Dear Editor,
We read with interest the recent letter by Hao1 that addressed the initial negative RT-PCR result in atypical patients. Since outbreak of unexplained pneumonia cases in Wuhan, China in December, 2019,2 the coronavirus disease 2019 (COVID-19) has spread to more than 90 countries. By 7th March, the infection of SARS-CoV-2 has influenced 101,918 patients globally.3 Recommended by World Health Organization (WHO), 4 a positive real-time reverse transcriptase polymerase chain-reaction (RT-PCR) result could confirm the diagnosis of suspected COVID-19 patients. However, there still lacks thoroughly research concerning viral shedding time among different samples in COVID-19 patients. Here we compared the viral shedding time of SARS-CoV-2 in different samples of intensive care unit (ICU) and non-ICU patients and analyzed their characteristics.
We analyzed a total of thirty-two COVID-19 patients admitted to Central Hospital of Xiangtan from January to February, 2020. Dynamic clinical samples including nasal swabs, blood, fecal, urine, saliva and tears were collected from each patient for surveillance. What is more, we recorded synchronous clinical and epidemiologic information with oral consent. Total RNA was extracted from clinical samples and we performed RT-PCR tests targeting SARS-CoV-2. The continuous variants were described by mean when they conform to Kolmogorov-Smirnov test. We did analysis of Chi-square tests and Mann-Whitney tests to compare differences among groups. P < 0.05 was considered significant. Statistical analyses and figures were conducted using the Stata 14 and GraphPad Prism 8.
From Table 1 , the thirty-two patients include eight ICU and twenty-four non-ICU patients, their age ranged from 34 to 54 years old. A proportion of 43.8% (14/32) patients were from Wuhan and none come back from other cities of Hubei province. Three patients had not been to Hubei province but infected by people from Hubei. Nine of thirty-two patients were identified as family clustered infection. Seven patients had no clear contact history. For the enrolled patients, 40.6% (13/32) of them carried underlying diseases, of which the common diseases were hypertension (5 patients) and diabetes (4 patients). The average onset days was five days and the most common initial symptom was cough (75. 0%, 24/32). Surprisingly, only 53.1% (17/32) patients presented with fever in the beginning. The other symptoms encompassed fatigue, headache, diarrhea, sore throat, muscular soreness and anhelation. Four patients were diagnosed as COVID-19 without symptoms.
Table 1.
Clinical and epidemiologic characteristics of COVID-19 patients.
| Patients (n = 32) | |
|---|---|
| Age-year | 41(34–54) |
| Male/Female-no. | 16/16 |
| BMI | 24.5 (22.6–26.5) |
| Epidemic-no. (%) | |
| From Hubei province | Wuhan 14(43.8%), other area of Hubei province 0(0.0%) |
| Not been to Hubei province, but infected by people from Hubei province | 3(9.4%) |
| Without any clear contact history | 7(21.9%) |
| Family cluster infection | 9(28.1%) |
| Hospital-related transmission rate (Xiangtan City) | 0(0.0%) |
| Underlying disease- no. (%) | |
| Diabetes | 4(12.5) |
| Hypertension | 5(15.6) |
| Cardiovascular diseases | 1(3.1) |
| Liver disease | 2(6.3) |
| Malignancy | 0(0.0) |
| Others | 1(3.1) |
| Initial symptom-no. (%) | |
| Fever | 17 (53.1) |
| Cough | 24 (75.0) |
| Fatigue | 5 (15.6) |
| Headache | 6 (18.8) |
| Diarrhea | 3 (9.4) |
| Sore throat | 7 (21.9) |
| Muscular soreness | 6 (18.8) |
| Anhelation | 10 (31.2) |
| No symptoms | 4 (12.5) |
As indicated by RT-PCR results, we obtained positive rate and viral shedding time of different samples in ICU and non-ICU patients. The positive rate of nasal swab in these patients was 100.0% (32/32) and three patients experienced negative result at the first tests. The positive rate of saliva (78.1%, 25/32) was significantly higher than that of tears (15.6%, 5/32) in enrolled patients (p<0.001). All the urine samples from thirty-two patients were negative. In the tests of blood, the positive rate was 87.5% (7/8) in ICU and 66.7% (16/24) in non-ICU patients respectively.
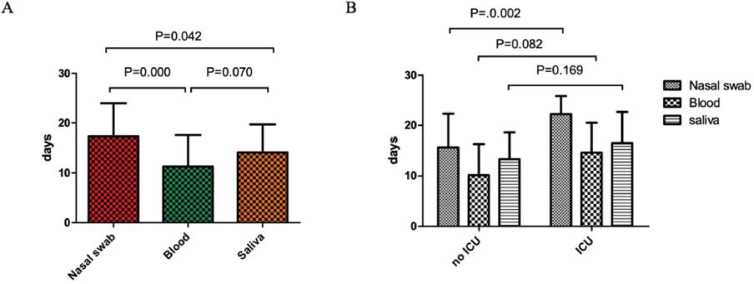
What is more, we performed consecutive analyses of nasal swab, blood and saliva in non-ICU and ICU groups. As shown in Fig. 1 (A), the viral shedding (from positive to negative) time of SARS-CoV-2 of nasal swab was significantly longer than that of blood (p = 0.000) and saliva (p = 0.05). Though shorter time of viral shedding in blood samples than saliva was observed, no significant p value was detected (p = 0.070). The viral shedding time of SARS-CoV-2 of nasal swab was 15.67±6.68 days in non-ICU group and 22.25±3.62 days in ICU group. For blood, the viral shedding time was 10.17±6.13 and 14.63±5.88 days in non-ICU and ICU patients respectively. Saliva in non-ICU and ICU patients took 13.33±5.27 and 16.50±6.19 days separately to converse to negative. As shown in Fig. 1(B), the viral shedding time of SARS-CoV-2 of nasal swab in non-ICU patients was significantly shorter than ICU patients (P = 0.02). No obvious significance was indicated in blood and saliva samples between non-ICU and ICU groups.
Fig. 1.
Comparisons of viral shedding time of SARS-CoV-2 in different samples and patients.
(A) Comparisons of viral shedding time of SARS-CoV-2 in different samples.
(B) Comparisons of viral shedding time of SARS-CoV-2 of different samples in non-ICU and ICU patients.
RT-PCR was a widely used tool in diagnosing SARS-CoV-2 infection. Guided by WHO,4 two consecutive negative RT-PCR result with at least two days apart should be achieved before discharge of patients. Several cases have been reported negative of RT-PCR tests before confirmation.1 In our study, one patient experienced five continuous negative results before admission and then the sixth nasal swab on admission finally supported his diagnosis. Considering this situation, the molecular diagnosis should be made with other clinical samples or other etiologic tests including serological tests, CRISPR, or metagenomic sequencing. The viral shedding time of SARS-CoV-2 was reported around two weeks.5 The longer time consumed to turn from positive to negative in nasal swabs than in blood and saliva samples was in accordance with the respiratory transmission characteristics of the disease, and indicated that a longer surveillance needed in respiratory samples for nucleic acid testing.
What is more, in our study, we categorized patients into two groups: ICU and non-ICU. In ICU patients, the viral shedding time of blood, nasal and saliva sample all exceeded two weeks, illustrating a relatively longer period than non-ICU patients. These differences of viral shedding time in ICU and non-ICU patients might be correlated with virus load, severity and invasive operations of patients.
In conclusion, our study originally illustrated that nasal swab samples consumed more time to turn negative than blood and saliva samples. What is more, viral shedding time of SARS-CoV-2 in ICU patients was longer than that of non-ICU patients, especially nasal swab samples. A combination tests of different samples might provide us with further information concerning the transmission characteristics of COVID-19 patients.
Declaration of Competing Interest
All authors report no potential conflict of interest.
Acknowledgments
Acknowledgments
We thank the patients for cooperating with our investigation and acknowledge the professionalism and compassion demonstrated by all the healthcare workers involved in patients’ care.
Funding
This work was supported by National Natural Science Foundation of China [grant number 82041010] and Shanghai Science and Technology Association [grant number 20411950400].
References
- 1.Hao W. Clinical features of atypical 2019 novel coronavirus pneumonia with an initially negative RT-PCR assay. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.008. PubMed PMID: 32092387. Epub 2020/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. PubMed PMID: 32015507. Epub 2020/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Available from:https://ncov.dxy.cn/ncovh5/view/pneumonia?scene=2&clicktime=1579582186&enterid=1579582186&from=timeline&isappinstalled=0.
- 4.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. [cited 394 10196]. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
- 5.To K.K., Tsang O.T., Chik-Yan Yip C., Chan K.H., Wu T.C., Chan J.M.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. PubMed PMID: 32047895. Epub 2020/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]