Abstract
Recent international epidemics of coronavirus-associated illnesses underscore the urgent medical and public health need for vaccine development and regulatory body approved therapies. In particular, the current coronavirus disease 2019 (COVID-19) pandemic has quickly intensified interest in developing treatment options to mitigate impact on human life. Remdesivir (GS-5734™) is a broad-spectrum antiviral drug that is now being tested as a potential treatment for COVID-19 in international, multi-site clinical trials. Currently available evidence about the antiviral effects of remdesivir against coronaviruses is primarily based on in vitro and in vivo studies (including some on a chemically related compound, GS-441524™), which have demonstrated largely favorable findings. As the pandemic progresses, information from human compassionate use cases will continue to accumulate before the clinical trials are concluded. It is imperative for public health practitioners and the One Health community to stay up to date on the most promising potential therapeutic options that are under investigation. Thus, the purpose of this review is to synthesize the knowledge to date about remdesivir as a therapeutic option for coronaviruses, with a special focus on information relevant to the One Health community.
Keywords: Remdesivir, GS-5734, Coronavirus, COVID-19, SARS-CoV-2, Compassionate use
1. Introduction
The first human coronaviruses (HCoVs) were identified in the 1960s [1], and for the majority of the past six decades, HCoVs have generally maintained a relatively innocuous reputation as a cause of the common cold [2]. However, public perception and awareness of HCoVs has shifted considerably in the last twenty years, with the onset of three high profile outbreaks that have garnered international attention and have subsequently led to renewed interest in potential therapeutic options for HCoV-related illnesses. In 2002–2003, severe acute respiratory syndrome coronavirus (SARS-CoV) infected more than 8000 people, reaching 26 countries and resulting in a 10% case fatality rate [3]. In the ~7 years between April 2012 and November 2019, there were 2494 laboratory-confirmed cases of infection with Middle East respiratory syndrome coronavirus (MERS-CoV) that reached 27 countries and had a mortality rate of approximately 34% [4], though serologic studies have implied that the prevalence of MERS-CoV may have been underestimated [5]. Most recently, the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019 in Wuhan and its subsequent spread to over 160 countries across the world has generated intense interest in vaccine development and treatment options for coronavirus disease 2019 (COVID-19) [6,7].
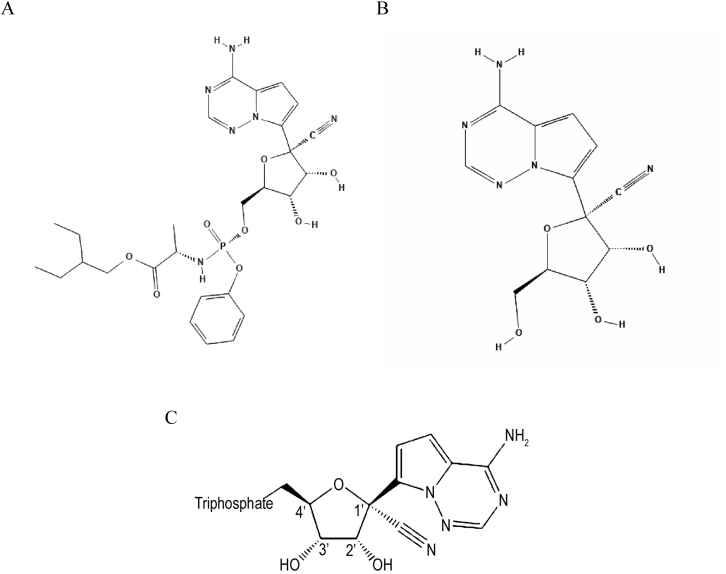
Remdesivir (GS-5734, Fig. 1a) is an investigational broad-spectrum small-molecule antiviral drug that has demonstrated activity against RNA viruses in several families, including Coronaviridae (such as SARS-CoV, MERS-CoV, and strains of bat coronaviruses capable of infecting human respiratory epithelial cells), Paramyxoviridae (such as Nipah virus, respiratory syncytial virus, and Hendra virus), and Filoviridae (such as Ebola virus) [[8], [9], [10], [11], [12]]. Originally developed to treat Ebola virus infection [8], remdesivir is a prodrug of the parent adenosine analog, GS-441524 (Fig. 1b), both of which are metabolized into an active nucleoside triphosphate (NTP) by the host (Fig. 1c) [13]. The parent nucleoside, GS-441524, has displayed antiviral activity against SARS-CoV, Marburg virus, and feline infectious peritonitis virus, among others [9,[14], [15], [16]]. A number of studies have examined the effects of these two drugs on coronaviruses (CoVs) both in vitro and in vivo using mouse and non-human primate animal models [[9], [10], [11],13,17,18].
Fig. 1.
Chemical structures of: a) Remdesivir⁎; b) GS-441524⁎⁎; c) triphosphate metabolite of both drugs†.
* Original source of image: PubChem Database, National Center for Biotechnology Information. PubChem Database. Remdesivir, CID = 121304016, https://pubchem.ncbi.nlm.nih.gov/compound/cid-121304016 (accessed on Mar. 21, 2020).
** Original source of image: PubChem Database, National Center for Biotechnology Information. PubChem Database. GS-441524, CID = 44468216, https://pubchem.ncbi.nlm.nih.gov/compound/44468216 (accessed on Mar. 21, 2020).
†Adapted from Reference [50].
There are currently no antiviral drugs approved for the treatment of CoV-specific illnesses. Given the rapidly evolving pandemic of COVID-19, it is crucial for public health practitioners and the One Health community to stay up to date on potential therapeutic options that are under investigation [19]. Based on existing data, remdesivir is a promising candidate, and multi-site clinical trials of remdesivir are now underway among hospitalized adults with COVID-19 [20]. This review summarizes the knowledge to date on remdesivir as a therapeutic option for CoVs.
2. Overview of coronaviruses
Viruses classified under the Coronaviridae family are enveloped positive-sense, single-stranded RNA viruses that exhibit high genetic diversity [21]. Within the Orthocoronavirinae subfamily, there are four genera: alphacoronavirus, betacoronavirus, gammacoronavirus, and deltacoronavirus. Viruses in this family can infect a wide variety of host species, such as birds, humans, and non-human mammals, including dromedary camels, alpacas, domestic pigs, dogs, cats, ferrets, minks, and bats [5,11,12,16,[22], [23], [24]]. However, to our knowledge, only viruses of the alpha- and betacoronavirus genera infect humans [2], though those in the gamma- and deltacoronavirus genera have indirect effects through economic impacts on the agricultural production of poultry and pigs [12]. Host specificity is believed to be largely dependent upon variation in the CoV spike attachment glycoprotein [23]. Although the infectivity of most strains is host species-specific, host range is wide across different CoVs, and some bat CoVs rely on the same host receptor (angiotensin-converting enzyme-2; ACE-2) as human CoVs to facilitate entry into cells [11,25,26]. It has been hypothesized that the CoV propensity for host-switching may partly be attributable to recombination events that alter the spike protein, which, in turn, affects interactions with host receptors (e.g., ACE-2) [27,28].
Historically, HCoVs were largely considered to be relatively low virulence viruses that produced less severe, self-limiting disease, and were predominantly known as the second most prevalent cause of colds and upper respiratory infections (URIs), after rhinoviruses [2,29]. The endemic human CoVs that cumulatively account for about 10–30% of URIs are HCoV-229E and HCoV-NL63, which are both alphacoronaviruses, and HCoV-OC43 and HCoV-HKU1, which are both betacoronaviruses [2]. Among patients with URIs severe enough to warrant hospitalization, one study found that approximately 5% of cases were attributable to rhinoviruses or HCoVs, but a substantial proportion of these hospitalized patients had underlying pulmonary or cardiac comorbidities that may have exacerbated their conditions [29].
The virus underlying COVID-19 (SARS-CoV-2) is a betacoronavirus that is closely related to SARS-CoV. These CoVs share ~80% RNA sequence identity [30,31]. The similarity is even greater between the viruses when comparing the sequences specific to a key drug target, the RNA-dependent RNA polymerase (RdRP) (>90% sequence identity) [30]. By contrast, MERS-CoV shares about 50% genomic sequence identity with SARS-CoV-2 [31], and with the exception of some bat strains, many animal CoVs are even less similar [12].
3. Remdesivir mechanism of action
As a nucleoside analog, remdesivir acts as an RdRp inhibitor, targeting the viral genome replication process. The RdRp is the protein complex CoVs use to replicate their RNA-based genomes. After the host metabolizes remdesivir into active NTP, the metabolite competes with adenosine triphosphate (ATP; the natural nucleotide normally used in this process) for incorporation into the nascent RNA strand [32]. The incorporation of this substitute into the new strand results in premature termination of RNA synthesis, halting the growth of the RNA strand after a few more nucleotides are added. Although CoVs have a proofreading process that is able to detect and remove other nucleoside analogs, rendering them resistant to many of these drugs, remdesivir seems to outpace this viral proofreading activity, thus maintaining antiviral activity [30]. Unsurprisingly, Agostini et al. reported that a mutant murine hepatitis virus (MHV) devoid of proofreading ability was more sensitive to remdesivir [13]. The opposite is also possible—that mutations that improve proofreading or otherwise increase fidelity of the base-pairing process may result in remdesivir resistance [33]. In fact, Agostini et al. also induced mutations in MHV (through passage in remdesivir) that conferred strong resistance against the drug, but these mutated strains were outcompeted by wild-type MHV in coinfected cell cultures that were not exposed to remdesivir [13]. How well this experiment would represent a situation in which a resistance mutation developed naturally, though, is unclear. Nonetheless, some evidence suggests that remdesivir could have an additional mechanism of action that has yet to be discovered, which, if true, may allow for partial antiviral activity to continue despite viral mutations that enhance replication fidelity [[11], [12], [13]].
4. Summary of studies on remdesivir and coronaviruses
The existing studies on the potential antiviral effects of remdesivir that are of highest relevance to the virus underlying the current pandemic, SARS-CoV-2, are those that have been conducted on genetically similar coronaviruses. As discussed above, within the Coronaviridae family of viruses, there is a substantial amount of genetic heterogeneity, even among human strains [34]. This genetic diversity is posited to be attributable to the large viral genome and the molecular nuances of the viral RNA replication processes (e.g., RdRp infidelity) [21]. Although remdesivir is a broad-spectrum antiviral, its efficacy against other families of viruses may not be generalizable to its effectiveness against SARS-CoV-2, and attempts to predict the level of potential generalizability are hampered by the possibility that the drug may have secondary mechanisms of action that are yet to be elucidated. As a result, this review focuses on studies of the effects of remdesivir on CoVs specifically; however, some information about research on other lesser related viruses (e.g., Ebola) is briefly mentioned for additional context on the drug.
4.1. In vitro studies
The majority of in vitro efficacy studies conducted to date have reported positive results on the anti-CoV activity of remdesivir and GS-441524 (Table 1). Commonly used metrics in these studies are the half maximal effective concentration (EC50), which is the drug concentration at which half of the maximum response is attained after exposure, or the half maximal inhibitory concentration (IC50), which is the drug concentration at which half of the peak inhibiting effect of the drug against a specific viral function is achieved. Lower EC50 and IC50 values indicate higher potency. Typical outcomes measured in such studies include the amount of viral RNA (e.g., copies of certain open reading frames) in the culture, the number of virus-infected cells in the culture, or changes in viral replication rates.
Table 1.
Summary of in vitro studies on remdesivir (GS-5734) efficacy against coronaviruses.
| Study | Coronavirus | Cell line⁎ | EC50 or IC50⁎⁎ |
|---|---|---|---|
| Remdesivir | |||
| Sheahan et al. [11] | MERS-CoV | Calu-3 2B4 | IC50 = 0.025 μM |
| HAE | IC50 = 0.074 μM | ||
| SARS-CoV | HAE | IC50 = 0.069 μM | |
| Agostini et al. [13] | SARS-CoV | HAE | EC50 = 0.07 μM |
| MERS-CoV | HAE | EC50 = 0.07 μM | |
| MHV† | DBT | EC50 = 0.03 μM | |
| Brown et al. [12] | HCoV-OC43 | Huh7 | EC50 = 0.15 μM |
| HCoV-229E | Huh7 | EC50 = 0.024 μM | |
| LLC-PK1 | EC50 = 3.8 μM | ||
| PDCoV‡ | LLC-PK1 | Not reached | |
| Huh7 | EC50 = 0.02 μM | ||
| Sheahan et al. [10] | MERS-CoV | Calu-3 2B4 | EC50 = 0.09 μM |
| Wang et al. [17] | SARS-CoV-2 | Vero E6 | EC50 = 0.77 μM |
| GS-441524 | |||
| Murphy et al. [14] | FIPV¥ | CRFK | EC50 = 0.78 μM |
| Agostini et al. [13] | SARS-CoV | HAE | EC50 = 0.18 μM |
| MERS-CoV | HAE | EC50 = 0.86 μM | |
| MHV† | DBT | EC50 = 1.1 μM | |
Calu-3: human bronchial epithelial cells; HAE: human airway epithelial cells; DBT: mouse delayed brain tumor; Huh7: human liver cells; LLC-PK1: porcine kidney cells; Vero E6: African green monkey kidney epithelial cells; CRFK: feline kidney cells.
EC50 = Half maximal effective concentration; IC50 = half maximal inhibitory concentration. EC50 or IC50 provided as reported by each respective study.
MHV = murine hepatitis virus.
PDCoV = porcine deltacoronavirus.
FIPV = feline infectious peritonitis virus.
In studies of SARS-CoV and MERS-CoV in human respiratory epithelial cell cultures, remdesivir has demonstrated strong antiviral activity (EC50 ≈ 0.07 μM for either virus) with relative consistency, and has been shown to be capable of inhibiting MERS-CoV replication at levels below those that would result in unacceptable cytotoxicity [11,13]. Interestingly, a study published in February 2020 by Wang et al. was the first, to our knowledge, to examine the effect of remdesivir against SARS-CoV-2, the HCoV involved in the current pandemic [17]. This study investigated the impact of seven different drugs on viral titers, cytotoxicity, and infection rates, using Vero E6 cells (a cell line originating from African green monkey kidney epithelial cells). They found low potency of most of these drugs for inhibiting SARS-CoV-2. EC50 values ranged widely from 109.5 μM for ribavirin to 1.13 μM for choloquine and 0.77 μM for remdesivir, which were the two drugs that required the lowest concentrations for blocking viral infection.
A few in vitro studies have also investigated the utility of remdesivir against non-human CoVs. For example, a study by Murphy et al. reported anti-CoV activity of remdesivir's parent nucleoside, GS-441524, against an alphacoronavirus that only infects wild and domestic cats, the feline infectious peritonitis (FIP) virus (EC50 = 0.78 μM; Table 1) [14]. In 2019, Brown et al. observed some antiviral effects of remdesivir against SARS-like bat CoVs (specifically, Bat-CoV HKU3, Bat-CoV SCH014, and Bat-CoV WIV1) and MERS-like bat CoVs (specifically, Bat-CoV HKU5) [12].
Further, this study detected a weaker antiviral effect against porcine deltacoronavirus (PDCoV; Table 1), particularly when tested in porcine cell lines. Among currently known CoVs, PDCoV has the least similar RdRp sequence compared to SARS-CoV and MERS-CoV (only 67–69% similarity in amino acid sequence) [12]. In fact, the RdRp of PDCoV has a different amino acid at a specific site (phenylalanine to leucine at residue 483), a dissimilarity that has been previously associated with increased resistance to remdesivir in other CoVs [12,13]. Therefore, it was important to attempt to disentangle whether the lower antiviral activity observed could be related to a viral characteristic of PDCoV or if it could be attributable to differences in the cell lines used. While diminished potency of remdesivir against PDCoV was noted in cultures of porcine kidney epithelial cells (LLC-PK1), in a human liver cell line (Huh7), the EC50 was relatively low (EC50 not reached in LLC-PK1 vs. EC50 = 0.02 μM in Huh7; Table 1). Similarly, the EC50 was substantially higher for antiviral activity against an endemic HCoV (229E) in the porcine cell line (3.8 μM compared to 0.02 μM in Huh7), again indicating the likely lower potency of the drug in this context. Thus, the observed variations in remdesivir potency against the same viruses in the different cell lines may imply that there is some factor associated with the cell line, rather than the virus, that may be affecting the drug's antiviral activity. In fact, the authors state that these findings may imply that LLC-PK1 cells lack a cellular process necessary for remdesivir antiviral activity, which could also support the hypothesis that remdesivir may have another mechanism of action that is not presently understood.
4.2. In vivo studies: animal models & veterinary studies
Animal studies on remdesivir efficacy against CoVs have utilized transgenic mice and rhesus macaques [10,11,13,18]. For example, using a mouse model with a Ces1c-knockout (which better emulates human metabolism of remdesivir compared to wild-type mice) and a humanized MERS-CoV receptor, Sheahan et al. found that both prophylactic and therapeutic remdesivir had protective effects against MERS-CoV replication and associated pathology, generally resulting in less lung damage and better pulmonary function compared to controls [10]. Interestingly, among mice that were infected with a higher virus quantity (i.e., more plaque-forming units), those receiving prophylactic remdesivir one day before infection had significantly better 6-day survival than infected control mice that did not receive remdesivir. However, the mice were sacrificed before longer follow-up data could be obtained. Similarly, in a rhesus macaque model of MERS-CoV pathogenesis, prophylactic and therapeutic administration of remdesivir demonstrated favorable results, reducing respiratory tract viral titers and pulmonary pathology [18]. Prophylactic use was initiated 24 h prior to viral challenge, whereas therapeutic use was initiated 12 h after challenge. While respiratory rate was not substantially different between the prophylactic, therapeutic, and control groups, x-ray scores, representing the severity of pulmonary infiltrates, were significantly better in the prophylactic and therapeutic groups, comparing each to controls [18]. Incidentally, remdesivir has also been associated with beneficial effects, including reduced severity of respiratory signs and improved survival, against challenge with Nipah virus, which is a non-CoV virus in the Paramyxoviridae family, in African green monkeys [21].
In a Ces1c-knockout mouse model of SARS-CoV infection, prophylactic and therapeutic remdesivir treatment resulted in lower lung viral titers, and if therapy was administered 1 day post-infection (which is before peak viral replication), it was associated with greater pulmonary function, compared to controls [11]. However, there were no differences in disease pathology or survival if treatment was administered 2 days post-infection.
Some veterinary studies have also examined the effects of the parent nucleoside, GS-441524, in cats with FIP, a condition associated with very high mortality in cats [14,16]. Gilead Sciences (Foster City, California) had provided this drug to researchers at the University of California, Davis to evaluate its potential utility in veterinary indications [35]. Subsequently, GS-441524 became an intermediate in the production of the prodrug, remdesivir, which has superior ability to transport the active compound into cells [9,18]. Because both drugs yield the same active metabolite in host cells, some prior studies on the antiviral activity of remdesivir also investigate GS-441524 [15,18], and/or discuss its relevance in the veterinary studies [9,12].
In 2018, Murphy et al. examined the efficacy of GS-441524 against FIP in 12 experimentally infected cats [14]. Controls were not included, as historical data on untreated cats were available, demonstrating a mortality rate close to 100% [14,36]. After experimental challenge with the FIP virus, 2 cats did not develop FIP-related signs and remained untreated [14]. In the 10 infected cats, treatment was initiated upon onset of FIP-associated clinical signs (~10–18 days post infection), and continued for 2 weeks. Of these cats, half (n = 5) were treated with a 5 mg/kg dose of GS-441524 administered subcutaneously once per day, and the other half (n = 5) were treated with 2 mg/kg daily. If disease recurred after initial treatment, cats were re-treated using the same regimen for another 2 weeks. Follow-up continued for 8 months. All treated cats demonstrated favorable responses to GS-441524 treatment within 24–48 h, regardless of dose, and were still alive and clinically unremarkable 8 months post infection. Two cats (one from each dose group) experienced disease recurrence at 4 weeks and 6 weeks after initial treatment and were re-treated. Adverse events related to treatment, other than temporary discomfort at the injection site, were not observed. This study was based on a small number of animals, and it is unclear whether experimental infection is a relevant representation of naturally-occurring FIPV infection in cats. Future studies with additional follow-up time would be helpful for assessing whether unexpected long-term sequelae are observed in the cats, since such information on surviving animals is sparse given the high fatality rate.
In a field trial of treatment with GS-441524 for naturally occurring FIP, Pedersen et al. treated 31 cats with an initial dose of 2 mg/kg daily [16]. Within the first week of treatment, 4 cats (13%) with severe disease died or were euthanized, a fifth cat died after 26 days, and another was euthanized about 2-weeks post relapse due to the development of neurological signs and a lack of response to treatment. The remaining cats completed at least 12 weeks of treatment, but 8 experienced relapses requiring re-treatment with GS-441524. Dose escalation from 2 mg/kg to 4 mg/kg daily was required for 5 cats. A total of 25 treated cats (81%) survived FIP for at least 44 weeks of follow up, indicating that GS-441524 (and presumably, its prodrug remdesivir) is also a promising therapeutic candidate for treatment of alphacoronavirus-related disease in cats. Most cats in this study had “wet” or effusive FIP, the hallmark of which is the accumulation of fluids in the abdominal or thoracic cavities. There were only 3 cats in the study with abdominal non-effusive (“dry”) FIP, which is a more chronic condition, and another 4 cats initially had dry FIP that progressed into wet FIP. One cat in the dry FIP group and one in the dry-to-wet group did not survive. Because of the small number of cats with dry FIP, conclusions cannot be drawn about whether remdesivir has greater efficacy in the treatment of some clinical manifestations of FIP than others. A strength of this study is that it was conducted among animals with naturally-occurring CoV infection as a field trial, better representing real-world circumstances.
4.3. Human data
The first patient to present with COVID-19 in the U.S. was treated with intravenous remdesivir under the compassionate use clause after developing pneumonia in January 2020 [37]. This 35-year-old male had been infected during travel to Wuhan and returned to the U.S. where he was hospitalized for more than 12 days. He was treated with intravenous remdesivir on the seventh day, and his condition was reportedly improving on the eighth day. No adverse events related to remdesivir use were noted. The case report was written prior to the patient's discharge.
Human data on remdesivir use for COVID-19 will likely continue to become available from compassionate use cases before the clinical trial is completed. In fact, there are many anecdotal reports already circulating about treatment of COVID-19 patients with remdesivir. According to a recent newspaper article, 17 passengers on the Diamond Princess cruise ship were treated with intravenous remdesivir for 10 days and were alive at the time of article release. One of the physicians involved in their treatment, who is affiliated with the U.S. National Institutes of Health (NIH), felt that the patients were less dependent on ventilators after receiving the drug. Nevertheless, no conclusions can be drawn based on such reports.
A preprint of a case series on the first 12 COVID-19 patients in the U.S. (who had disease onset between January 14 and 29, 2020) recently became available, though it had not been peer-reviewed at the time of writing [38]. This report, authored by a team from the Centers for Disease Control (CDC), described the demographic and clinical characteristics of the patients, as well as information on course of disease and clinical management. Seven of the 12 patients (58%) were hospitalized, and three of these patients received remdesivir intravenously at the onset of worsening symptoms (two on the eleventh day of illness and one on the seventh day). Treatment with remdesivir was tolerated, though temporary gastrointestinal upset and elevated aminotransferase levels were observed in all three patients after administration. Treatment was discontinued after respiratory symptoms improved (total treatment durations of 4 days, 5 days, and 10 days). It should be noted that one of these three patients is the same patient described in the case report discussed above [37].
Some safety data were formally collected during a clinical trial conducted in the Democratic Republic of Congo that randomized 175 patients to be treated with remdesivir for Ebola [39]. Incidentally, randomization to remdesivir treatment was discontinued in this trial after an interim analysis showed superior survival for two other trial drugs, despite preclinical and compassionate use data having been favorable for remdesivir against the Ebola virus prior to the trial [9]. Interestingly, patients who received remdesivir had slower rates of viral clearance compared to patients who received single-dose antivirals (specifically, MAb114 and REGN-EB3), which the authors hypothesized may be related to the fact that the treatment plan for remdesivir involved multiple intravenous infusions. However, for now, information on the potential efficacy of remdesivir specifically against CoVs is largely limited to in vitro and animal studies, though COVID-19 related knowledge is evolving quickly.
Overall, past studies on other CoVs may have limited generalizability to the virus underlying the current pandemic because of the high genetic diversity of the Coronaviridae family, although broad-spectrum drugs tend to be directed at well-conserved targets [11,40]. In addition to this issue, there are a number of factors that can impact how predictive findings from in vitro or in vivo models may be of clinical efficacy against SARS-CoV-2. For example, drugs that may be effective in vitro may not have clinical utility if the therapeutic dose induces severe adverse events in the patient. Alternatively, if the treatment dose does not attain an effective serum concentration in patients, or if EC50 is greater than the achievable maximum serum concentration (Cmax), then the drug is less likely to have therapeutic utility. With regard to animal models, how closely the model represents disease pathogenesis and drug metabolism in humans can be challenging to gauge.
4.4. Clinical trials
Multiple clinical trials are underway on the use of remdesivir for treatment of COVID-19 [41]. The NIH-sponsored clinical trial, ongoing in the U.S. and the Republic of Korea, is a double-blinded, placebo-controlled trial in which patients are randomized to receive either placebo or an initial dose of 200 mg of intravenous remdesivir on the first day, followed by a maintenance dose of 100 mg per day, through discharge up to a maximum of 10 total treatment days [20]. The primary outcome of the trial, as described in the U.S. National Library of Medicine clinical trials registry, will be expressed as the proportion of patients in each category of a seven-category clinical severity scale on the fifteenth day post treatment initiation (Table 2) [42]. Additionally, Gilead Sciences is sponsoring a remdesivir study among patients with severe COVID-19 with a composite primary outcome measure of fever normalization and oxygen normalization [43].
Table 2.
Ordinal seven-point scale used as primary outcome in the U.S. National Institutes of Health sponsored clinical trial (NCT04280705) on remdesivir⁎.
|
Outcome: proportion of participants in each of the below ratings (Day 15) | |
|---|---|
| Severity rating | Category description⁎ |
| 1 | Death |
| 2 | Hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation |
| 3 | Hospitalized, on non-invasive ventilation or high flow oxygen devices |
| 4 | Hospitalized, requiring supplemental oxygen |
| 5 | Hospitalized, not requiring supplemental oxygen |
| 6 | Not hospitalized, limitation on activities |
| 7 | Not hospitalized, no limitations on activities |
Please note that information is taken verbatim from the U.S. National Library of Medicine clinical trials registry [42].
Two double-blinded placebo-controlled trials are also recruiting in Hubei Province, China [44,45]. One targets hospitalized patients with mild-to-moderate COVID-19 [44], while the other is focused on severe cases [45]. The primary outcome measure for the study on mild or moderate cases is time to clinical recovery, as defined by normalization of body temperature, respiratory rate, and oxygen saturation, and the resolution of cough for at least 72 h [44]. In the study on severe cases, time to clinical improvement is the primary outcome and is defined using a six-category scale, ranging from discharge to death [45].
5. Conclusions
While previous studies on remdesivir are promising, formal clinical evaluation is strongly warranted. In general, there are many reasons why favorable preclinical data can fail to translate directly into human clinical trial results [46], such as inadvertent use of irrelevant models, inability to achieve effective serum drug concentrations in patients, or the occurrence of unanticipated severe adverse events among patients. Therefore, postulating on expected results of the trials is extremely challenging.
Nonetheless, there are hundreds of clinical trials ongoing internationally on different drugs that utilize various mechanisms of action [41,51], including trials on other nucleosides inhibitors (e.g., ribavirin), protease inhibitors (e.g., lopinavir/ritonavir), and interleukin-6 receptor inhibitors (e.g., sarilumab) [41,47]. Another well-known candidate that is being evaluated in multiple trials against COVID-19 is chloroquine (or hydroxychloroquine), which is already approved as an antimalarial (and for extraintestinal amebiasis) [41,48]. Results of the clinical trials currently underway in the U.S. and China will provide crucial information about whether remdesivir represents a viable treatment option for COVID-19 [20,49]. If the trial findings are ultimately positive, it will be imperative to ensure that the drug is produced on a commercial scale capable of meeting the demand generated by both the current pandemic and future outbreaks. Such a change in production may also allow for the added benefit of the drug becoming more available for agricultural and veterinary use for relevant indications.
Funding statement
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors report no relevant conflicts of interest. E.S. Amirian was previously employed by McKesson Specialty Health, where she conducted health economics and outcomes research contract studies. However, none of these studies involved Gilead Sciences.
Acknowledgements
The authors acknowledge Megan Rafferty for her technical assistance.
References
- 1.Vassilara F., Spyridaki A., Pothitos G., Deliveliotou A., Papadopoulos A. A rare Case of human coronavirus 229E associated with acute respiratory distress syndrome in a healthy adult. Case Rep. Infect. Dis. 2018;2018:6796839. doi: 10.1155/2018/6796839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections-more than just the common cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization SARS (Severe Acute Respiratory Syndrome) https://www.who.int/ith/diseases/sars/en/ Available from: (accessed on 9 March 2020)
- 4.World Health Organization Middle East Respiratory Syndrome Coronavirus (MERS-CoV): MERS Monthly Summary, November 2019. https://www.who.int/emergencies/mers-cov/en/ Available from: (accessed on 9 March 2020)
- 5.Muller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect. Dis. 2015;15(5):559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanmugaraj B., Malla A., Phoolcharoen W. Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens. 2020;9(2) doi: 10.3390/pathogens9020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zowalaty M.E.E., Jarhult J.D. From SARS to COVID-19: a previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans – call for a one health approach. One Health. 2020;9:100124. doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L. GS-5734 and its parent nucleoside analog inhibit filo-, Pneumo-, and paramyxoviruses. Sci. Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., 3rd, Sims A.C., Feng J.Y. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir. Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy B.G., Perron M., Murakami E., Bauer K., Park Y., Eckstrand C. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet. Microbiol. 2018;219:226–233. doi: 10.1016/j.vetmic.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho A., Saunders O.L., Butler T., Zhang L., Xu J., Vela J.E. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 2012;22(8):2705–2707. doi: 10.1016/j.bmcl.2012.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen N.C., Perron M., Bannasch M., Montgomery E., Murakami E., Liepnieks M. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019;21(4):271–281. doi: 10.1177/1098612X19825701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2020 doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marty A.M., Jones M.K. The novel coronavirus (SARS-CoV-2) is a one health issue. One Health. 2020;9:100123. doi: 10.1016/j.onehlt.2020.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institutes of Health NIH Clinical Trial of Remdesivir to Treat COVID-19 Begins. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins Available from: (accessed on 10 March 2020)
- 21.Woo P.C., Lau S.K., Huang Y., Yuen K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. (Maywood) 2009;234(10):1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 22.Perera K.D., Galasiti Kankanamalage A.C., Rathnayake A.D., Honeyfield A., Groutas W., Chang K.O. Protease inhibitors broadly effective against feline, ferret and mink coronaviruses. Antivir. Res. 2018;160:79–86. doi: 10.1016/j.antiviral.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong L., Li J., Zhou Q., Xu Z., Chen L., Zhang Y. A new bat-HKU2-like coronavirus in swine, China, 2017. Emerg. Infect. Dis. 2017;23(9) doi: 10.3201/eid2309.170915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J. Virol. 2010;84(7):3134–3146. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolles M., Donaldson E., Baric R. SARS-CoV and emergent coronaviruses: viral determinants of interspecies transmission. Curr. Opin. Virol. 2011;1(6):624–634. doi: 10.1016/j.coviro.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Sahly H.M., Atmar R.L., Glezen W.P., Greenberg S.B. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin. Infect. Dis. 2000;31(1):96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020 doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchdoerfer R.N., Ward A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019;10(1):2342. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vabret A., Dina J., Mourez T., Gouarin S., Petitjean J., van der Werf S. Inter- and intra-variant genetic heterogeneity of human coronavirus OC43 strains in France. J. Gen. Virol. 2006;87(Pt 11):3349–3353. doi: 10.1099/vir.0.82065-0. [DOI] [PubMed] [Google Scholar]
- 35.Wogan L. Legal Treatment for Cat Disease Known as FIP Still Years Away. 2019. https://news.vin.com/VINNews.aspx?articleId=54548 Available from:
- 36.Pedersen N.C., Eckstrand C., Liu H., Leutenegger C., Murphy B. Levels of feline infectious peritonitis virus in blood, effusions, and various tissues and the role of lymphopenia in disease outcome following experimental infection. Vet. Microbiol. 2015;175(2–4):157–166. doi: 10.1016/j.vetmic.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First Case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The COVID-19 Investigation Team . 2020. First 12 Patients with Coronavirus Disease 2019 (COVID-19) in the United States. Preprint. [DOI] [PubMed] [Google Scholar]
- 39.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D. A randomized, controlled trial of ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3(10) doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. National Library of Medicine Clinical Trials Registry Coronavirus Trials. https://clinicaltrials.gov/ct2/results?cond=Coronavirus&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=&Search=Apply Available from: (accessed on 23 March 2020)
- 42.U.S. National Library of Medicine Clinical Trials Registry Adaptive COVID-19 Treatment Trial. https://clinicaltrials.gov/ct2/show/NCT04280705 Available from: (accessed on 23 March 2020)
- 43.U.S. National Library of Medicine Clinical Trials Registry Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) https://clinicaltrials.gov/ct2/show/NCT04292899 Available from: (accessed on 13 March 2020)
- 44.U.S. National Library of Medicine Clinical Trials Registry Mild/Moderate 2019-nCoV Remdesivir RCT. 2020. https://clinicaltrials.gov/ct2/show/NCT04252664 Available from: (accessed on 14 March 2020)
- 45.U.S. National Library of Medicine Clinical Trials Registry, Severe 2019-nCoV Remdesivir RCT 2020. https://clinicaltrials.gov/ct2/show/NCT04257656 Available from: (accessed on 14 March 2020)
- 46.Bolker J.A. Animal models in translational research: Rosetta stone or stumbling block? Bioessays. 2017;39(12) doi: 10.1002/bies.201700089. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q., Wang Y., Qi C., Shen L., Li J. Clinical trial analysis of 2019-nCoV therapy registered in China. J. Med. Virol. 2020 doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Food and Drug Association Chloroquine Label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/006002s044lbl.pdf Available from:
- 49.Grady D. Gilead to expand coronavirus drug trials to other countries. New York Times. 2020 https://www.nytimes.com/2020/02/26/health/coronavirus-gilead-drug-trials.html Available from: (accessed on 14 March 2020) [Google Scholar]
- 50.Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. Mechanism of inhibition of ebola virus RNA-dependent RNA polymerase by Remdesivir. Viruses. 2019;11(4) doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devlin S.I.H. Hopes rise over experimental drug’s effectiveness against coronavirus. The Guardian. 2020 https://www.theguardian.com/world/2020/mar/10/hopes-rise-over-experimental-drugs-effectiveness-against-coronavirus Available from: (accessed on 14 March 2020) [Google Scholar]