This pooled analysis analyzes the association between breastfeeding (ie, ever/never, duration, timing) and ovarian cancer risk overall and by histotype.
Key Points
Question
Is breastfeeding associated with risk of ovarian cancer overall and by histotype?
Findings
In this pooled analysis including 9973 women with ovarian cancer and 13 843 controls from 13 case-control studies, breastfeeding was associated with a 24% reduced risk of invasive epithelial ovarian cancer. Longer breastfeeding duration and shorter time since last breastfeeding episode were associated with a further decrease in risk.
Meaning
This large study with extensive information on breastfeeding provides epidemiological evidence that breastfeeding, a potentially modifiable factor, may confer significant reduction in ovarian cancer risk, including high-grade serous, the deadliest subtype.
Abstract
Importance
Breastfeeding has been associated with a reduced risk of epithelial ovarian cancer in multiple studies, but others showed no association. Whether risk reduction extends beyond that provided by pregnancy alone or differs by histotype is unclear. Furthermore, the observed associations between duration and timing of breastfeeding with ovarian cancer risk have been inconsistent.
Objective
To determine the association between breastfeeding (ie, ever/never, duration, timing) and ovarian cancer risk overall and by histotype.
Design, Setting, and Participants
A pooled analysis of parous women with ovarian cancer and controls from 13 case-control studies participating in the Ovarian Cancer Association Consortium was performed. Odds ratios (ORs) and 95% CIs of the overall association were calculated using multivariable logistic regression and polytomous logistic regression for histotype-specific associations. All data were collected from individual sites from November 1989 to December 2009, and analysis took place from September 2017 to July 2019.
Exposures
Data on breastfeeding history, including duration per child breastfed, age at first and last breastfeeding, and years since last breastfeeding were collected by questionnaire or interview and was harmonized across studies.
Main Outcomes and Measures
Diagnosis of epithelial ovarian cancer.
Results
A total of 9973 women with ovarian cancer (mean [SD] age, 57.4 [11.1] years) and 13 843 controls (mean [SD] age, 56.4 [11.7] years) were included. Breastfeeding was associated with a 24% lower risk of invasive ovarian cancer (odds ratio [OR], 0.76; 95% CI, 0.71-0.80). Independent of parity, ever having breastfed was associated with reduction in risk of all invasive ovarian cancers, particularly high-grade serous and endometrioid cancers. For a single breastfeeding episode, mean breastfeeding duration of 1 to 3 months was associated with 18% lower risk (OR, 0.82; 95% CI, 0.76-0.88), and breastfeeding for 12 or more months was associated with a 34% lower risk (OR, 0.66; 95% CI, 0.58-0.75). More recent breastfeeding was associated with a reduction in risk (OR, 0.56; 95% CI, 0.47-0.66 for <10 years) that persisted for decades (OR, 0.83; 95% CI, 0.77-0.90 for ≥30 years; P for trend = .02).
Conclusions and Relevance
Breastfeeding is associated with a significant decrease in risk of ovarian cancer overall and for the high-grade serous subtype, the most lethal type of ovarian cancer. The findings suggest that breastfeeding is a potentially modifiable factor that may lower risk of ovarian cancer independent of pregnancy alone.
Introduction
Ovarian cancer survival remains poor with 5-year survival less than 50%, mostly owing to late detection.1 Prevention is crucial for reducing mortality from this disease. With few modifiable risk factors beyond oral contraceptive (OC) use,1,2 identifying additional modifiable factors is needed to tailor prevention strategies.
Numerous studies have investigated the association between breastfeeding and ovarian cancer risk, with some showing a significant decrease in risk and others showing no association,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31 leading the World Cancer Research Fund International to describe evidence of the association as limited.2 Furthermore, relationships between ovarian cancer and breastfeeding patterns, including number of breastfeeding episodes, breastfeeding duration, and timing of breastfeeding, have been inconsistent. Prior studies have had insufficient sample size to evaluate heterogeneity by disease histotype. More than 90% of ovarian cancers are epithelial in origin,1 with the major histotypes exhibiting varied risk profiles29 and likely having distinct etiologic pathways. Therefore, we evaluated associations between breastfeeding and epithelial ovarian cancer risk overall and by histotype using data from 13 case-control studies participating in the Ovarian Cancer Association Consortium.
Methods
Study Population
The Ovarian Cancer Association Consortium was established in 2005 to promote collaborative research on epidemiologic factors associated with ovarian cancer. Our analysis includes data from 13 Ovarian Cancer Association Consortium studies32,33,34,35,36,37,38,39,40,41,42,43,44 with information on breastfeeding history (eTable 1 in Supplement). All studies are population-based and used in-person interviews for data collection except the Australian Ovarian Cancer Study37 and the German Ovarian Cancer Study,41 which used self-administered questionnaire for data collection. All studies frequency matched by age categories. The Hawaii Ovarian Cancer Case-Control Study,34 Family Registry for Ovarian Cancer and Genetic Epidemiology of Ovarian Cancer study,36 and University of Southern California Study of Lifestyle and Women’s Health43 additionally matched for race. The New Jersey Ovarian Cancer Study32 did not have any matching variables. All participants provided informed consent, and participating institutions obtained approval from relevant ethics committees. We excluded women who were nulliparous (n = 6309), missing parity information (n = 233), missing breastfeeding status (n = 480), and had nonepithelial tumors (n = 115). All data were collected from individual sites from November 1989 to December 2009.
Study Variables
Detailed information on breastfeeding history for each pregnancy lasting 6 months or longer, including ever breastfeeding (yes/no), breastfeeding duration (months), and age at each pregnancy was self-reported. Ever breastfeeding was defined as any breastfeeding, regardless of duration. A single breastfeeding episode refers to breastfeeding offspring from a given pregnancy (including twins and multiples). Total duration of breastfeeding was calculated by summing durations of individual breastfeeding episodes. If duration of any breastfeeding episode was unknown, total duration was considered unknown (n = 51). Mean breastfeeding duration per episode was obtained by dividing total duration by number of breastfeeding episodes. Timing of supplementation by formula, milk, or other foods was assessed in 5 studies (Connecticut Ovarian Cancer Study,38 Diseases of the Ovary and their Evaluation,40 Hawaii Ovarian Cancer Case-Control Study,34 North Carolina Ovarian Cancer Study,44 and University of Southern California Study of Lifestyle and Women’s Health43), although specific wording varied by sites. To define the duration of exclusive breastfeeding consistently across studies, we calculated the duration as the time between birth and initial supplementation based on these questions. Analyses of ages at first and last breastfeeding episode were restricted to girls and women 15 years or older at first or last breastfeeding episode. Other relevant variables include age at diagnosis/interview, decade of participant’s birth, self-reported race, attained education, total duration of OC use, parity, history of endometriosis, tubal ligation, menopausal status, young adult body mass index (calculated as weight in kilograms divided by height in meters squared), and family history of ovarian cancer.
Statistical Analyses
We used unconditional logistic regression to estimate the odds ratios (ORs) and 95% CIs for associations between breastfeeding and ovarian cancer risk for each site. We used indicator variables to account for missing data (race/ethnicity, n = 881; OC use, n = 264). In the main multivariate model, we adjusted for age (continuous), race/ethnicity (white, black, Asian, other/unknown), OC use (never, <1 year, 1 to <5 years, 5 to <10 years, ≥10 years, unknown), parity (continuous), and decade of birth (to account for possible changes in breastfeeding practices over time). We also considered adjustments for additional ovarian cancer risk factors including education (>high school, high school, some college, college, unknown), tubal ligation (no, yes, unknown), endometriosis (no, yes, unknown), body mass index at young adulthood (<20, 20 to <25, 25 to <30, ≥30, unknown), and family history of ovarian cancer (no, yes, unknown). However, these factors did not change the association between breastfeeding and ovarian cancer risk more than 10% and were not included in final models. We used random-effects meta-analyses of invasive cancers and borderline tumors and likelihood ratio tests to assess heterogeneity of the associations between study sites. Because we observed no significant heterogeneity, we used the pooled data set for all analyses adjusted for study site. We performed polytomous logistic regression to evaluate the associations between breastfeeding and cancer risk by invasive (low-grade serous, high-grade serous, mucinous, endometrioid, clear cell, other) and borderline (serous, mucinous) histotypes and likelihood ratio tests to evaluate differences in the associations by histotype. Among invasive serous tumors, low grade was defined as grade of 1 and high grade as grade of 2 or higher.
Because parity and breastfeeding are highly correlated, we created variables to evaluate each possible combination of parity and breastfeeding (eg, 1 liveborn with no breastfeeding, 1 liveborn with breastfeeding) to evaluate the independent association of breastfeeding with outcomes. We performed stratified analyses by age (≤50 years, >50 years), body mass index at young adulthood (<25, 25 to <30, ≥30), history of endometriosis, family history of ovarian cancer, and race/ethnicity. To evaluate interactions, we created crossproduct terms involving breastfeeding and the potential effect modifier and calculated likelihood-ratio statistics comparing models with and without the interaction terms. We tested for trend using Wald tests of continuous variables (months of breastfeeding, number of breastfeeding episodes, ages at first and last breastfeeding episodes, time since last breastfeeding episode) among women who reported ever having breastfed. Additionally, we conducted a sensitivity analysis using residuals to examine the association of breastfeeding patterns independent of parity. We computed residuals from a linear regression model with parity as the independent variable and number of breastfeeding episodes/total duration of breastfeeding as dependent variables. Residuals of breastfeeding episodes or total breastfeeding duration were next grouped into quintiles and used as the exposure of interest in multivariate models. To address potential confounding by parity, we restricted the analysis to women with exactly 2 births and examined whether breastfeeding both vs 1 child was associated with differential risk, adjusting for total duration of breastfeeding.
When examining the associations between ages at first and last breastfeeding episodes and ovarian cancer risk, we additionally adjusted for total breastfeeding duration and ages at first or last birth, respectively. We examined whether ages at first and last breastfeeding were independently associated with risk by simultaneously including both variables in the model. We additionally adjusted for age at last birth when examining the association between time since last breastfeeding and ovarian cancer risk, and we performed the duration and timing analyses restricted to high-grade serous histotype.
We used SAS version 9.4 (SAS Institute) for logistic regression analyses. For polytomous logistic regression, we used Stata/IC, version 15.1 (StataCorp). All P values are 2-sided, with .05 as the significance threshold. Analysis was performed from September 2017 to July 2019.
Results
A total of 9973 women with ovarian cancer and 13 843 controls were included in the analysis. Among parous controls, the prevalence of ever having breastfed ranged from 41% to 93% across studies, and mean breastfeeding duration per episode ranged from 3.4 to 8.7 months (eTable 1 in the Supplement). The mean (SD) age was 57.4 (11.1) years for women with ovarian cancer and 56.4 (11.7) years for controls, and 21 103 participants (89%) identified as white (eTable 2 in the Supplement). Compared with controls, those with ovarian cancer were older, more likely to be postmenopausal, primiparous, have never used OCs, have a history of endometriosis, and have a family history of ovarian cancer.
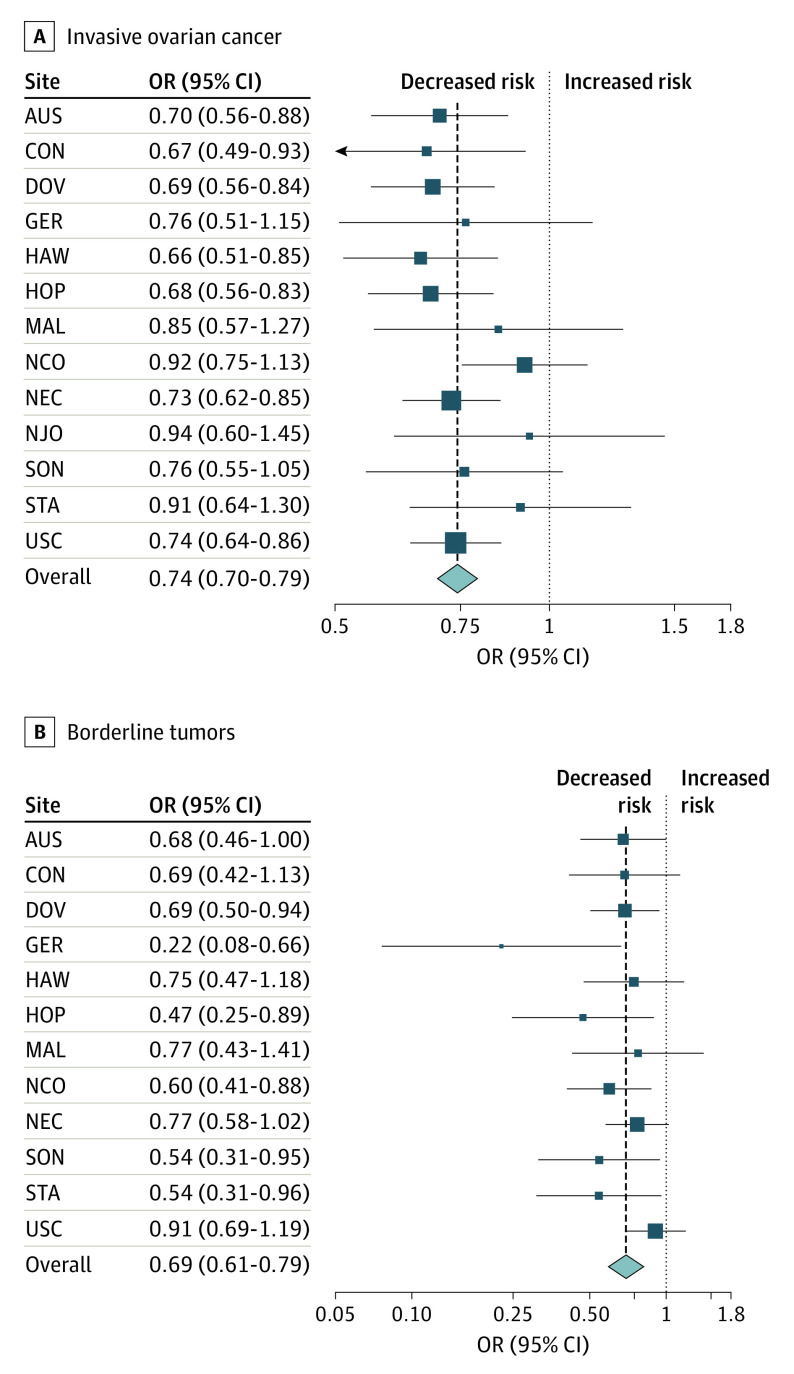
After multivariable adjustment, ever having breastfed was inversely associated with risks of both invasive (OR, 0.76; 95% CI, 0.71-0.80) and borderline (OR, 0.72; 95% CI, 0.64-0.81) tumors compared with never breastfeeding (Table 1). Associations were consistent across study sites for both invasive and borderline tumors (Figure). Among invasive tumors, the association was statistically significant for high-grade serous (OR, 0.75; 95% CI, 0.70-0.81), endometrioid (OR, 0.73; 95% CI, 0.64-0.84), and clear cell tumors (OR, 0.78; 95% CI, 0.64-0.96). We observed similar, although not statistically significant, associations for low-grade serous and no significant inverse association for mucinous tumors. For borderline tumors, there was a statistically significant inverse association for both mucinous (OR, 0.68; 95% CI, 0.57-0.82) and serous tumors (OR, 0.77; 95% CI, 0.66-0.89). In the 5 studies34,38,40,43,44 with data on exclusive breastfeeding, we observed a similar inverse association with ovarian cancer risk for women who breastfed exclusively for at least 3 months (OR, 0.81; 95% CI, 0.72-0.92) and breastfed but not exclusively for 3 months (OR, 0.70; 95% CI, 0.62-0.79) compared with women who never breastfed.
Table 1. Pooled ORs and 95% CIs for the Association Between Breastfeeding and Ovarian Cancer Risk in the Ovarian Cancer Association Consortium.
| Variable | Women with ovarian cancer, No. (%) | Breastfed, ever vs never, OR (95% CI)a |
|---|---|---|
| Invasive | ||
| Overall | 7991 (100) | 0.76 (0.71-0.80) |
| Serous | ||
| Low-grade | 324 (4) | 0.78 (0.61-1.01) |
| High-grade | 4718 (59) | 0.75 (0.70-0.81) |
| Mucinous | 508 (6) | 0.93 (0.76-1.14) |
| Endometrioid | 1027 (13) | 0.73 (0.64-0.84) |
| Clear cell | 452 (6) | 0.78 (0.64-0.96) |
| Other | 962 (12) | 0.75 (0.65-0.87) |
| P value for heterogeneity | NA | .46 |
| Borderline | ||
| Overall | 1843 (100) | 0.72 (0.64-0.81) |
| Serous | 1080 (61) | 0.77 (0.66-0.89) |
| Mucinous | 686 (39) | 0.68 (0.57-0.82) |
| P value for heterogeneity | NA | .31 |
Abbreviations: NA, not applicable; OR, odds ratio.
Adjusted for age, race/ethnicity, oral contraceptive use, parity, birth decade, and study site.
Figure. Association Between Breastfeeding and Epithelial Ovarian Cancer in 13 Case-Control Studies.
Summary odds ratios (ORs) and 95% CIs for the association between ever breastfeeding and risk of invasive ovarian cancer (A) or borderline tumors (B) estimated by random-effects meta-analysis of 13 studies from the Ovarian Cancer Association Consortium (1989-2009) adjusted for age, race/ethnicity, oral contraceptive use, parity, and birth decade. AUS indicates Australian Ovarian Cancer Study37; CON, Connecticut Ovarian Cancer Study38; DOV, Diseases of the Ovary and their Evaluation40; GER, German Ovarian Cancer Study41; HAW, Hawaii Ovarian Cancer Case-Control Study34; HOP, Hormones and Ovarian Cancer Prediction Study35; MAL, Malignant Ovarian Cancer Study33; NCO, North Carolina Ovarian Cancer Study44; NEC, New England Case Control Study42; NJO, New Jersey Ovarian Cancer Study32; SON, Southern Ontario Ovarian Cancer Study39; STA, Family Registry for Ovarian Cancer and Genetic Epidemiology of Ovarian Cancer36; USC, University of Southern California Study of Lifestyle and Women’s Health.43
To examine the association between breastfeeding and ovarian cancer risk within parity strata, we created a crossclassified variable of parity and breastfeeding (eFigure in the Supplement). Among primiparous women, ever breastfeeding was associated with 14% lower risk of all invasive disease (OR, 0.86; 95% CI, 0.75-0.99) and 16% lower risk of high-grade serous tumors (OR, 0.84; 95% CI, 0.71-0.99) compared with never breastfeeding. For multiparous women, we observed a similar inverse association for both all invasive and high-grade serous tumors.
In stratified analyses, we observed no statistically significant effect modification by age, body mass index at young adulthood, history of endometriosis, and family history of ovarian cancer (eTable 3 in the Supplement). Furthermore, we observed significant interaction by race/ethnicity (P for interaction = .01), with the strongest association in white women (OR, 0.73; 95% CI, 0.69-0.79) and no significant association among black women (OR, 0.92; 95% CI, 0.66-1.29) or Asian women (OR, 0.81; 95% CI, 0.58-1.12).
Compared with women who never breastfed, longer mean duration of breastfeeding per episode was inversely associated with invasive ovarian cancer risk (Table 2). Mean breastfeeding duration of less than 3 months per child was associated with 18% lower risk (OR, 0.82; 95% CI, 0.76-0.88) while mean breastfeeding duration of more than 12 months per child was associated with 34% lower risk (OR, 0.66; 95% CI, 0.58-0.75; P for trend = .001). Similar associations were observed in analyses restricted to high-grade serous tumors (Table 2) and in regression analyses of residuals controlled for parity (data not shown). In addition, we stratified mean breastfeeding duration per episode by parity (1, 2, and ≥3 live births) and observed associations similar in magnitude (Table 2). Similarly, total breastfeeding duration was significantly associated with reduced ovarian cancer risk (P for trend <.001 among women who breastfed; data not shown).
Table 2. Pooled ORs and 95% CIs for the Association Between Mean Duration of Breastfeeding per Episode and Invasive Ovarian Cancer Risk Stratified by Parity in the Ovarian Cancer Association Consortium.
| Variable | Controls, No. (%) | All invasive | High-grade serous | ||
|---|---|---|---|---|---|
| Women with ovarian cancer, No. (%) | OR (95% CI) | Women with ovarian cancer, No. (%) | OR (95% CI) | ||
| Mean breastfeeding duration per episode, moa,b | |||||
| Never breastfed | 4426 (32) | 3257 (41) | 1 [Reference] | 1978 (42) | 1 [Reference] |
| 0 to <3 | 3473 (25) | 1879 (24) | 0.82 (0.76-0.88) | 1067 (23) | 0.79 (0.72-0.86) |
| 3 to <6 | 2553 (19) | 1311 (17) | 0.75 (0.69-0.82) | 776 (17) | 0.76 (0.68-0.84) |
| 6 to <9 | 1454 (11) | 676 (9) | 0.69 (0.62-0.76) | 394 (8) | 0.68 (0.60-0.78) |
| 9 to <12 | 914 (7) | 399 (5) | 0.66 (0.58-0.76) | 235 (5) | 0.68 (0.58-0.79) |
| ≥12 | 934 (7) | 400 (5) | 0.66 (0.58-0.75) | 218 (5) | 0.66 (0.56-0.78) |
| P value for trendc | NA | NA | .001 | NA | .01 |
| Mean breastfeeding duration per episode among women with 1 birth, mod | |||||
| Never breastfed | 881 (40) | 716 (47) | 1 [Reference] | 389 (50) | 1 [Reference] |
| 0 to <3 | 573 (26) | 364 (24) | 0.90 (0.75-1.07) | 188 (24) | 0.94 (0.76-1.18) |
| 3 to <6 | 316 (14) | 200 (13) | 0.85 (0.69-1.06) | 92 (12) | 0.81 (0.61-1.07) |
| 6 to <9 | 161 (7) | 93 (6) | 0.74 (0.55-0.98) | 41 (5) | 0.68 (0.46-1.00) |
| 9 to <12 | 130 (6) | 67 (4) | 0.68 (0.49-0.94) | 36 (5) | 0.78 (0.52-1.17) |
| ≥12 | 169 (8) | 88 (6) | 0.66 (0.49-0.89) | 37 (5) | 0.64 (0.43-0.95) |
| P value for trendc | NA | NA | .01 | NA | .01 |
| Mean breastfeeding duration per episode among women with 2 births, mod | |||||
| Never breastfed | 1726 (32) | 1257 (42) | 1 [Reference] | 765 (44) | 1 [Reference] |
| 0 to <3 | 1325 (24) | 700 (23) | 0.83 (0.73-0.94) | 377 (21) | 0.76 (0.65-0.88) |
| 3 to <6 | 1087 (20) | 493 (16) | 0.69 (0.60-0.79) | 279 (16) | 0.68 (0.57-0.80) |
| 6 to <9 | 611 (11) | 277 (9) | 0.67 (0.57-0.80) | 171 (10) | 0.70 (0.57-0.86) |
| 9 to <12 | 349 (6) | 131 (4) | 0.57 (0.45-0.71) | 72 (4) | 0.52 (0.39-0.68) |
| ≥12 | 372 (7) | 161 (5) | 0.67 (0.54-0.82) | 90 (5) | 0.66 (0.51-0.86) |
| P value for trendc | NA | NA | .02 | NA | .13 |
| Mean breastfeeding duration per episode among women with 3 births, mod | |||||
| Never breastfed | 1013 (29) | 750 (39) | 1 [Reference] | 470 (40) | 1 [Reference] |
| 0 to <3 | 899 (26) | 469 (24) | 0.77 (0.66-0.90) | 268 (23) | 0.72 (0.60-0.87) |
| 3 to <6 | 640 (19) | 342 (17) | 0.77 (0.65-0.91) | 226 (19) | 0.85 (0.69-1.04) |
| 6 to <9 | 408 (12) | 174 (9) | 0.66 (0.53-0.82) | 106 (9) | 0.68 (0.53-0.88) |
| 9 to <12 | 262 (8) | 118 (6) | 0.74 (0.57-0.95) | 71 (6) | 0.76 (0.56-1.03) |
| ≥12 | 220 (6) | 66 (3) | 0.51 (0.38-0.70) | 41 (3) | 0.58 (0.40-0.84) |
| P value for trendc | NA | NA | .04 | NA | .42 |
Abbreviations: NA, not applicable; OR, odds ratio.
Adjusted for age, race/ethnicity, oral contraceptive use, parity, birth decade, and study site.
Mean duration of breastfeeding per child breastfed.
Among women who breastfed.
Adjusted for age, race, oral contraceptive use, birth decade, and study site.
Among women who breastfed, older ages at first (OR, 0.90; 95% CI, 0.85-0.96, per 5 years of age increase at first breastfeeding; P for trend = .001) and last breastfeeding episode (OR, 0.94; 95% CI, 0.89-0.99, per 5 years of age increase at last breastfeeding; P for trend = .02) were associated with lower risk of invasive ovarian cancer (eTable 4 in the Supplement). When adjusted for ages at first and last birth together, both associations were attenuated. Breastfeeding was associated with a substantial reduction in ovarian cancer risk for decades after the last breastfeeding episode, although the magnitude of risk reduction attenuated over time. Compared with women who never breastfed, time since last breastfeeding episode of less than 10 years was associated with 44% lower risk (OR, 0.56; 95% CI, 0.47-0.66), while time since last breastfeeding episode of 30 years or less was associated with 17% lower risk (OR, 0.83; 95% CI, 0.77-0.90; P for trend = .02). Associations for age at breastfeeding and timing of breastfeeding were similar for high-grade serous tumors (eTable 4 in the Supplement) and when restricted to women with 2 births (eTable 5 in the Supplement).
Discussion
In this largest study of breastfeeding and ovarian cancer risk to date and to our knowledge, including 9973 parous women with ovarian cancer from 13 studies, a history of breastfeeding was associated with 24% decrease in invasive cancer risk and 28% decrease in borderline tumor risk, with consistent risk reduction across study sites. Furthermore, mean breastfeeding duration per episode was inversely associated with risk, with 18% reduction in risk for women who breastfed less than 3 months per live birth and 34% reduction for women who breastfed 12 months or longer per live birth. Similar associations were observed for high-grade serous tumors, the deadliest ovarian cancer histotype.
Consistent with some but not all prior studies,3,4,5,6,7,8,9,10,11,12,13,14,15,17,20,22,24,26,27,29,30,44,45,46,47,48,49,50,51,52,53,54,55 we observed an inverse association between mean breastfeeding duration per episode and risk of invasive and borderline ovarian tumors. Statistically significant risk reduction associated with a mean breastfeeding duration of less than 3 months per episode suggests even a short duration of breastfeeding is beneficial. We did not observe a statistically significant difference in risk associated with nonexclusive breastfeeding compared with exclusive breastfeeding; however, sample size was limited and exclusive breastfeeding definitions varied between studies.56 Associations between breastfeeding and ovarian cancer risk were stronger with older ages at first and last breastfeeding episodes, consistent with previous reports.57,58,59 However, these associations were attenuated after mutual adjustment for ages at first and last breastfeeding. While risk reduction was greatest for women who had breastfed within the past 10 years, the association persisted more than 30 years, a pattern also seen with time since last OC use and last birth.59
Our findings indicate that breastfeeding is associated with lower risk of high-grade serous cancers, the most common and fatal subtype. While previous studies4,5,29,46,47 reported inconsistent findings for serous histotypes, most did not separate low- from high-grade serous tumors, which are thought to have separate etiologic pathways.29,60 The Million Women Study5 separately analyzed low- and high-grade serous tumors and reported a lower risk of both histotypes with longer total and mean duration per episode of breastfeeding. However, the associations did not reach statistical significance. Similar to ovarian cancer overall, we observed a reduced risk of high-grade serous tumors with as little as 3 months’ mean breastfeeding duration per episode and greater risk reduction with longer breastfeeding duration, older ages at first and last breastfeeding episodes, and recent (<10 years) breastfeeding. The inverse association with time since last breastfeeding persisted for more than 30 years. While we observed similar associations with breastfeeding patterns and endometrioid ovarian cancers, fewer cases of other invasive histotypes limited our ability to evaluate them with the same level of detail.
Biological mechanisms through which breastfeeding could reduce ovarian cancer risk are not well understood. To date, the leading hypothesis has been that ovulation suppression during breastfeeding inhibits epithelial cell division and proliferation, thereby reducing the opportunity to initiate or promote carcinogenesis. This may especially be pertinent in the first few months post partum when immune function and tumor surveillance mechanisms remain suppressed.61 However, we observed a stronger inverse association with longer breastfeeding duration, suggesting anovulation cannot entirely explain the association because ovulation typically returns once solids are introduced. Several lines of evidence suggest that breastfeeding may also be associated with long-term modulation of inflammatory, immune, or metabolic pathways, which could influence ovarian cancer risk.62,63
Strengths and Limitations
Important strengths of this study include detailed data on breastfeeding patterns, including information on duration and timing of each breastfeeding episode. We included a large number of women with ovarian cancer and controls from established studies with geographic diversity and extensive data on demographic, reproductive, and lifestyle variables. The large sample size enabled us to examine breastfeeding patterns in detail and specifically to assess high-grade serous tumors, the most common and fatal histotype. In addition, our pooled analysis allowed us to harmonize variables for consistency across the 13 studies, adjust for a single set of confounders, evaluate histotype-specific associations, and assess potential effect modification in stratified analyses. Importantly, we were also able to disentangle the association between breastfeeding and ovarian cancer risk from that of parity.
Our study has some limitations, including the potential for differential self-reporting of breastfeeding information by disease status, which could distort the magnitude of associations. Our results may also be influenced by selection bias as controls participating in these studies may differ from women with ovarian cancer by factors influencing breastfeeding or ovarian cancer risk, including unknown factors that could not be accounted for in the analysis. Despite adjustment for potential confounders, unmeasured confounding may persist but is unlikely to substantively alter our findings given our robust associations. Validation in prospective cohorts is needed to address these concerns. Finally, because our study population included predominantly white women, we could not sufficiently evaluate details of breastfeeding patterns in black women, Asian women, and less common racial and ethnic groups.20 The association between breastfeeding and ovarian cancer needs to be investigated in large populations of other races and ethnicities.
Conclusions
In conclusion, breastfeeding is associated with a significant decrease in ovarian cancer risk overall and for high-grade serous tumors, the most lethal subtype. The World Health Organization recommends exclusive breastfeeding for at least 6 months and continued breastfeeding with complementary foods for 2 or more years.2,64 Our results support these recommendations, while noting that breastfeeding fewer than 3 months per child is still associated with significant ovarian cancer risk reduction.
eTable 1. Characteristics of 13 case-control studies from the Ovarian Cancer Association Consortium (1989-2009) included in the pooled analysis of breastfeeding and ovarian cancer risk
eTable 2. Characteristics of participants from 13 studies from the Ovarian Cancer Association Consortium (1989-2009) included in the pooled analysis of breastfeeding and ovarian cancer risk
eTable 3. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the association between breastfeeding history and invasive ovarian cancer risk by selected characteristics in the Ovarian Cancer Association Consortium
eTable 4. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the association between age and time since breastfeeding and invasive ovarian cancer risk in the Ovarian Cancer Association Consortium
eTable 5. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the association between age and time since breastfeeding and invasive ovarian cancer risk among women with 2 births in the Ovarian Cancer Association Consortium
eFigure. Cross-classification of parity and breastfeeding in relation to invasive ovarian cancer and high-grade serous ovarian cancer risk in the Ovarian Cancer Association Consortium
References
- 1.American Cancer Society Cancer Facts & Figures 2019. American Cancer Society; 2019. [Google Scholar]
- 2.World Cancer Research Fund/ American Institute for Cancer Research Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. World Cancer Research Fund International; 2018. [Google Scholar]
- 3.Chiaffarino F, Pelucchi C, Negri E, et al. Breastfeeding and the risk of epithelial ovarian cancer in an Italian population. Gynecol Oncol. 2005;98(2):304-308. doi: 10.1016/j.ygyno.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 4.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes Control. 2007;18(5):517-523. doi: 10.1007/s10552-007-0130-2 [DOI] [PubMed] [Google Scholar]
- 5.Gaitskell K, Green J, Pirie K, et al. ; Million Women Study Collaborators . Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. Int J Cancer. 2018;142(2):281-289. doi: 10.1002/ijc.31063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greggi S, Parazzini F, Paratore MP, et al. Risk factors for ovarian cancer in central Italy. Gynecol Oncol. 2000;79(1):50-54. doi: 10.1006/gyno.2000.5909 [DOI] [PubMed] [Google Scholar]
- 7.Gronwald J, Byrski T, Huzarski T, et al. Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat. 2006;95(2):105-109. doi: 10.1007/s10549-005-9051-5 [DOI] [PubMed] [Google Scholar]
- 8.Gwinn ML, Lee NC, Rhodes PH, Layde PM, Rubin GL. Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. J Clin Epidemiol. 1990;43(6):559-568. doi: 10.1016/0895-4356(90)90160-Q [DOI] [PubMed] [Google Scholar]
- 9.Harlow BL, Weiss NS, Roth GJ, Chu J, Daling JR. Case-control study of borderline ovarian tumors: reproductive history and exposure to exogenous female hormones. Cancer Res. 1988;48(20):5849-5852. [PubMed] [Google Scholar]
- 10.Harris R, Whittemore AS, Itnyre J; Collaborative Ovarian Cancer Group . Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. III: epithelial tumors of low malignant potential in white women. Am J Epidemiol. 1992;136(10):1204-1211. doi: 10.1093/oxfordjournals.aje.a116428 [DOI] [PubMed] [Google Scholar]
- 11.Hartge P, Schiffman MH, Hoover R, McGowan L, Lesher L, Norris HJ. A case-control study of epithelial ovarian cancer. Am J Obstet Gynecol. 1989;161(1):10-16. doi: 10.1016/0002-9378(89)90221-4 [DOI] [PubMed] [Google Scholar]
- 12.Hirose K, Tajima K, Hamajima N, et al. Comparative case-referent study of risk factors among hormone-related female cancers in Japan. Jpn J Cancer Res. 1999;90(3):255-261. doi: 10.1111/j.1349-7006.1999.tb00741.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huusom LD, Frederiksen K, Høgdall EV, et al. Association of reproductive factors, oral contraceptive use and selected lifestyle factors with the risk of ovarian borderline tumors: a Danish case-control study. Cancer Causes Control. 2006;17(6):821-829. doi: 10.1007/s10552-006-0022-x [DOI] [PubMed] [Google Scholar]
- 14.Jordan SJ, Green AC, Whiteman DC, Webb PM; Australian Ovarian Cancer Study Group . Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol. 2007;107(2):223-230. doi: 10.1016/j.ygyno.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Kurta ML, Moysich KB, Weissfeld JL, et al. Use of fertility drugs and risk of ovarian cancer: results from a U.S.-based case-control study. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1282-1292. doi: 10.1158/1055-9965.EPI-12-0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le DC, Kubo T, Fujino Y, et al. Reproductive factors in relation to ovarian cancer: a case-control study in Northern Vietnam. Contraception. 2012;86(5):494-499. doi: 10.1016/j.contraception.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin JR, Risch HA, Lubinski J, et al. ; Hereditary Ovarian Cancer Clinical Study Group . Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet Oncol. 2007;8(1):26-34. doi: 10.1016/S1470-2045(06)70983-4 [DOI] [PubMed] [Google Scholar]
- 18.Mink PJ, Folsom AR, Sellers TA, Kushi LH. Physical activity, waist-to-hip ratio, and other risk factors for ovarian cancer: a follow-up study of older women. Epidemiology. 1996;7(1):38-45. doi: 10.1097/00001648-199601000-00008 [DOI] [PubMed] [Google Scholar]
- 19.Modugno F, Goughnour SL, Wallack D, et al. Breastfeeding factors and risk of epithelial ovarian cancer. Gynecol Oncol. 2019;153(1):116-122. doi: 10.1016/j.ygyno.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moorman PG, Alberg AJ, Bandera EV, et al. Reproductive factors and ovarian cancer risk in African-American women. Ann Epidemiol. 2016;26(9):654-662. doi: 10.1016/j.annepidem.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori M, Harabuchi I, Miyake H, Casagrande JT, Henderson BE, Ross RK. Reproductive, genetic, and dietary risk factors for ovarian cancer. Am J Epidemiol. 1988;128(4):771-777. doi: 10.1093/oxfordjournals.aje.a115030 [DOI] [PubMed] [Google Scholar]
- 22.Mori M, Nishida T, Sugiyama T, et al. Anthropometric and other risk factors for ovarian cancer in a case-control study. Jpn J Cancer Res. 1998;89(3):246-253. doi: 10.1111/j.1349-7006.1998.tb00555.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pięta B, Chmaj-Wierzchowska K, Opala T. Past obstetric history and risk of ovarian cancer. Ann Agric Environ Med. 2012;19(3):385-388. [PubMed] [Google Scholar]
- 24.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Salmeron-Castro J, Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Res. 1999;59(15):3658-3662. [PubMed] [Google Scholar]
- 25.Titus-Ernstoff L, Rees JR, Terry KL, Cramer DW. Breast-feeding the last born child and risk of ovarian cancer. Cancer Causes Control. 2010;21(2):201-207. doi: 10.1007/s10552-009-9450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsilidis KK, Allen NE, Key TJ, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2011;105(9):1436-1442. doi: 10.1038/bjc.2011.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tung KH, Goodman MT, Wu AH, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. 2003;158(7):629-638. doi: 10.1093/aje/kwg177 [DOI] [PubMed] [Google Scholar]
- 28.Weiderpass E, Sandin S, Inoue M, et al. Risk factors for epithelial ovarian cancer in Japan: results from the Japan Public Health Center-based Prospective Study cohort. Int J Oncol. 2012;40(1):21-30. [DOI] [PubMed] [Google Scholar]
- 29.Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888-2898. doi: 10.1200/JCO.2016.66.8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittemore AS, Harris R, Itnyre J; Collaborative Ovarian Cancer Group . Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II: invasive epithelial ovarian cancers in white women. Am J Epidemiol. 1992;136(10):1184-1203. doi: 10.1093/oxfordjournals.aje.a116427 [DOI] [PubMed] [Google Scholar]
- 31.Wilailak S, Vipupinyo C, Suraseranivong V, et al. Depot medroxyprogesterone acetate and epithelial ovarian cancer: a multicentre case-control study. BJOG. 2012;119(6):672-677. doi: 10.1111/j.1471-0528.2012.03298.x [DOI] [PubMed] [Google Scholar]
- 32.Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. doi: 10.1186/1472-6874-11-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glud E, Kjaer SK, Thomsen BL, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004;164(20):2253-2259. doi: 10.1001/archinte.164.20.2253 [DOI] [PubMed] [Google Scholar]
- 34.Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15(4):1055-1060. doi: 10.1677/ERC-08-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23(2):311-319. doi: 10.1097/EDE.0b013e3182456ad3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuire V, Felberg A, Mills M, et al. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160(7):613-618. doi: 10.1093/aje/kwh284 [DOI] [PubMed] [Google Scholar]
- 37.Merritt MA, Green AC, Nagle CM, Webb PM; Australian Cancer Study (Ovarian Cancer); Australian Ovarian Cancer Study Group . Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122(1):170-176. doi: 10.1002/ijc.23017 [DOI] [PubMed] [Google Scholar]
- 38.Risch HA, Bale AE, Beck PA, Zheng W. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1738-1741. doi: 10.1158/1055-9965.EPI-06-0272 [DOI] [PubMed] [Google Scholar]
- 39.Risch HA, Marrett LD, Howe GR. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am J Epidemiol. 1994;140(7):585-597. doi: 10.1093/oxfordjournals.aje.a117296 [DOI] [PubMed] [Google Scholar]
- 40.Bodelon C, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23(12):1985-1994. doi: 10.1007/s10552-012-0076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95(6):370-374. doi: [DOI] [PubMed] [Google Scholar]
- 42.Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65(13):5974-5981. doi: 10.1158/0008-5472.CAN-04-3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124(6):1409-1415. doi: 10.1002/ijc.24091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moorman PG, Calingaert B, Palmieri RT, et al. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am J Epidemiol. 2008;167(9):1059-1069. doi: 10.1093/aje/kwn006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. 1989;60(4):592-598. doi: 10.1038/bjc.1989.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan SJ, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Breast-feeding and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23(6):919-927. doi: 10.1007/s10552-012-9963-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modugno F, Ness RB, Wheeler JE. Reproductive risk factors for epithelial ovarian cancer according to histologic type and invasiveness. Ann Epidemiol. 2001;11(8):568-574. doi: 10.1016/S1047-2797(01)00213-7 [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Wu PC, Lang JH, Ge WJ, Hartge P, Brinton LA. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992;21(1):23-29. doi: 10.1093/ije/21.1.23 [DOI] [PubMed] [Google Scholar]
- 49.Mills PK, Riordan DG, Cress RD. Epithelial ovarian cancer risk by invasiveness and cell type in the Central Valley of California. Gynecol Oncol. 2004;95(1):215-225. doi: 10.1016/j.ygyno.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 50.Ness RB, Grisso JA, Klapper J, et al. ; SHARE Study Group . Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. Am J Epidemiol. 2000;152(3):233-241. doi: 10.1093/aje/152.3.233 [DOI] [PubMed] [Google Scholar]
- 51.Riman T, Dickman PW, Nilsson S, et al. Risk factors for epithelial borderline ovarian tumors: results of a Swedish case-control study. Gynecol Oncol. 2001;83(3):575-585. doi: 10.1006/gyno.2001.6451 [DOI] [PubMed] [Google Scholar]
- 52.Risch HA, Weiss NS, Lyon JL, Daling JR, Liff JM. Events of reproductive life and the incidence of epithelial ovarian cancer. Am J Epidemiol. 1983;117(2):128-139. doi: 10.1093/oxfordjournals.aje.a113523 [DOI] [PubMed] [Google Scholar]
- 53.Rosenblatt KA, Thomas DB; The WHO Collaborative Study of Neoplasia and Steroid Contraceptives . Lactation and the risk of epithelial ovarian cancer. Int J Epidemiol. 1993;22(2):192-197. doi: 10.1093/ije/22.2.192 [DOI] [PubMed] [Google Scholar]
- 54.Yen ML, Yen BL, Bai CH, Lin RS. Risk factors for ovarian cancer in Taiwan: a case-control study in a low-incidence population. Gynecol Oncol. 2003;89(2):318-324. doi: 10.1016/S0090-8258(03)00088-X [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Xie X, Lee AH, Binns CW. Prolonged lactation reduces ovarian cancer risk in Chinese women. Eur J Cancer Prev. 2004;13(6):499-502. doi: 10.1097/00008469-200412000-00006 [DOI] [PubMed] [Google Scholar]
- 56.Perez A, Vela P, Masnick GS, Potter RG. First ovulation after childbirth: the effect of breast-feeding. Am J Obstet Gynecol. 1972;114(8):1041-1047. doi: 10.1016/0002-9378(72)90866-6 [DOI] [PubMed] [Google Scholar]
- 57.Adami HO, Hsieh CC, Lambe M, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344(8932):1250-1254. doi: 10.1016/S0140-6736(94)90749-8 [DOI] [PubMed] [Google Scholar]
- 58.Whiteman DC, Siskind V, Purdie DM, Green AC. Timing of pregnancy and the risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(1):42-46. [PubMed] [Google Scholar]
- 59.Wu AH, Pearce CL, Lee AW, et al. Timing of births and oral contraceptive use influences ovarian cancer risk. Int J Cancer. 2017;141(12):2392-2399. doi: 10.1002/ijc.30910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine Ovarian Cancers: Evolving Paradigms in Research and Care. National Academies Press;2016. [PubMed] [Google Scholar]
- 61.Groer MW, El-Badri N, Djeu J, Williams SN, Kane B, Szekeres K. Suppression of natural killer cell cytotoxicity in postpartum women: time course and potential mechanisms. Biol Res Nurs. 2014;16(3):320-326. doi: 10.1177/1099800413498927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81-88. doi: 10.1055/s-0028-1103034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang D, Li N, Xi Y, Zhao Y, Wang T. Diabetes mellitus and risk of ovarian cancer: a systematic review and meta-analysis of 15 cohort studies. Diabetes Res Clin Pract. 2017;130:43-52. doi: 10.1016/j.diabres.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Maternal, newborn, child and adolescent health. Accessed February 27, 2020. https://www.who.int/maternal_child_adolescent/topics/child/nutrition/breastfeeding/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of 13 case-control studies from the Ovarian Cancer Association Consortium (1989-2009) included in the pooled analysis of breastfeeding and ovarian cancer risk
eTable 2. Characteristics of participants from 13 studies from the Ovarian Cancer Association Consortium (1989-2009) included in the pooled analysis of breastfeeding and ovarian cancer risk
eTable 3. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the association between breastfeeding history and invasive ovarian cancer risk by selected characteristics in the Ovarian Cancer Association Consortium
eTable 4. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the association between age and time since breastfeeding and invasive ovarian cancer risk in the Ovarian Cancer Association Consortium
eTable 5. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the association between age and time since breastfeeding and invasive ovarian cancer risk among women with 2 births in the Ovarian Cancer Association Consortium
eFigure. Cross-classification of parity and breastfeeding in relation to invasive ovarian cancer and high-grade serous ovarian cancer risk in the Ovarian Cancer Association Consortium