Highlights
-
•
Pathogenesis of cytokine release syndrome (CRS) in severe COVID-19 patients.
-
•
Key role of interleukin-6 (IL-6) in CRS.
-
•
IL-6 receptor (IL-6R) antagonist tocilizumab suggested as a possible drug for severe COVID-19.
Keywords: COVID-19, Cytokine release syndrome, Tocilizumab, IL-6, IL-6R
Abstract
Since December 2019, a viral pneumonia, named coronavirus disease 2019 (COVID-19), from Wuhan, China, has swept the world. Although the case fatality rate is not high, the number of people infected is large and there is still a large number of patients dying. With the collation and publication of more and more clinical data, a large number of data suggest that there are mild or severe cytokine storms in severe patients, which is an important cause of death. Therefore, treatment of the cytokine storm has become an important part of rescuing severe COVID-19 patients. Interleukin-6 (IL-6) plays an important role in cytokine release syndrome. If it is possible to block the signal transduction pathway of IL-6, it is expected to become a new method for the treatment of severe COVID-19 patients. Tocilizumab is an IL-6 receptor (IL-6R) blocker that can effectively block the IL-6 signal transduction pathway and thus is likely to become an effective drug for patients with severe COVID-19.
1. Introduction
In December 2019, several patients with unexplained pneumonia appeared in Wuhan, China, and a viral pneumonia currently sweeping the world was in the process of development. Several days later, the virus was identified as a new Betacoronavirus, which was officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The disease caused by SARS-CoV-2 has been named coronavirus disease 2019 (COVID-19). As of 14 March 2020, the virus has caused 81 026 infections in China with a case fatality rate of 3.9% (3194/81 026). A total of 55 095 confirmed cases have been reported in other countries in the world, with a mortality rate of 4.1% (2238/55 095), which does not differ much from that in China. Although most patients present with mild symptoms that are not life-threatening, the number of deaths is still high owing to the large population base.
The first COVID-19 pathology observed bilateral diffuse alveolar injury with cytomyxoid fibroma exudate, and subsequent peripheral flow cytometry analysis found a decrease in CD4+ and CD8+ T-cells but an increase in the Th17 cell proportion [2]. Th17 cells are helper T-cells differentiated from Th0 cells mainly stimulated by interleukin-6 (IL-6) and IL-23 [3]. A study to be published (Jing Liu et al.) including 40 COVID-19 patients (of whom 13 were severe) suggests that severe cases show a sustained decrease in the proportion of lymphocytes compared with mild cases. In addition, CD8+ T-cells decreased and inflammatory cytokines [IL-6, IL-10, IL-2 and interferon-gamma (IFNγ)] in the peripheral blood increased in severe cases.
Taken together, we have a reasonable hypothesis that cytokine storms play an important role in severe COVID-19 cases. Therefore, neutralising key inflammatory factors in cytokine release syndrome (CRS) will be of great value in reducing mortality in severe cases.
2. Brief introduction to cytokine release syndrome
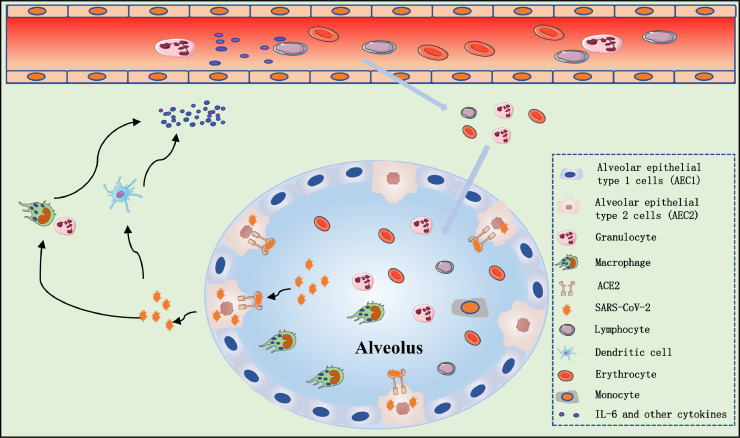
CRS is a systemic inflammatory response that can be caused by infection, some drugs and other factors, characterised by a sharp increase in the level of a large number of pro-inflammatory cytokines [4], [5], [6]. CRS is more common in immune system-related diseases and immune-related therapy such as chimeric antigen receptor T-cell (CAR-T) therapy, organ transplantation sepsis [7] and viral infections. SARS-CoV-2 binds to alveolar epithelial cells. The virus then activates the innate and adaptive immune systems, resulting in the release of a large number of cytokines, including IL-6. In addition, vascular permeability is increased by these pro-inflammatory factors, resulting in a large amount of fluid and blood cells entering the alveoli, resulting in dyspnoea and even respiratory failure [8], [9], [10] (Fig. 1 ). The first gross examination autopsy report of a COVID-19 death reported that a bronzed appearance of both lungs and a large amount of grey-white viscous liquid overflow could be seen after incision [11].
Fig. 1.
Possible mechanism of cytokine release syndrome in severe coronavirus disease 2019 (COVID-19) patients. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects alveolar epithelial cells [mainly alveolar epithelial type 2 (AEC2) cells] through the angiotensin-converting enzyme 2 (ACE2) receptor. Destruction of epithelial cells and the increase of cell permeability lead to release of the virus. SARS-CoV-2 activates the innate immune system; macrophages and other innate immune cells not only capture the virus but also release a large number of cytokines and chemokines, including interleukin-6 (IL-6). Adaptive immunity is also activated by antigen-presenting cells (mainly dendritic cells). T- and B-cells not only play an antiviral role but also directly or indirectly promote the secretion of inflammatory cytokines. In addition, under the stimulation of inflammatory factors, a large number of inflammatory exudates and erythrocytes enter the alveoli, resulting in dyspnoea and respiratory failure.
3. Interleukin-6 and its role in cytokine release syndrome
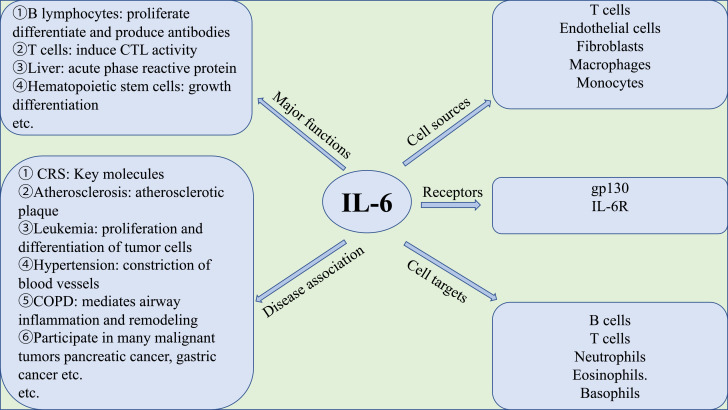
IL-6 is an important member of the cytokine network and plays a central role in acute inflammation [12]. IL-6, discovered by Weissenbach et al. in 1980 [13], is a multifunctional cytokine that plays an important role in human metabolism, autoimmune cell differentiation, disease treatment, etc. [14]. A brief introduction to IL-6 is shown in Fig. 2 .
Fig. 2.
Brief introduction to interleukin-6 (IL-6). CTL, cytotoxic T lymphocyte; CRS, cytokine release syndrome; COPD, chronic obstructive pulmonary disease; gp130, glycoprotein 130; IL-6R, interleukin-6 receptor.
3.1. Structure and characteristics of interleukin-6
IL-6 is a small polypeptide consisting of four α helices. It has a molecular weight of 19–28 kDa and comprises 184 amino acid residues, usually in monomer form, with an isoelectric point of 5.0, glycosylation sites and two disulfide bonds. The gene encoding IL-6 is located on chromosome 7p15–21, including 4 introns and 5 exons [15].
IL-6 can be produced by almost all stromal cells and immune system cells, including B-lymphocytes, T-lymphocytes, macrophages, monocytes, dendritic cells, mast cells, and other non-lymphocytic cells such as fibroblasts, endothelial cells, keratinocytes, glomerular mesangial cells and tumour cells [16]. The main activators of IL-6 expression are IL-1β and tumour necrosis factor-alpha (TNFα) [14]. However, there are also other ways to promote the synthesis of IL-6, such as Toll-like receptors (TLRs), prostaglandins, adipokines, stress response and other cytokines [14].
In the early stage of infectious inflammation, IL-6 is produced by monocytes and macrophages stimulated by TLRs. In non-infectious inflammation, such as burns or traumatic injuries, it can also be produced by cells stimulated by TLRs. This acute IL-6 expression plays a central role in host defence by stimulating various cell populations.
3.2. Signal transduction pathway of interleukin-6
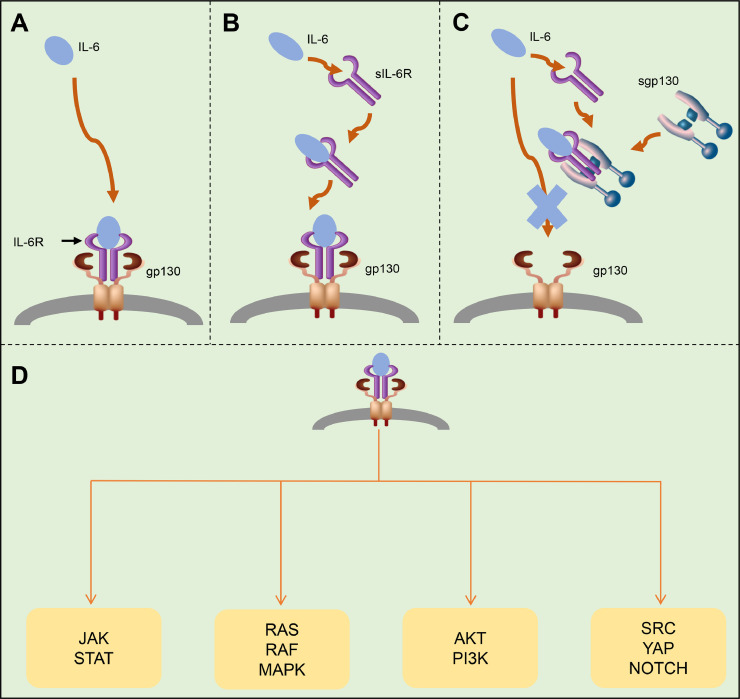
IL-6 plays a central role in the cytokine storm. It is a multi-effective cytokine with both anti-inflammatory and pro-inflammatory effects. There are three main pathways of IL-6 signal transduction [14,17]: (i) classical signal transduction (Fig. 3 A); (ii) trans-signal transduction (Fig. 3B); and trans-presentation (Fig. 3C).
Fig. 3.
Signal transduction pathways of interleukin-6 (IL-6): (A) classical signal transduction; (B) trans signal transduction; and (C) trans-presentation. (D) The next step is to activate the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway (STAT1, STAT3 and, to a lesser extent, STAT5); in addition, the RAS-RAF, SRC-YAP-NOTCH and AKT-PI3K pathways are also activated. These promote complex biological functions such as proliferation, differentiation, oxidative stress and immune regulation, etc. IL-6R, interleukin-6 receptor; gp130, glycoprotein 130; sIL-6R, soluble interleukin-6 receptor; sgp130, soluble glycoprotein 130;
In the classical signal transduction pathway [18], IL-6 binds to the IL-6 receptor (IL-6R) to form a complex and then binds to the transmembrane glycoprotein 130 (gp130) to initiate intracellular signal transduction. The IL-6R exists not only in transmembrane form but also in a soluble form (sIL-6R). IL-6 binds to these two forms and then interacts with gp130 to trigger downstream signal transduction and gene expression [19], [20], [21]. In the trans-signal transduction pathway, the binding affinity of sIL-6R to IL-6 is similar to that of IL-6R, and this complex binds to gp130, which initiates intracellular signal transduction. In the classical signal pathway, many cells cannot respond to the IL-6 signal because they do not express IL-6R, but some of these cells can be stimulated by sIL-6R–IL-6 complex to respond to the IL-6 signal and cause cell signal transduction [22,23]. The trans-presentation signal is suppressed by extracellular soluble gp130 (sgp130), and sgp130 can form a complex with sIL-6R to prevent sIL-6R from binding to membrane-bound gp130 [16]. The next step is to activate the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway (STAT1, STAT3 and, to a lesser extent, STAT5) [22], [23], [24], [25]; in addition, the RAS-RAF [23,26], SRC-YAP-NOTCH [27] and AKT-PI3K [28,29] pathways are also activated (Fig. 3D). This promotes complex biological functions such as proliferation, differentiation, oxidative stress, immune regulation, etc. [16].
3.3. Biological functions of interleukin-6
IL-6 can promote T-cell population expansion and activation and B-cell differentiation, regulate the acute phase response, and affect the hormone-like properties of vascular disease, lipid metabolism, insulin resistance, mitochondrial activity, neuroendocrine system and neuropsychological behaviour [14]. In addition, IL-6 promotes the differentiation of osteoclasts and angiogenesis, the proliferation of keratinocytes and glomerular membrane cells, and the growth of myeloma and plasmacytoma cells [14,25].
3.3.1. Effect on B lymphocytes [30]
IL-6 can induce B-cells to proliferate, differentiate and produce antibodies. IL-6 is especially required when B-cells are activated by antigen and differentiate into effector cells producing immunoglobulin M (IgM), IgG and IgA antibodies.
3.3.2. Effect on T lymphocytes [31]
IL-6 is the terminal helper factor of cytotoxic T lymphocytes (CTLs), which can induce CTL activity and cause immature thymocytes to develop into CTLs. In addition, IL-6 is a pro-inflammatory regulator of T-cells. IL-6 can promote Th17 cell lineage and function, inhibit the induction of regulatory T-cells (Tregs), and promote the development of the self-reactive pro-inflammatory CD4+ T-cell response. IL-6 combined with transforming growth factor-beta (TGFβ) can promote the development and function of Th17 cells, whilst Th17 cells are related to the occurrence and development of many self-inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, etc. [31], [32], [33].
3.3.3. Effect on hepatocytes [34,35]
IL-6 is a strong inducer of acute-phase reactive proteins. It can induce hepatocytes to synthesise acute-phase reactive protein at the gene transcription level, especially serum amyloid A and C-reactive protein (CRP).
3.3.4. Effect on haematopoietic stem cells [36]
IL-6 can co-operate with other cytokines to promote the growth of early bone marrow stem cells, enhance the differentiation of blood cells, and promote their colony formation.
3.3.5. Participate in the occurrence of immune abnormalities [22,37]
Hypergammaglobulinemia, myocardial myxoma, bladder cancer and chronic rheumatoid arthritis, among others, are accompanied by abnormally increased levels of IL-6.
3.3.6. Participate in the occurrence and development of cardiovascular diseases [38]
Myocardial ischaemia, coronary atherosclerosis, angina pectoris, congestive heart failure and hypertension, among others, are accompanied by abnormally increased IL-6 levels.
4. Interleukin-6 receptor antagonist tocilizumab
The classical IL-6 signal is limited to cells (macrophages, neutrophils, T-cells, etc.) that express the IL-6R and plays a leading role in the low level of IL-6. The combination of IL-6 and cell-related IL-6R leads to gp130 homologous dimerisation and initiates downstream pathways. However, when the level of IL-6 increases, the IL-6 signal is widely expressed because gp130 is ubiquitous. Binding of tocilizumab to cell-related IL-6R and sIL-6R can inhibit classical and trans signals. Thus, it can inhibit CRS.
Tocilizumab is a recombinant humanised anti-human IL-6R monoclonal antibody of the IgG1 subtype. Tocilizumab specifically binds soluble and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits sIL-6R- and mIL-6R-mediated signal transduction. It has been approved for the treatment of rheumatoid arthritis [39] and systemic juvenile idiopathic arthritis [40]. In addition, it has also been reported to play a role in Castleman disease [41] and Crohn's disease [42]. It is worth noting that tocilizumab is effective in the treatment of patients with severe CRS [43,44]. The recommended dose of tocilizumab is 8 mg/kg by intravenous (i.v.) drip every 4 weeks for adult with rheumatoid arthritis, which can be used in combination with methotrexate or other antirheumatic drugs. When liver enzyme abnormalities occur or the neutrophil or platelet counts decrease, the dose of tocilizumab can be reduced to 4 mg/kg. For systemic juvenile idiopathic arthritis, the dose of tocilizumab is 12 mg/kg (for body weight <30 kg) or 8 mg/kg (for body weight ≥30 kg). An i.v. drip every 2 weeks is recommended, with an infusion time of >1 h.
The safety of tocilizumab was studied in five phase III double-blind controlled trials and its extended period (data come from the treatment of rheumatoid arthritis) [45]. The total control population included all patients in the double-blind period of each core study from randomised grouping to the first change of treatment regimen, or the completion of a 2-year treatment period. Among them, the double-blind control period of four studies was 6 months, and the other double-blind control period was 2 years. In these double-blind controlled trials, 774 patients received tocilizumab 4 mg/kg combined with methotrexate (MTX) and 1870 patients received with tocilizumab 8 mg/kg combined with MTX or other disease-modifying antirheumatic drugs (DMARDs). A total of 288 patients were treated with tocilizumab 8 mg/kg alone. In a 6-month controlled trial, the incidence of infection events in patients receiving tocilizumab 8 mg/kg + DMARD or placebo + DMARD was 127 cases/100 patient-years and 112 cases/100 patient-years, respectively. Among the total exposed population, the overall incidence of infection events in the tocilizumab + DMARD group was 108 cases/100 patient-years. The 6-month controlled trial also showed that the incidence of severe infection (bacteria, viruses and fungi) in the 8 mg/kg + DMARD group was 5.3/100 patient-years, whilst that in the placebo + DMARD group was 3.9/100 patient-years. In the monotherapy trial, the incidence of severe infection was 3.6 cases/100 patient-years in the tocilizumab group and 1.5 cases/100 patient-years in the MTX group. Regarding the safety of tocilizumab in the treatment of patients with severe COVID-19, a recent study included 21 patients with a mean age of 56.8 ± 16.5 years (range 25–88 years) [46]. There were no complications associated with tocilizumab treatment and no history of illness deterioration or death. Overall, the risk of secondary infection with tocilizumab is not too high.
The largest clinical data from China's Centers for Disease Control and Prevention show that of the 44 672 confirmed cases included, 2683 (12.8%) had hypertension, 1102 (5.3%) had diabetes mellitus and 873 (4.3%) had other cardiovascular diseases [47]. A total of 1023 deaths (2.3%) occurred among confirmed cases; the crude death rate with no reported complications was 0.9% whereas the mortality rate of patients with complications was much higher (10.5% of patients with cardiovascular disease, 7.3% of patients with diabetes mellitus and 6.0% of patients with hypertension). There is some controversy about whether tocilizumab increases the risk of cardiovascular disease. Data from several randomised controlled trials (RCTs) and real-world evidence (RWE) studies have been published. A study by Giles et al. included 3080 patients aged >50 years with more than one risk factor for cardiovascular disease who met the diagnosis of active rheumatoid arthritis, of whom 1538 were treated with tocilizumab and 1542 were treated with etanercept [48]. After an average follow-up of 3.2 years, 83 major adverse cardiovascular events (MACEs) occurred in the tocilizumab group (5.4%), whilst 78 MACEs occurred in the etanercept group (5.1%). The resulting hazard ratio (HR) was 1.05 [95% confidence interval (CI) 0.77–1.43]. The authors concluded that tocilizumab had a higher cardiovascular risk than etanercept. Interestingly, two RWE studies came to a different conclusion from the aforementioned RCT. In the study by Kim et al., cardiovascular events in rheumatoid arthritis patients newly initiating tocilizumab or tumour necrosis factor inhibitors (TNFi) were compared; after strict propensity score matching, 9218 tocilizumab initiators and 18 810 TNFi initiators were included [49]. The incidence rate of cardiovascular events was 0.52 per/100 person-years for tocilizumab and 0.59 per/100 person-years for TNFi. The combined HR was 0.84 (95% CI 0.56–1.26). Another RWE study by Xie et al. came to a similar conclusion that there was no significant difference in the risk of cardiovascular events associated with the use of tocilizumab compared with TNFi [HR (Medicare database) = 0.79 (95% CI 0.65–0.96); HR (MarketScan database) = 0.84 (95% CI 0.52–1.37)] [50]. Although the conclusion of the RCT study was slightly different from RWE studies, the 95% CIs of all studies were not significant (i.e. the 95% CI contains 1), so there is insufficient evidence to conclude that tocilizumab increases cardiovascular events.
In August 2017, the US Food and Drug Administration (FDA) approved tocilizumab for the treatment of CRS caused by chimeric antigen receptor T-cell (CAR-T) immunotherapy [51]. A 7-year-old girl with acute lymphoblastic leukaemia (ALL) developed a severe cytokine storm following CAR-T treatment. Subsequent treatment with tocilizumab dramatically improved her condition and did not affect the efficacy of CAR-T [43]. Another study reported that a male patient with ALL developed haemophagocytic lymphohistiocytosis following treatment with blinatumomab. The patient developed severe respiratory failure and methemoglobinemia. Subsequent treatment with tocilizumab successfully saved the patient's life [52].
SARS-CoV-2, SARS-CoV and MERS-CoV are coronaviruses, and CRS of varying degrees has occurred in severe patients with severe acute respiratory syndrome (SARS) [53], [54], [55] and Middle East respiratory syndrome (MERS) [56]. All of them had high expression of IL-6. Currently, a clinical trial in China with a small sample size (Chinese Clinical Trial Registry ID: ChiCTR2000029765; http://www.chictr.org.cn/showprojen.aspx?proj=49409) has shown good efficacy of tocilizumab [46]. All 21 patients included met the criteria for severe or critical COVID-19 [57], including one of the following: shortness of breath; respiratory rate >30 bpm; oxygen saturation <93% while resting; or PaO2/FiO2 ratio ≤300 mmHg. Critical criteria included one of the following: respiratory failure requiring mechanical ventilation; shock; and admission to the intensive care unit (ICU) with other organ failure. After a few days of treatment, the body temperature of patients with fever (all 21 patients initially had a fever) returned to normal and all other symptoms were significantly improved. Of 20 patients, 15 (75%) had reduced their oxygen intake and 1 patient did not require oxygen. Imaging examination by computed tomography (CT) showed that 90.5% (19/21) of the patients had absorption of pulmonary lesions. Laboratory examination showed that the proportion of peripheral blood lymphocytes and CRP returned to normal. An inadequacy of the study is that only the level of IL-6 in peripheral blood before treatment with tocilizumab was reported (mean value 132.38 ± 278.54 pg/mL), but the level of IL-6 after treatment was not.
Finally, from analysis of the possible mechanism of COVID-19 and small sample clinical data, tocilizumab has good efficacy. From a pharmacoeconomic viewpoint, we suggest that it should be used in critically ill COVID-19 patients with significantly elevated IL-6.
In conclusion, CRS occurs in a large number of patients with severe COVID-19, which is an important cause of death. IL-6 is the key molecule of CRS, therefore the IL-6R antagonist tocilizumab may be an important drug to save patients' lives.
Acknowledgments
Funding: This study was supported by the China Mega-Project for Infectious Diseases [grant nos. 2017ZX10203202 and 2013ZX10002005] and the China Mega-Project for Innovative Drugs [grant no. 2016ZX09101065].
Competing interests: None declared.
Ethical approval: Not required.
Editor: Jean-Marc Rolain
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Feb 18 doi: 10.1016/S2213-2600(20)30076-X. [Epub ahead of print]Erratum in: Lancet Respir Med 2020 Feb 25 [Epub ahead of print]. doi: 10.1016/S2213-2600(20)30085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miossec P., Kolls J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 4.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 6.Teijaro J.R. Cytokine storms in infectious diseases. Semin Immunopathol. 2017;39:501–503. doi: 10.1007/s00281-017-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 8.Leiva-Juarez M.M., Kolls J.K., Evans S.E. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018;11:21–34. doi: 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudsen L., Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol. 2018;150:661–676. doi: 10.1007/s00418-018-1747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brune K., Frank J., Schwingshackl A., Finigan J., Sidhaye V.K. Pulmonary epithelial barrier function: some new players and mechanisms. Am J Physiol Lung Cell Mol Physiol. 2015;308:L731–L745. doi: 10.1152/ajplung.00309.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q., Wang R.S., Qu G.Q., Wang Y.Y., Liu P., Zhu Y.Z. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi [Journal of Forensic Medicine] 2020;36:19–21. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Scheller J. Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006;195:173–183. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 13.Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia coli cloning studies. Proc Natl Acad Sci U S A. 1980;77:7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. Erratum in: Nat Immunol 2017;18:1271. [DOI] [PubMed] [Google Scholar]
- 15.Scheller J., Garbers C., Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014;26:2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 18.Baran P., Hansen S., Waetzig G.H., Akbarzadeh M., Lamertz L., Huber H.J. The balance of interleukin (IL)-6, IL-6•soluble IL-6 receptor (sIL-6R), and IL-6•sIL-6R•sgp130 complexes allows simultaneous classic and trans-signaling. J Biol Chem. 2018;293:6762–6775. doi: 10.1074/jbc.RA117.001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briso E.M., Dienz O., Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J Immunol. 2008;180:7102–7106. doi: 10.4049/jimmunol.180.11.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell I.L., Erta M., Lim S.L., Frausto R., May U., Rose-John S. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J Neurosci. 2014;34:2503–2513. doi: 10.1523/JNEUROSCI.2830-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S.A. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 22.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villarino A.V., Kanno Y., O'Shea J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zegeye M.M., Lindkvist M., Falker K., Kumawat A.K., Paramel G., Grenegård M. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun Signal. 2018;16:55. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su H., Lei C.T., Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol. 2017;8:405. doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Müller-Newen G., Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi K., Wu L.W., Grivennikov S.I., de Jong P.R., Lian I., Yu F.X. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada O., Ozaki K., Akiyama M., Kawauchi K. JAK-STAT and JAK-PI3K-mTORC1 pathways regulate telomerase transcriptionally and posttranslationally in ATL cells. Mol Cancer Ther. 2012;11:1112–1121. doi: 10.1158/1535-7163.MCT-11-0850. [DOI] [PubMed] [Google Scholar]
- 29.Thiem S., Pierce T.P., Palmieri M., Putoczki T.L., Buchert M., Preaudet A. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 2013;123:767–781. doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987;6:2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones B.E., Maerz M.D., Buckner J.H. IL-6: a cytokine at the crossroads of autoimmunity. Curr Opin Immunol. 2018;55:9–14. doi: 10.1016/j.coi.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eu J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64:1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Fraunberger P., Wang Y., Holler E., Parhofer K.G., Nagel D., Walli A.K. Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock. 2006;26:10–12. doi: 10.1097/01.shk.0000215319.06866.bd. [DOI] [PubMed] [Google Scholar]
- 36.Campard D., Vasse M., Rose-John S., Poyer F., Lamacz M., Vannier J.P. Multilevel regulation of IL-6R by IL-6–sIL-6R fusion protein according to the primitiveness of peripheral blood-derived CD133+ cells. Stem Cells. 2006;24:1302–1314. doi: 10.1634/stemcells.2005-0173. [DOI] [PubMed] [Google Scholar]
- 37.Kraakman M.J., Kammoun H.L., Allen T.L., Deswaerte V., Henstridge D.C., Estevez E. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 2015;21:403–416. doi: 10.1016/j.cmet.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Qu D., Liu J., Lau C.W., Huang Y. IL-6 in diabetes and cardiovascular complications. Br J Pharmacol. 2014;171:3595–3603. doi: 10.1111/bph.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarro G., Taroumian S., Barroso N., Duan L., Furst D. Tocilizumab in rheumatoid arthritis: a meta-analysis of efficacy and selected clinical conundrums. Semin Arthritis Rheum. 2014;43:458–469. doi: 10.1016/j.semarthrit.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Yokota S., Miyamae T., Imagawa T., Iwata N., Katakura S., Mori M. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52:818–825. doi: 10.1002/art.20944. [DOI] [PubMed] [Google Scholar]
- 41.Nishimoto N., Kanakura Y., Aozasa K., Johkoh T., Nakamura M., Nakano S. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 42.Ito H., Takazoe M., Fukuda Y., Hibi T., Kusugami K., Andoh A. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989–996. doi: 10.1053/j.gastro.2004.01.012. discussion 947. [DOI] [PubMed] [Google Scholar]
- 43.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winkler U., Jensen M., Manzke O., Schulz H., Diehl V., Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- 45.Genentech, Inc . Genentech, Inc.; South San Francisco, CA: 2019. Prescribing information. ACTEMRA® (tocilizumab) injection, for intravenous or subcutaneous use.https://www.gene.com/download/pdf/actemra_prescribing.pdf [accessed 11 March 2020] [Google Scholar]
- 46.Xu X.L., Han M.F., Li T.T., Sun W., Wang D.S., Fu B.Q., et al. Effective treatment of severe COVID-19 patients with tocilizumab. http://chinaxiv.org/abs/202003.00026[accessed 11 March 2020]. [DOI] [PMC free article] [PubMed]
- 47.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- 48.Giles J.T., Sattar N., Gabriel S., Ridker P.M., Gay S., Warne C. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2020;72:31–40. doi: 10.1002/art.41095. [DOI] [PubMed] [Google Scholar]
- 49.Kim S.C., Solomon D.H., Rogers J.R., Gale S., Klearman M., Sarsour K. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol. 2017;69:1154–1164. doi: 10.1002/art.40084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie F., Yun H., Levitan E.B., Muntner P., Curtis J.R. Tocilizumab and the risk of cardiovascular disease: direct comparison among biologic disease-modifying antirheumatic drugs for rheumatoid arthritis patients. Arthritis Care Res. 2019;71:1004–1018. doi: 10.1002/acr.23737. [DOI] [PubMed] [Google Scholar]
- 51.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teachey D.T., Rheingold S.R., Maude S.L., Zugmaier G., Barrett D.M., Seif A.E. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castilletti C., Bordi L., Lalle E., Rozera G., Poccia F., Agrati C. Coordinate induction of IFN-α and -γ by SARS-CoV also in the absence of virus replication. Virology. 2005;341:163–169. doi: 10.1016/j.virol.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi S.Q., Peng J.P., Li Y.C., Qin C., Liang G.D., Xu L. The expression of membrane protein augments the specific responses induced by SARS-CoV nucleocapsid DNA immunization. Mol Immunol. 2006;43:1791–1798. doi: 10.1016/j.molimm.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tseng C.T., Perrone L.A., Zhu H., Makino S., Peters C.J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol. 2005;174:7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- 56.Zheng Y., Wang Q.Y. Bioinformatics analysis on molecular mechanism of ribavirin and interferon-α in treating MERS-CoV [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:291–293. doi: 10.3760/cma.j.issn.0254-6450.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 57.The State Council of the People's Republic of China. Diagnosis and treatment protocol for novel coronavirus pneumonia (seventh edition). http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d47598711a0d4cbf874a9.pdf[accessed 12 March 2020].