This cohort study assesses presurgical and postsurgical patient- and clinical-level factors associated with the delayed administration of postoperative radiation therapy among patients with head and neck squamous cell carcinoma.
Key Points
Question
What variables are associated with delayed initiation of postoperative radiotherapy after surgical treatment for head and neck squamous cell carcinoma that could be incorporated into clinically useful nomograms for pretreatment counseling and risk adjustment?
Findings
In this cohort study of 60 776 patients with head and neck squamous cell carcinoma, delayed postoperative radiotherapy initiation was associated with race/ethnicity, insurance type, tumor site, US region, facility type, clinical stage, length of stay, and care fragmentation. Presurgical and postsurgical nomograms based on these variables were developed and externally validated.
Meaning
Findings of this study suggest that a nomogram using presurgical information can improve pretreatment counseling and targeted intervention delivery for patients at high risk for postoperative radiotherapy initiation delay, whereas a nomogram also using postsurgical data can drive institutional quality improvement initiatives and enhance risk-adjusted comparisons of delay rates across facilities.
Abstract
Importance
The standard of care for initiation of postoperative radiotherapy (PORT) in head and neck squamous cell carcinoma (HNSCC) is within 6 weeks of surgical treatment. Delays in guideline-adherent PORT initiation are common, associated with mortality, and a measure of quality care, but patient-specific tools to estimate the risk of these delays are lacking.
Objective
To develop and validate 2 nomograms (that use presurgical and postsurgical data) for predicting delayed PORT initiation.
Design, Setting, and Participants
This cohort study obtained patient data from January 1, 2004, to December 31, 2015, from the National Cancer Database. Adults aged 18 years or older with a newly diagnosed HNSCC who underwent surgical treatment and PORT at a Commission on Cancer–accredited facility were included. Data analysis was conducted from June 2, 2019, to January 29, 2020.
Exposures
Surgical treatment and PORT.
Main Outcomes and Measures
The primary outcome measure was PORT initiation more than 6 weeks after the surgical intervention. Multivariable logistic regression models were created in a random selection of 80% of the sample (derivation cohort) and were internally validated with bootstrapping, assessed for discrimination by calibration plots and the concordance (C) index, and externally validated in the remaining 20% of the sample (validation cohort).
Results
The study included 60 766 adults with HNSCC who were grouped into derivation and validation cohorts. The derivation cohort comprised 48 625 patients (mean [SD] age, 59.59 [11.3] years; 36 825 men [75.7%]) selected randomly from the full sample, whereas 12 151 patients (mean [SD] age, 59.63 [11.2] years; 9266 men [76.3%]) composed the validation cohort. The rate of PORT delay was 55.8% (n=27140) in the derivation cohort and 56.7% (n=6900) in the validation cohort. Both nomograms created to predict the risk of PORT initiation delay used variables, including race/ethnicity, insurance type, tumor site, and facility type. The nomogram based on presurgical variables included clinical stage and severity of comorbidity, whereas the nomogram with postsurgical variables included US region, length of stay, and care fragmentation between surgical and radiotherapy facilities. For the presurgical nomogram, the concordance indices were 0.670 (95% CI, 0.664-0.676) in the derivation cohort and 0.674 (95% CI, 0.662-0.685) in the validation cohort. For the nomogram with postsurgical variables, the concordance indices were 0.691 (95% CI, 0.686-0.696) in the derivation cohort and 0.694 (95% CI, 0.685-0.704) in the validation cohort.
Conclusions and Relevance
This study found that a nomogram developed with presurgical data to generate personalized estimates of PORT initiation delay may improve pretreatment counseling and the delivery of interventions to patients at high risk for such a delay. A nomogram including postsurgical data can drive institutional quality improvement initiatives and enhance risk-adjusted comparisons of delay rates across facilities.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is diagnosed in 65 000 individuals and results in 14 600 deaths per year in the United States.1 It is a disease in which advanced stage presentation is common1 and treatment delays are prevalent.2 Despite aggressive multimodal therapy consisting of a combination of surgical intervention, radiotherapy, and chemotherapy,3 outcomes remain poor for HNSCC, with only 50% of patients with locally advanced HNSCC surviving beyond 5 years.1 For patients with locoregionally advanced, surgically treated HNSCC, the treatment package includes surgical intervention followed by postoperative radiotherapy (PORT) with or without concurrent chemotherapy. Within this treatment package, starting PORT within 6 weeks of the surgical procedure is the standard of care according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines3 and is key to obtaining optimal mortality and morbidity outcomes and optimizes health care resources.2
Delays in starting PORT after surgical treatment for HNSCC are associated with an increased risk of cancer recurrence and decreased survival,2,4,5 independent of the treatment package time (ie, time from the surgical procedure to the completion of PORT).6 Delayed PORT initiation is also the primary factor in prolonged treatment package time.7 A study found that 56% of patients with HNSCC did not receive guideline-adherent, timely PORT.8 As such, the care delivery pathway for PORT, which is potentially modifiable through an intervention that addresses the structure of cancer care delivery, might be a target and might have the potential to decrease mortality for patients with HNSCC.2,6,9 However, knowledge of models for predicting PORT initiation delays is lacking, preventing the implementation of presurgical interventions targeting those patients at highest risk.
The time interval from surgical treatment to PORT initiation (≤6 weeks) is the only measure of timely care in the NCCN Guidelines for HNSCC.3 Although patient, medical, surgical, and socioeconomic factors may play a role in delays in starting PORT, the delays themselves reflect the underlying cancer care delivery processes10 and have been consistently associated with worse oncologic outcomes by a variety of systematic reviews.2,4 Delivery of timely PORT has, therefore, been proposed as a measure of the quality of HNSCC care delivery.11 However, rates of PORT initiation delay vary widely across institutions, reflecting the differences in case mix and health care delivery settings.8
Use of nomograms to predict PORT initiation delay may be a solution to both problems. A nomogram is a graphic depiction of a statistical model that quantifies the risk of an event. It can provide health care practitioners a user-friendly interface to streamline risk assessment and to efficiently communicate personalized risk assessments to patients, thereby improving clinical decision-making.12 A presurgical nomogram can generate personalized estimates of the risk of PORT initiation delay, thereby enhancing pretreatment counseling and guiding the implementation of interventions for patients at high-risk for late PORT administration. On the other hand, a nomogram incorporating postsurgical information can be used to compare rates of PORT initiation delay across institutions with different case mixes and patient populations. For example, a nomogram can help calculate an expected risk of PORT initiation delay for each patient at an institution; this estimated risk of delay can then be compared with the observed rate of delay at the institution across its mix of cases. Such a tool would enhance the validity and fairness of risk adjustment (eg, by adjusting for case mix for institutions that systematically treat patients with lower socioeconomic status) and facilitate the use of PORT initiation delay rate as a measure of quality HNSCC care delivery.
Therefore, the objectives of this study were to develop and validate 2 nomograms for predicting nonadherence with the NCCN Guidelines for timely initiation of PORT. One nomogram was based on information available in the presurgical setting, and the other one incorporated both presurgical and postsurgical information.
Methods
This cohort study obtained patient data from January 1, 2004, to December 31, 2015, from the National Cancer Database, a hospital-based cancer registry that is jointly maintained by the American College of Surgeons Commission on Cancer and the American Cancer Society. The database captures data from more than 1500 Commission on Cancer–accredited hospitals in the US and is generalizable to US patients with HNSCC.13 Because the data used in this study contained no personal identifiers, the study was exempt from review, and thus from the informed consent requirement, by the Medical University of South Carolina Institutional Review Board.
Study Population
The study sample included patients aged 18 years or older with a newly diagnosed HNSCC who underwent a curative-intent surgical procedure and PORT with or without chemotherapy. The HNSCC diagnoses were filtered with International Classification of Diseases for Oncology, Third Revision, topography codes for the oral cavity, oropharynx, hypopharynx, and larynx as well as histologic codes for squamous cell carcinoma (eTable in the Supplement). Patients who received the following interventions were excluded (because of our concerns about their clinical relevance and the data accuracy): induction chemotherapy; brachytherapy, stereotactic radiotherapy, or an unspecified radiation modality; palliative-intent treatment; definitive surgical intervention more than 180 days after diagnosis; and PORT initiation more than 180 days after the surgical procedure (eFigure in the Supplement). The final sample was divided into a derivation cohort (a random selection of 80% of the sample) and a validation cohort (the remaining 20% of the sample).
Study Outcomes and Measures
Variables were categorized as in a previous study and were described elsewhere.8 The primary end point was PORT initiation delay, defined in the NCCN Guidelines as the initiation of PORT more than 6 weeks (42 days) after surgical treatment.3 In the National Cancer Database, time to PORT is calculated as the interval between definitive surgical treatment of the primary cancer site and initiation of radiation therapy. The following variables were evaluated for their association with delayed PORT initiation: age, sex, race/ethnicity, urban or rural status, educational attainment, household income, distance from treatment facility, insurance type, severity of comorbidities (Charlson/Deyo comorbidity score), primary tumor site, clinical and pathological stage according to the AJCC [American Joint Committee on Cancer] Cancer Staging Manual (sixth edition for diagnosis before 2010 and seventh edition for diagnosis in 2010 or later), surgical margins, postoperative length of stay (LOS), 30-day readmission, administration of concurrent chemotherapy, treatment facility type, fragmentation of care between surgical and radiotherapy facilities (surgical procedure and PORT provided at different facilities), and US region.14 Patients with oropharyngeal squamous cell carcinoma were not subdivided by human papillomavirus status because previous studies did not demonstrate its association with PORT initiation delay.8
Statistical Analysis
Descriptive characteristics were summarized using frequency and percentage (No. [%]) for categorical variables and mean (SD) for continuous variables. Multivariable logistic regression analysis was used to create models to estimate the risk of PORT initiation delay. Effect size estimates for the role of each of the variables in the models were presented as adjusted odds ratios (ORs), and measures of precision of point estimates were presented as 95% CIs. Final models were selected on the basis of clinical judgment and comparison of concordance (C) indices and then were internally validated by bootstrapping with 200 or 500 resamples.
Calibration and discrimination statistics were used to evaluate model performance. The accuracy of the model’s prediction was evaluated by estimating the model’s calibration. The model’s discriminative ability was assessed by the C index; a C index of 0.5 represented agreement owing to random chance, and a C index of 1 represented perfect discrimination. Models were created using the derivation data set and were validated using the validation data set. The 2 nomograms were created from the logistic regression models by assigning points to each included variable in proportion to its effect size.
All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc), 2-sided testing was performed, and the nomograms were generated using R package RMS (R Foundation for Statistical Computing). A 2-tailed P = .05 was considered statistically significant. Data analysis was conducted from June 2, 2019, to January 29, 2020.
Results
A total of 60 776 patients with HNSCC were included in this study and divided into derivation and validation cohorts. The derivation cohort was composed of 48 625 patients (mean [SD] age, 59.59 [11.3] years; 36 825 men [75.7%]) selected randomly from the full sample, whereas 12 151 patients (mean [SD] age, 59.63 [11.2] years; 9266 men [76.3%]) composed the validation cohort. Table 1 shows the characteristics of the 2 cohorts.
Table 1. Characteristics of the Study Sample.
| Variable | Cohort, No. (%) | |
|---|---|---|
| Derivation (n = 48 625) | Validation (n = 12 151) | |
| Timely PORT initiation | ||
| Yes | 21 485 (44.2) | 5251 (43.2) |
| No | 27 140 (55.8) | 6900 (56.7) |
| Age, mean (SD), y | 59.59 (11.3) | 59.63 (11.2) |
| Sex | ||
| Male | 36 825 (75.7) | 9266 (76.3) |
| Female | 11 800 (24.3) | 2885 (23.7) |
| Race/ethnicitya | ||
| White non-Hispanic | 38 893 (85.1) | 9688 (84.6) |
| White Hispanic | 1822 (4.0) | 479 (4.2) |
| Black | 4059 (8.9) | 1036 (9.1) |
| Other | 933 (2.0) | 247 (2.2) |
| Insurance typea | ||
| Private | 23 945 (51.1) | 5998 (51.3) |
| Medicare | 4898 (10.5) | 1175 (10.1) |
| Medicaid | 15 549 (33.2) | 3912 (33.5) |
| Uninsured | 2450 (5.2) | 601 (5.1) |
| Urban or rural statusa | ||
| Metro | 37 706 (79.8) | 9517 (80.5) |
| Urban | 8508 (18.0) | 2075 (17.6) |
| Rural | 1066 (2.3) | 234 (2.0) |
| Educational attainment, quartile | ||
| Highest | 8286 (17.2) | 1972 (16.4) |
| 2nd highest | 13 077 (27.2) | 3295 (27.4) |
| 2nd lowest | 15 885 (33.0) | 3965 (33.0) |
| Lowest | 10 910 (22.7) | 2788 (23.2) |
| Household income, quartilea | ||
| Lowest | 9029 (18.8) | 2196 (18.3) |
| 2nd lowest | 11 848 (24.6) | 2893 (24.1) |
| 2nd highest | 13 006 (27.0) | 3269 (27.2) |
| Highest | 14 238 (29.6) | 3654 (30.4) |
| Distance from treatment facility, mean (SD), miles | 33.5 (109.8) | 34.3 (123.7) |
| Charlson/Deyo comorbidity score | ||
| 0 | 38 558 (79.3) | 9604 (79.0) |
| 1 | 7978 (16.4) | 1984 (16.3) |
| ≥2 | 2089 (4.3) | 563 (4.6) |
| Primary tumor site | ||
| Oral cavity | 15 557 (32.0) | 3808 (31.3) |
| Oropharynx | 20 176 (41.9) | 5082 (41.8) |
| Hypopharynx | 1280 (2.6) | 334 (2.8) |
| Larynx | 11 612 (23.9) | 2927 (24.1) |
| AJCC clinical stagea | ||
| I | 6164 (16.4) | 1540 (16.4) |
| II | 5735 (15.3) | 1437 (15.3) |
| III | 7554 (20.1) | 1912 (20.4) |
| IV | 18 116 (48.2) | 4506 (48.0) |
| AJCC pathological stagea | ||
| I | 3243 (9.2) | 787 (8.9) |
| II | 3321 (9.4) | 891 (10.1) |
| III | 6524 (18.6) | 1648 (18.7) |
| IV | 22 091 (62.8) | 5475 (62.2) |
| Surgical marginsa | ||
| Negative | 29 375 (71.2) | 7418 (71.8) |
| Positive | 11 905 (28.8) | 2912 (28.2) |
| Postoperative LOS, da | ||
| 0-3 | 23 728 (59.1) | 5943 (59.4) |
| 4-7 | 7019 (17.5) | 1691 (16.8) |
| 8-14 | 6518 (16.2) | 1623 (16.2) |
| 15-21 | 1582 (3.9) | 415 (4.2) |
| >21 | 1305 (3.3) | 339 (3.4) |
| 30-d Hospital readmissiona | ||
| No | 43 742 (94.3) | 10 934 (94.1) |
| Yes | 2622 (5.7) | 685 (5.9) |
| Radiotherapy modality | ||
| External beam | 21 925 (45.1) | 5527 (45.5) |
| IMRT | 23 990 (49.3) | 5949 (49.0) |
| Conformal or 3-D therapy | 1805 (3.7) | 453 (3.7) |
| Other | 905 (1.9) | 222 (1.8) |
| Concurrent chemoradiation | ||
| None | 24 473 (50.3) | 6167 (50.8) |
| Yes | 24 152 (49.7) | 5957 (49.2) |
| Treatment facility type | ||
| Academic | 21 381 (44.0) | 5381 (44.3) |
| Nonacademic | 27 244 (56.0) | 6770 (55.7) |
| Surgical procedure and PORT at same facility | ||
| Yes | 24 385 (50.2) | 6005 (49.4) |
| No | 24 240 (49.9) | 6146 (50.6) |
| US regiona | ||
| Northeast | 9477 (20.1) | 2344 (19.9) |
| Midwest | 13 829 (29.3) | 3392 (28.8) |
| South | 16 806 (35.7) | 4272 (36.3) |
| West | 7030 (14.9) | 1768 (15.0) |
Abbreviations: 3-D, 3-dimensional; AJCC, American Joint Committee on Cancer; IMRT, intensity-modulated radiation therapy; LOS, length of stay; PORT, postoperative radiotherapy.
SI conversion factor: To convert miles to kilometers, multiply by 1.6.
Values do not sum to a complete data set because of missing or unknown values.
In the derivation cohort, 27 140 patients (55.8%) experienced a PORT initiation delay. A multivariable logistic regression model was created to estimate the presurgical risk of PORT initiation delay (Table 2). Race/ethnicity (black: OR, 1.39; 95% CI, 1.27-1.51, insurance type (Medicaid: OR, 1.71 [95% CI, 1.57-1.85]; uninsured: OR, 1.45 [95% CI, 1.30-1.62]), Charlson/Deyo comorbidity score of 2 or higher (OR, 1.43; 95% CI, 1.28-1.60), primary tumor site (non–oral cavity), stage IV cancer (OR, 2.14; 95% CI, 2.00-2.30), and facility type (academic: OR, 1.38; 95% CI, 1.32-1.45) were associated with an increased risk of PORT initiation delay. The calibration plot (Figure 1A) demonstrates that the predicted risk of delayed PORT initiation closely approximated the observed risk. Model discrimination, as quantified by the C index, was 0.670 (95% CI, 0.664-0.676). The optimism outputs from interval validation with 200 resamples (eg, R2, slope, and intercept) indicated no overfitting of the model; bias-corrected C indices generated by bootstrap validations were similar (0.670; 95% CI, 0.665-0.677). We generated the first nomogram to provide a presurgical, personalized estimate of PORT initiation delay (Figure 1B), whereby points in the nomogram were assigned in proportion to the effect sizes in the first multivariable logistic regression analysis model. Points were allocated for each variable, summed, and then used to calculate a patient-specific, presurgical risk of PORT initiation delay (Figure 1C).
Table 2. Multivariable Logistic Regression Models Using Presurgical and Postsurgical Variables .
| Variable | OR (95% CI) |
|---|---|
| Presurgical variables | |
| Race/ethnicity | |
| White non-Hispanic | 1 [Reference] |
| White Hispanic | 1.38 (1.22-1.57) |
| Black | 1.39 (1.27-1.51) |
| Other | 1.17 (0.99-1.38) |
| Insurance type | |
| Private | 1 [Reference] |
| Medicaid | 1.71 (1.57-1.85) |
| Medicare | 1.10 (1.04-1.15) |
| Uninsured | 1.45 (1.30-1.62) |
| Primary tumor site | |
| Oral cavity | 1 [Reference] |
| Oropharynx | 0.41 (0.39-0.43) |
| Hypopharynx | 0.64 (0.55-0.74) |
| Larynx | 0.39 (0.36-0.41) |
| AJCC clinical stage | |
| I | 1 [Reference] |
| II | 1.54 (1.41-1.67) |
| III | 1.87 (1.72-2.02) |
| IV | 2.14 (2.00-2.30) |
| Charlson/Deyo comorbidity score | |
| 0 | 1 [Reference] |
| 1 | 0.71 (0.67-0.76) |
| >2 | 1.43 (1.28-1.60) |
| Treatment facility type | |
| Nonacademic | 1 [Reference] |
| Academic | 1.38 (1.32-1.45) |
| C index original | 0.670 (0.664-0.676) |
| Bootstrap-corrected C index | 0.670 (0.665-0.677) |
| Presurgical and postsurgical variables | |
| Race/ethnicity | |
| White non-Hispanic | 1 [Reference] |
| White Hispanic | 1.26 (1.11-1.42) |
| Black | 1.37 (1.26-1.49) |
| Other | 1.08 (0.92-1.28) |
| Insurance type | |
| Private | 1 [Reference] |
| Medicaid | 1.62 (1.49-1.76) |
| Medicare | 1.11 (1.05-1.17) |
| Uninsured | 1.50 (1.34-1.67) |
| Primary tumor site | |
| Oral cavity | 1 [Reference] |
| Oropharynx | 0.60 (0.57-0.64) |
| Hypopharynx | 0.65 (0.57-0.76) |
| Larynx | 0.41 (0.39-0.44) |
| US region | |
| Northeast | 1 [Reference] |
| Midwest | 0.71 (0.66-0.75) |
| South | 0.78 (0.73-0.83) |
| West | 0.91 (0.84-0.98) |
| Treatment facility type | |
| Nonacademic | 1 [Reference] |
| Academic | 1.29 (1.23-1.35) |
| Postoperative LOS, d | |
| 0-3 | 1 [Reference] |
| 4-7 | 1.75 (1.64-1.68) |
| 8-14 | 2.72 (2.53-2.92) |
| 15-21 | 4.14 (3.59-4.79) |
| >21 | 6.13 (5.15-7.31) |
| Surgical procedure and PORT at same facility | |
| Yes | 1 [Reference] |
| No | 1.36 (1.29-1.42) |
| C index original | 0.691 (0.686-0.696) |
| Bootstrap-corrected C index | 0.700 (0.695-0.706) |
Abbreviations: AJCC, American Joint Committee on Cancer; C index, concordance index; LOS, length of stay; OR, odds ratio; PORT, postoperative radiotherapy.
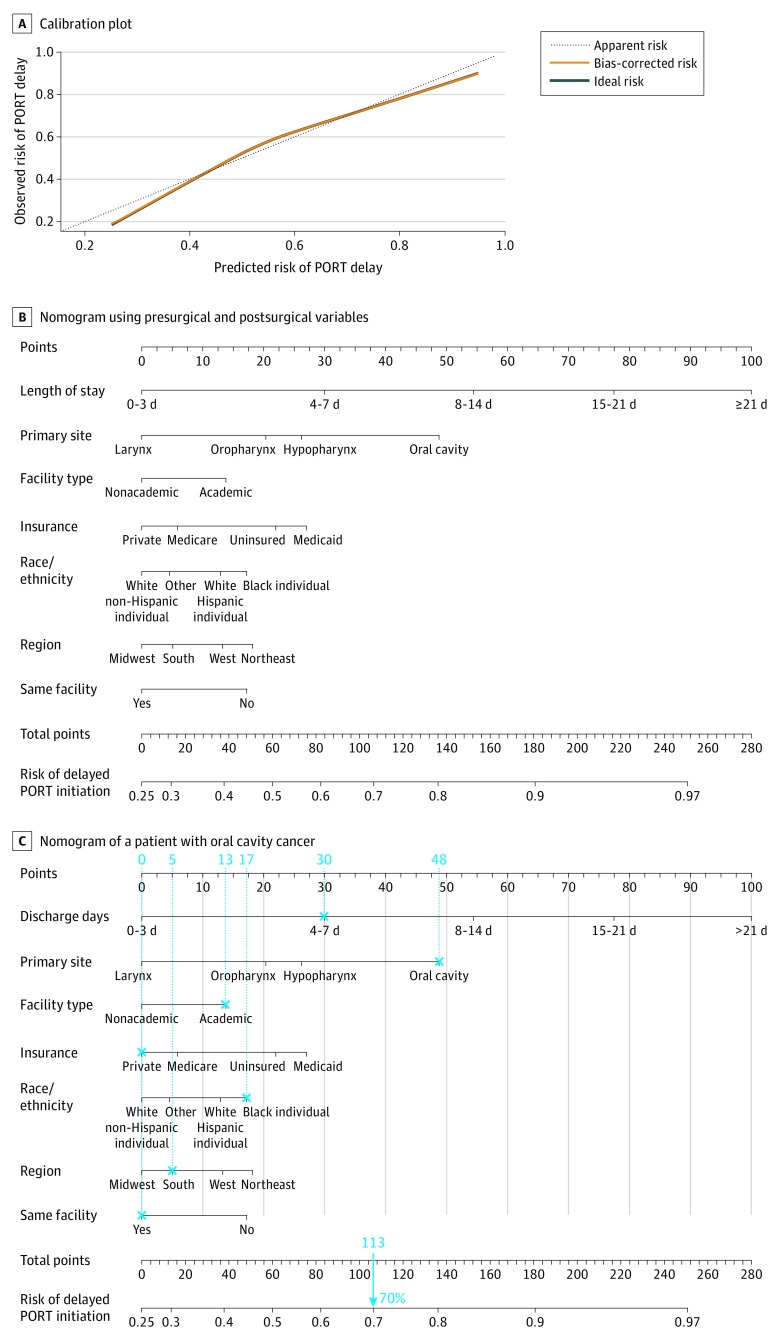
Figure 1. Calibration Model and Nomogram Based on Presurgical Variables.
A, The dashed line through the origin point represents a perfectly calibrated model in which the predicted risk of postoperative radiotherapy (PORT) initiation delay is the observed risk of PORT initiation delay. B, Each clinical variable has a certain number of points (top row), ranging from 0 to 100. These points are added to generate a total number of points, which then corresponds to the risk (percentage) of delayed PORT initiation. C, This illustration of a nomogram shows the calculation of the presurgical risk for a patient with stage IV laryngeal cancer who was a black individual, was treated at an academic institution, had Medicare insurance, had a Charlson/Deyo comorbidity score of 1, and had a total score of 180 points that corresponded to a 67% risk of PORT initiation delay.
AJCC indicates American Joint Committee on Cancer; CDC, Centers for Disease Control and Prevention.
Multivariable logistic regression analysis with the addition of postsurgical information was performed to create the second model (Table 2). In addition to variables that were also included in the presurgical model (ie, race/ethnicity, insurance type, primary tumor site, and facility type), US region (non-Northeast), postoperative LOS (15-21 days: OR, 4.14 [95% CI, 3.59-4.79]; >21 days: OR, 6.13 [95% CI, 5.15-7.31]), and fragmentation of care between the surgical and radiotherapy facilities (OR, 1.36; 95% CI, 1.29-1.42) were associated with PORT initiation delay in the second model. The model for PORT initiation delay was also well calibrated (Figure 2A). The C index was 0.691 (95% CI, 0.686-0.696) and similar to the bias-corrected C-indices generated by bootstrap validations (0.700; 95% CI, 0.695-0.706). A second nomogram that incorporated postsurgical information was created from the second multivariable model (Figure 2B). Figure 2C shows an example of a postsurgical nomogram used to calculate the risk of delayed PORT initiation for a patient with oral cavity cancer.
Figure 2. Calibration Model and Nomogram Based on Presurgical and Postsurgical Variables.
A, The dashed line through the origin point represents a perfectly calibrated model in which the predicted risk of postoperative radiotherapy (PORT) initiation delay is the observed risk of PORT initiation delay. B, Each clinical variable has a certain number of points (top row), ranging from 0 to 100. These points are added to generate a total number of points, which then corresponds to the risk (percentage) of delayed PORT initiation. C, This illustration shows a nomogram for a patient with oral cavity cancer who was a black individual, had a 4- to 7-day length of stay, was treated at a single academic medical center, had private insurance, lived in the South (US), and had a total score of 113 points that corresponded to a 70% risk of PORT initiation delay.
AJCC indicates American Joint Committee on Cancer; CDC, Centers for Disease Control and Prevention.
To externally validate the 2 nomograms, the models generated in the derivation cohort were applied to the validation cohort, which consisted of a random selection of 20% (n = 12 151) of the sample (Table 1). In the validation cohort, the rate of delayed PORT initiation was 56.7% (n = 6900). When applied to the validation cohort, the first nomogram, which estimated individual pretreatment risk of PORT initiation delay, had an uncorrected C index of 0.674 (95% CI, 0.662-0.685) and a bootstrap-corrected C index of 0.673 (95% CI, 0.661-0.684). The second nomogram, which incorporated postsurgical information, had an uncorrected C index of 0.694 (95% CI, 0.685-0.704) and a bootstrap-corrected C index of 0.702 (95% CI, 0.697-0.707) in the validation cohort.
Discussion
The prevalence of PORT initiation delay found in this study (55.8% in the derivation cohort and 56.7% in the validation cohort) reinforces the finding from previous research that failure to deliver timely, guideline-adherent PORT is a common problem.8 The importance of eliminating PORT initiation delays after surgical treatment for HNSCC is further accentuated by the association of delays with mortality, the potential of targeting delays to improve oncologic outcomes,2,6,9 and the finding that delays are a marker of high-quality HNSCC care delivery.11
This cohort study used a large, nationally representative data set13 to develop and validate 2 nomograms for predicting nonadherence to NCCN Guidelines for timely initiation of PORT, with the first based on presurgical information and the second on both presurgical and postoperative variables. The first nomogram can provide personalized estimates of PORT initiation delay to enhance preoperative counseling and guide interventions for patients at highest risk for delay. The second nomogram can adjust the risk of PORT initiation delay rates by case-mix differences (eg, for institutions that systematically treat patients with lower socioeconomic status), thereby enhancing the validity of such delays as an institutional measure of high-quality HNSCC care delivery and identifying targets for quality improvement initiatives.
The presurgical nomogram suggested that stage IV cancer and oral cavity site were 2 of the key variables associated with delayed PORT initiation. These variables are easily identifiable and allow for the targeting of the care processes associated with presurgical referrals to radiation and dental oncologic treatments, which increase the rate of timely PORT administration.10,15 There are many reasons that individuals present with advanced stage cancer, but it is possible that delays in presentation are associated with symptom awareness, risk perception, and other psychosocial barriers to timely care,16,17 all of which may play a role in PORT initiation delay.
Identifying patients at high risk for delay can be viewed as a first step. In an innovative study, Shew et al18 used a machine learning algorithm to identify patients at high-risk for PORT initiation delay. The present study adds to the existing literature by providing a method for generating personalized estimates of PORT initiation delay, which can enhance preoperative counseling and guide interventions for patients at highest risk for delay. However, capitalizing fully on this information requires further research into individualized strategies that can be implemented early for those with high risk. In a landmark study, Divi et al15 showed that a quality improvement intervention for patient engagement, timely dental extractions, and timely radiation oncologist consults is associated with decreased PORT initiation delays. As an interactive decision-making tool, the presurgical nomogram could be integrated into such an intervention to enhance patient engagement, facilitate communication about timely PORT, and strengthen the therapeutic alliance. This first nomogram could also be delivered through a web-based or smartphone platform and seamlessly integrated into clinical workflow to help patients and clinicians personalize PORT initiation delay risk into an easily interpretable and communicable data point.19
Guideline concordance and timeliness are 2 indicators of high-quality care. The delivery of timely PORT has, therefore, been proposed as a marker of high-quality care delivery for head and neck cancer.11 However, PORT initiation delay rates vary widely across institutions and are high even at high-volume cancer programs.10,15 Frameworks must be developed to facilitate accurate comparisons of delay rates across institutions and to drive improvements in the structure and processes of HNSCC care delivery. The second nomogram, which included postsurgical data, is a first step toward quantifying delay rates that acknowledge key differences in case mix. In their study, Swegal et al20 developed a hospital-specific observed-to-expected ratio for adhering to NCCN Guidelines on the care for elderly patients with laryngeal squamous cell carcinoma. Swegal et al20 acknowledged that population differences may be associated with guideline adherence and that observed-to-expected ratios may be a way to perform a risk-adjusted evaluation of quality, thereby enhancing the validity and perceived fairness of measures of quality care.
In addition to adjusting for case mix, the second nomogram can decrease rates of PORT initiation delay. For example, LOS is associated with delay, but the exact mechanism of this association is unknown. The delay is likely associated with the interruption of key care delivery processes (eg, timely referrals and consultations).10 Duration of surgical intervention, postoperative infection, and unplanned reoperation have all been associated with prolonged LOS, particularly among patients undergoing a free flap procedure.21,22 Smoking status, although not available in the present data set, has a strong association with postoperative infections, prolonged LOS, and postdischarge readmission.23 Quality improvement efforts that target prolonged LOS and smoking cessation may decrease the rates of PORT initiation delay. In addition, data-driven benchmarks based on case acuity may help refine this LOS target when measuring quality across institutions.24
Our findings suggest that fragmentation of care between the surgical and radiotherapy facilities was also associated with delayed initiation of PORT. This study adds to the growing evidence of the negative implications of care fragmentation for patients undergoing surgical treatment and adjuvant therapy.8,10,25 The reasons that fragmentation of care is associated with a higher risk of PORT initiation delay are unknown but likely reflect the challenges among surgical, radiation, and medical oncologic practitioners in discussing altered postoperative anatomy, wound healing issues, safety of initiating PORT based on flap reconstruction, or areas at high risk to cover when planning treatment volumes. In addition, care fragmentation may also reflect the underlying challenges of patient indecision or the social determinants of health that preclude radiotherapy at the facility in which the surgical treatment was provided (which for HNSCC is often a high-volume, academic medical center). More research is required to understand and address the ways in which care fragmentation is predisposed to PORT initiation delay.
Limitations
This study has several limitations. Because it was a retrospective study, the reasons for delayed, non–guideline-adherent PORT initiation could not be discerned. Although the nomograms were developed using a random selection of the sample and were validated in a separate cohort, their clinical use must be externally validated and evaluated.26,27 Some potentially important variables were not available in the data set and thus could not be included in the nomograms, such as patient-level factors (eg, social support, financial stability, health literacy, history of mental illness, dental disease, cigarette smoking, or alcohol consumption) and surgical factors (eg, flap loss and wound infection, which may not be fully captured by LOS and readmission data). Although the C indices for both models suggest good performance,28 the inclusion of additional patient-level variables could result in more precise risk-prediction models. Because rates of PORT initiation delay are known to disproportionately burden racial/ethnic minorities,8 future research should include variables that capture aspects of culture (eg, collectivism, religiosity, and temporal orientation) that may differ between patients and members of the medical team, particularly in the racial/ethnic minority populations.29 Although fragmentation of care between the surgical and PORT facilities was a part of the institution-level risk-adjustment model, other sources of care fragmentation (eg, dental care) known to be associated with PORT initiation delay rates10,15 were missing from the data set and thus were not used in the models. Incorporation of additional variables into the models improved the precision and discriminative ability of the models, but it also made the nomograms more cumbersome and thus potentially decreased their clinical use. The optimal balance between precise risk estimation and clinical use was not known and should be addressed in future research.
Conclusions
This cohort study found that more than 50% of patients with HNSCC experienced a delay in PORT administration. Stage IV cancers, oral cavity subsite, extended LOS, and fragmentation of care appeared to be associated with such delays in HNSCC. Our findings suggest that the validated patient-level, presurgical nomogram can provide personalized risk estimates of PORT initiation delay to enhance pretreatment counseling and help target patients at high risk for delay. We believe that the validated nomogram that incorporated postsurgical data can be used to compare rates of PORT initiation delay across institutions with different case mixes and patient populations, facilitating the use of delay rate as a measure of high-quality HNSCC care delivery and driving quality improvement initiatives. Future research that incorporates more granular variables, such as patient risk behaviors and socioeconomic status, will help refine these nomograms.
eTable. International Classification of Disease for Oncology, 3rd Edition, Topography Codes Used to Identify Head and Neck Squamous Cell Carcinoma
eFigure. Cohort Derivation
References
- 1.American Cancer Society Cancer facts & figures 2019. Accessed August 10, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
- 2.Graboyes EM, Kompelli AR, Neskey DM, et al. Association of treatment delays with survival for patients with head and neck cancer: a systematic review. JAMA Otolaryngol Head Neck Surg. 2019;145(2):166-177. doi: 10.1001/jamaoto.2018.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Head and Neck Cancers. Version 1. 2019 ed. National Comprehensive Cancer Network; 2019. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21(3):555-563. doi: 10.1200/JCO.2003.04.171 [DOI] [PubMed] [Google Scholar]
- 5.Chen MM, Harris JP, Orosco RK, Sirjani D, Hara W, Divi V. Association of time between surgery and adjuvant therapy with survival in oral cavity cancer. Otolaryngol Head Neck Surg. 2018;158(6):1051-1056. doi: 10.1177/0194599817751679 [DOI] [PubMed] [Google Scholar]
- 6.Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi: 10.1002/cncr.31533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao HH, Schonewolf CA, Tan EX, et al. The impact of treatment package time on locoregional control for HPV+ oropharyngeal squamous cell carcinoma treated with surgery and postoperative (chemo)radiation. Head Neck. 2019;41(11):3858-3868. doi: 10.1002/hed.25914 [DOI] [PubMed] [Google Scholar]
- 8.Graboyes EM, Garrett-Mayer E, Sharma AK, Lentsch EJ, Day TA. Adherence to National Comprehensive Cancer Network Guidelines for time to initiation of postoperative radiation therapy for patients with head and neck cancer. Cancer. 2017;123(14):2651-2660. doi: 10.1002/cncr.30651 [DOI] [PubMed] [Google Scholar]
- 9.Houlton JJ. Defining optimal treatment times in head and neck cancer care: what are we waiting for? JAMA Otolaryngol Head Neck Surg. 2019;145(2):177-178. doi: 10.1001/jamaoto.2018.2838 [DOI] [PubMed] [Google Scholar]
- 10.Janz TA, Kim J, Hill EG, et al. Association of care processes with timely, equitable postoperative radiotherapy in patients with surgically treated head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2018;144(12):1105-1114. doi: 10.1001/jamaoto.2018.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer JD, Speedy SE, Ferris RL, Rademaker AW, Patel UA, Samant S. National evaluation of multidisciplinary quality metrics for head and neck cancer. Cancer. 2017;123(22):4372-4381. doi: 10.1002/cncr.30902 [DOI] [PubMed] [Google Scholar]
- 12.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173-e180. doi: 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janz TA, Graboyes EM, Nguyen SA, et al. A comparison of the NCDB and SEER database for research involving head and neck cancer. Otolaryngol Head Neck Surg. 2019;160(2):284-294. doi: 10.1177/0194599818792205 [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Data Base. Participant use data file (PUF). Data dictionary. Version PUF 2014. Accessed November 21, 2016. https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/puf%20data%20dictionary%20version%20puf%202014.ashx
- 15.Divi V, Chen MM, Hara W, et al. Reducing the time from surgery to adjuvant radiation therapy: an institutional quality improvement project. Otolaryngol Head Neck Surg. 2018;159(1):158-165. doi: 10.1177/0194599818768254 [DOI] [PubMed] [Google Scholar]
- 16.Liao DZ, Schlecht NF, Rosenblatt G, et al. Association of delayed time to treatment initiation with overall survival and recurrence among patients with head and neck squamous cell carcinoma in an underserved urban population. JAMA Otolaryngol Head Neck Surg. 2019. doi: 10.1001/jamaoto.2019.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graboyes EM, Hughes-Halbert C. Delivering timely head and neck cancer care to an underserved urban population-better late than never, but never late is better. JAMA Otolaryngol Head Neck Surg. 2019. doi: 10.1001/jamaoto.2019.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shew M, New J, Bur AM. Machine learning to predict delays in adjuvant radiation following surgery for head and neck cancer. Otolaryngol Head Neck Surg. 2019;160(6):1058-1064. doi: 10.1177/0194599818823200 [DOI] [PubMed] [Google Scholar]
- 19.Teng MS, Gupta V. Timely adjuvant postoperative radiotherapy: racing to a PORT in the storm. JAMA Otolaryngol Head Neck Surg. 2018;144(12):1114-1115. doi: 10.1001/jamaoto.2018.2266 [DOI] [PubMed] [Google Scholar]
- 20.Swegal WC, Herbert RJ, Eisele DW, Chang J, Bristow RE, Gourin CG. Observed-to-expected ratio for adherence to treatment guidelines as a quality of care indicator for laryngeal cancer. Laryngoscope. 2019;130(3):672-678. doi: 10.1002/lary.28104 [DOI] [PubMed] [Google Scholar]
- 21.Penel N, Mallet Y, Roussel-Delvallez M, Lefebvre JL, Yazdanpanah Y. Factors determining length of the postoperative hospital stay after major head and neck cancer surgery. Oral Oncol. 2008;44(6):555-562. doi: 10.1016/j.oraloncology.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Thomas WW, Brant J, Chen J, et al. Clinical factors associated with reoperation and prolonged length of stay in free tissue transfer to oncologic head and neck defects. JAMA Facial Plast Surg. 2018;20(2):154-159. doi: 10.1001/jamafacial.2017.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health, eds. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention; 2014. [PubMed] [Google Scholar]
- 24.Weber RS, Lewis CM, Eastman SD, et al. Quality and performance indicators in an academic department of head and neck surgery. Arch Otolaryngol Head Neck Surg. 2010;136(12):1212-1218. doi: 10.1001/archoto.2010.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amini A, Stokes WA, Jones BL, et al. Postoperative radiation performed at the same surgical facility associated with improved overall survival in oral cavity squamous cell carcinoma. Head Neck. 2019;41(7):2299-2308. doi: 10.1002/hed.25697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kattan MW, Hess KR, Amin MB, et al. ; Members of the AJCC Precision Medicine Core . American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin. 2016;66(5):370-374. doi: 10.3322/caac.21339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam RK, Kattan MW, Chin JL, et al. Prospective multi-institutional study evaluating the performance of prostate cancer risk calculators. J Clin Oncol. 2011;29(22):2959-2964. doi: 10.1200/JCO.2010.32.6371 [DOI] [PubMed] [Google Scholar]
- 28.Fakhry C, Zhang Q, Nguyen-Tân PF, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol. 2017;35(36):4057-4065. doi: 10.1200/JCO.2016.72.0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes Halbert C, Barg FK, Weathers B, et al. Differences in cultural beliefs and values among African American and European American men with prostate cancer. Cancer Control. 2007;14(3):277-284. doi: 10.1177/107327480701400311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. International Classification of Disease for Oncology, 3rd Edition, Topography Codes Used to Identify Head and Neck Squamous Cell Carcinoma
eFigure. Cohort Derivation