJ, Greve M, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology. 2019;126(6):841-848. doi: 10.1016/j.ophtha.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 6.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114(5):855-859. doi: 10.1016/j.ophtha.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Stewart MW. Predicted biologic activity of intravitreal bevacizumab. Retina. 2007;27(9):1196-1200. doi: 10.1097/IAE.0b013e318158ea28 [DOI] [PubMed] [Google Scholar]
- 8.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46(2):726-733. doi: 10.1167/iovs.04-0601 [DOI] [PubMed] [Google Scholar]
- 9.Sridhar J, Hsu J, Shahlaee A, et al. Topical dorzolamide-timolol with intravitreous anti-vascular endothelial growth factor for neovascular age-related macular degeneration. JAMA Ophthalmol. 2016;134(4):437-443. doi: 10.1001/jamaophthalmol.2016.0045 [DOI] [PubMed] [Google Scholar]
- 10.Byeon SH, Kwon OW, Song JH, Kim SE, Park YS. Prolongation of activity of single intravitreal bevacizumab by adjuvant topical aqueous depressant (Timolol-Dorzolamide). Graefes Arch Clin Exp Ophthalmol. 2009;247(1):35-42. doi: 10.1007/s00417-008-0917-1 [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Lee SC, Byeon SH, Koh HJ, Kim SS, Lee CS. Efficacy of adjuvant topical dorzolamide-timolol in patients with neovascular age-related macular degeneration refractory to anti-vascular endothelial growth factor therapy. Retina. 2019;39(10):1953-1958. doi: 10.1097/IAE.0000000000002293 [DOI] [PubMed] [Google Scholar]
- 12.Rahimy E, Ying GS, Pan W, Hsu J. Effect of intraocular pressure-lowering medications on neovascular age-related macular degeneration treatment outcomes in the Comparison of Age-related macular degeneration Treatment Trials. Retina. 2019;39(4):636-647. doi: 10.1097/IAE.0000000000002124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritter M, Simader C, Bolz M, et al. Intraretinal cysts are the most relevant prognostic biomarker in neovascular age-related macular degeneration independent of the therapeutic strategy. Br J Ophthalmol. 2014;98(12):1629-1635. doi: 10.1136/bjophthalmol-2014-305186 [DOI] [PubMed] [Google Scholar]
- 14.Simader C, Ritter M, Bolz M, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1237-1245. doi: 10.1016/j.ophtha.2013.12.029 [DOI] [PubMed] [Google Scholar]
- 15.Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ; FLUID Investigators . Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126(5):723-734. doi: 10.1016/j.ophtha.2018.11.025 [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Toth CA, Daniel E, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Macular morphology and visual acuity in the second year of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(4):865-875. doi: 10.1016/j.ophtha.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond). 2015;29(6):721-731. doi: 10.1038/eye.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brubaker RF, Ingram CJ, Schoff EO, Nau CB. Comparison of the efficacy of betaxolol-brinzolamide and timolol-dorzolamide as suppressors of aqueous humor flow in human subjects. Ophthalmology. 2000;107(2):283-287. doi: 10.1016/S0161-6420(99)00044-5 [DOI] [PubMed] [Google Scholar]
- 19.Ristori C, Filippi L, Dal Monte M, et al. Role of the adrenergic system in a mouse model of oxygen-induced retinopathy: antiangiogenic effects of beta-adrenoreceptor blockade. Invest Ophthalmol Vis Sci. 2011;52(1):155-170. doi: 10.1167/iovs.10-5536 [DOI] [PubMed] [Google Scholar]
- 20.Martini D, Monte MD, Ristori C, et al. Antiangiogenic effects of β2-adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J Neurochem. 2011;119(6):1317-1329. doi: 10.1111/j.1471-4159.2011.07530.x [DOI] [PubMed] [Google Scholar]
- 21.Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The β-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog Retin Eye Res. 2014;42:103-129. doi: 10.1016/j.preteyeres.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 22.Lavine JA, Sang Y, Wang S, Ip MS, Sheibani N. Attenuation of choroidal neovascularization by β(2)-adrenoreceptor antagonism. JAMA Ophthalmol. 2013;131(3):376-382. doi: 10.1001/jamaophthalmol.2013.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montero JA, Ruiz-Moreno JM, Sanchis-Merino E, Perez-Martin S. Systemic beta-blockers may reduce the need for repeated intravitreal injections in patients with wet age-related macular degeneration treated by bevacizumab. Retina. 2013;33(3):508-512. doi: 10.1097/IAE.0b013e3182695ba0 [DOI] [PubMed] [Google Scholar]
- 24.Traband A, Shaffer JA, VanderBeek BL. Systemic beta-blockers in neovascular age-related macular degeneration. Retina. 2017;37(1):41-46. doi: 10.1097/IAE.0000000000001226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006;141(5):850-858. doi: 10.1016/j.ajo.2005.12.030 [DOI] [PubMed] [Google Scholar]
- 26.Genead MA, McAnany JJ, Fishman GA. Topical dorzolamide for treatment of cystoid macular edema in patients with choroideremia. Retina. 2012;32(4):826-833. doi: 10.1097/IAE.0b013e3182215ae9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol. 2010;128(2):190-197. doi: 10.1001/archophthalmol.2009.398 [DOI] [PubMed] [Google Scholar]
- 28.Pikkel J, Beiran I, Ophir A, Miller B. Acetazolamide for central serous retinopathy. Ophthalmology. 2002;109(9):1723-1725. doi: 10.1016/S0161-6420(02)01157-0 [DOI] [PubMed] [Google Scholar]
- 29.Terashima H, Suzuki K, Kato K, Sugai N. Membrane-bound carbonic anhydrase activity in the rat corneal endothelium and retina. Jpn J Ophthalmol. 1996;40(2):142-153. [PubMed] [Google Scholar]
- 30.Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol. 2009;133(6):603-622. doi: 10.1085/jgp.200810169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris A, Jonescu-Cuypers CP, Kagemann L, et al. Effect of dorzolamide timolol combination versus timolol 0.5% on ocular bloodflow in patients with primary open-angle glaucoma. Am J Ophthalmol. 2001;132(4):490-495. doi: 10.1016/S0002-9394(01)01158-8 [DOI] [PubMed] [Google Scholar]
- 32.Harris A, Ciulla TA, Pratt LM, et al. The effects of dorzolamide on choroidal and retinal perfusion in non-exudative age related macular degeneration. Br J Ophthalmol. 2003;87(6):753-757. doi: 10.1136/bjo.87.6.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noergaard MH, Bach-Holm D, Scherfig E, et al. Dorzolamide increases retinal oxygen tension after branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2008;49(3):1136-1141. doi: 10.1167/iovs.07-0508 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eFigure 1. Distribution of changes in central subfoveal fluid height from the baseline visit
eFigure 2. Mean visual acuity at the baseline visit and subsequent three visits
eFigure 3. Mean change in visual acuity from the baseline visit
eTable. Adverse events summary
Data Sharing Statement.