Key Points
Question
What ocular structure changes in astronauts are associated with long-duration spaceflight (6 months), and do the changes persist 1 year after returning to Earth?
Findings
This cohort study of 11 astronauts found that long-duration spaceflight was associated with optic disc edema and peripapillary choroidal thickening during spaceflight, with reduced axial length and anterior chamber depth after spaceflight, and with a hyperopic shift observed 1 year after returning to Earth.
Meaning
This study prospectively expands on previously reported retrospective findings that peripapillary optic disc edema and choroid thickening may not be specifically associated with particular individuals but may present bilaterally, may occur in both sexes, and may be associated with persistent abnormalities 1 year after returning to Earth.
Abstract
Importance
During long-duration spaceflights, nearly all astronauts exhibit some change in ocular structure within the spectrum of spaceflight-associated neuro-ocular syndrome.
Objective
To quantitatively determine in a prospective study whether changes in ocular structures hypothesized to be associated with the development of spaceflight-associated neuro-ocular syndrome occur during 6-month missions on board the International Space Station (ISS).
Design, Setting, and Participants
The Ocular Health ISS Study of astronauts is a longitudinal prospective cohort study that uses objective quantitative imaging modalities. The present cohort study investigated the ocular structure of 11 astronauts before, during, and after a 6-month mission on board the ISS.
Main Outcomes and Measures
Changes in ocular structure (peripapillary edema, axial length, anterior chamber depth, and refraction) hypothesized to be associated with the development of spaceflight-associated neuro-ocular syndrome during 6-month missions on board the ISS were assessed. Statistical analyses were conducted from August 2018 to January 2019.
Results
Before launch, the 11 astronauts were a mean (SD) age of 45 (5) years, a mean (SD) height of 1.76 (0.05) m, and a mean (SD) weight of 75.3 (7.1) kg. Six astronauts did not have prior spaceflight experience, 3 had completed short-duration missions on board the Space Shuttle, and 2 had previous long-duration spaceflight missions on board the ISS. Their mean (SD) duration on board the ISS in the present study was 170 (19) days. Optic nerve head rim tissue and peripapillary choroidal thickness increased from preflight values during early spaceflight, with maximal change typically near the end of the mission (mean change in optic nerve head rim tissue thickness on flight day 150: 35.7 μm; 95% CI, 28.5-42.9 μm; P < .001; mean choroidal thickness change on flight day 150: 43 μm; 95% CI, 35-46 μm; P < .001). The mean postflight axial length of the eye decreased by 0.08 mm (95% CI, 0.10-0.07 mm; P < .001) compared with preflight measures, and this change persisted through the last examination (1 year after spaceflight: 0.05 mm; 95% CI, 0.07-0.03 mm; P < .001).
Conclusions and Relevance
This study found that spaceflight-associated peripapillary optic disc edema and choroid thickening were observed bilaterally and occurred in both sexes. In addition, this study documented substantial peripapillary choroid thickening during spaceflight, which has never been reported in a prospective study cohort population and which may be a contributing factor in spaceflight-associated neuro-ocular syndrome. Data collection on spaceflight missions longer than 6 months will help determine whether the duration of the mission is associated with exacerbating these observed changes in ocular structure or visual function.
This cohort study uses objective quantitative imaging techniques to prospectively assess whether ocular structure changes hypothesized to be associated with the development of spaceflight-associated neuro-ocular syndrome are observed during a 6-month mission on board the International Space Station or for up to 1 year after returning to Earth in astronauts.
Introduction
Spaceflight-associated neuro-ocular syndrome (SANS), as described by the National Aeronautics and Space Administration (NASA), develops in astronauts during long-duration missions (>1 month of spaceflight). It is characterized by changes in visual acuity (due to hyperopic shifts) and eye structure (eg, optic disc edema, choroidal folds, and globe flattening). In 2011, Mader and coworkers1 reported a case series of the initial SANS findings in 7 cases involving astronauts. Overall, clinically relevant optic disc edema, the defining characteristic of SANS, has been diagnosed in 16% to 19% of astronauts who have been examined using ophthalmoscopic imaging during and after long-duration spaceflight.2 In addition to being an intriguing pathophysiological phenomenon, SANS is a high-level concern for the aerospace medicine community because the length of missions is expected to increase substantially in the future.
A retrospective analysis of Lifetime Surveillance of Astronaut Health data collected from astronauts before and after long-duration (4-6 months) missions to the International Space Station (ISS) showed that global total retinal thickness at the optic nerve head increased by 8.5% (from 362 μm before spaceflight to 393 μm after spaceflight).3 In this same data set, peripapillary choroid thickness was 2.8% greater (before spaceflight, 253 μm; after spaceflight, 260 μm) after long-duration spaceflight compared with before spaceflight. A few published case studies have provided clues to the etiology and development of SANS, and anecdotal reports have suggested that only a subset of crewmembers develop optic disc edema, including indications that it occurs only in male astronauts. However, there is no reliable method to predict which crewmembers will develop disc edema, nor are there sufficient data to determine the appropriate countermeasures to prevent or treat the syndrome. To date, there has been only 1 case report describing the time course of certain ocular changes during spaceflight and characterizing the postflight course on the return to normal gravity.4 Thus, additional data are crucial to determine the cause of these changes and to develop appropriate countermeasures. In an effort to explain SANS findings and define the risk to mission success and long-term astronaut health, NASA’s Human Research Program, Space Medicine Operations, and the Biomedical Research and Environmental Sciences Division established the Ocular Health Study. The purpose of the present report is to quantitate ocular structural changes, including peripapillary retinal thickness, that develop in association with long-duration spaceflight and to document how long these changes persist after landing.
Methods
Eleven astronauts who spent a mean (SD) duration of 170 (19) days on board the ISS participated in this study, including astronauts from NASA, the European Space Agency, and the Japan Aerospace Exploration Agency. Before launch, astronauts were a mean (SD) age of 45 (5) years, a mean (SD) height of 1.76 (0.05) m, and a mean (SD) weight of 75.3 (7.1) kg. After returning to earth, astronauts weighed a mean (SD) of 74.8 (7.5) kg. Although 2 of the crewmembers participating in a 1-year mission were participants in the present study, only data for the 11 ISS astronauts who completed a 6-month mission are reported here because the duration of the mission may be associated with the recovery profile after spaceflight, which is an integral objective to assess in the present investigation. Of the 11 crewmembers described here, 6 did not have prior spaceflight experience, 3 had completed short-duration missions on board the Space Shuttle, and 2 had previous long-duration spaceflight missions on board the ISS. This study was approved by the NASA Johnson Space Center Institutional Review and Human Research Multilateral Review Boards. Participants provided informed written consent that was obtained consistent with the Declaration of Helsinki, and research participants did not receive compensation or other incentives to participate in the study.
Posterior segment images of both eyes were acquired using optical coherence tomography (OCT) (Spectralis; Heidelberg Engineering) from all crewmembers before, during, and after spaceflight. A high-resolution OCT radial scan pattern (20°, set of 12 B-scans, and 16 automatic real-time tracking levels) and a circle pattern (12°, 100 automatic real-time tracking levels) centered over the optic nerve head were acquired 21 to 3 months before spaceflight in the NASA Flight Medical Clinic. These baseline scans were transmitted to the ISS, which has the same OCT model system, to ensure appropriate alignment for follow-up examinations. Ophthalmoscopic images were acquired before and after spaceflight (Canon CR-2 Plus AF) and during spaceflight (OIS EyeScan; Ophthalmic Imaging Systems). Inflight data were acquired within a 10-day window of flight days 10, 30, 60, 90, 120, and 150. Postflight testing occurred within a 7-day window after returning to Earth, a 10-day window of the 30th and 90th day after returning, and a 30-day window of the 180th and 365th day after returning. These testing windows were selected to enable scheduling flexibility with NASA research and NASA operations while maintaining an organized study design. The mean (SD) ambient carbon dioxide level on board the ISS was estimated to be 2.8 (0.2) mm Hg (based on similar methods as reported by Law and coworkers5).
The OCT image segmentation of the Bruch membrane and the inner limiting membrane were manually corrected for any errors in automatic segmentation. For each radial B-scan, the Bruch membrane opening (BMO) was marked and used to compute the minimum rim width (MRW) as the minimum distance from the BMO to the inner limiting membrane. Subsequently, a best-fit ellipse to the BMO was used as a reference to compute the total retinal thickness (TRT) from annular eccentricities corresponding to regions of the BMO to 250 μm, 250 to 500 μm, 500 to 1000 μm, and 1000 to 1500 μm (Figure 1) by using a TRT map interpolated from the radial scan segmentation. For circular scans, after compensating for the peripapillary B-scan image contrast, the junction between the choroid and sclera was manually defined and used to calculate choroid thickness.
Figure 1. Optic Nerve Head Minimum Rim Width and Total Retinal Thickness Quantification.
Analyzed regions of interest are depicted as indicated by the colored annuli. BMO represents Bruch membrane opening.
Complete eye examinations were conducted by a NASA Flight Medicine Clinic optometrist (C.R.G.) before and after ISS missions, including cycloplegic refraction, optical biometry, and OCT (MedB1.10).6 Axial length and anterior chamber depth (ACD) were measured before and after spaceflight using optical biometry (IOLMaster 500; Zeiss). All participants passed NASA Flight Medicine Clinic’s ocular examinations and were cleared to participate in their assigned mission, and none of the participants had a history of systemic disease (eg, hypertension, connective tissue disorders, iron deficiency, diabetes, or renal disease) or had ever used any medication that could produce elevated intracranial pressure (ICP) (eg, vitamin A, tetracycline, corticosteroids, or nalidixic acid).
We evaluated the effects associated with spaceflight (ie, before vs after each flight) in separate mixed-effects statistical models per dependent variable, with a priori simple interaction terms comparing each postflight observation to each preflight observation, adjusting for astronauts’ prior weightlessness exposure (flight days exposed) as a continuously scaled covariate. Astronauts’ closest preflight (within 1 year) data were used for these analyses and compared with postflight data. Given our nesting of right and left eye observations within each astronaut and repeated observations over time, each model included random y-intercepts to accommodate the nesting of repeated measures within each astronaut for eye (left, right) and for time, with degrees of freedom calculated per our repeated-measures design. Each statistical test underwent a rigorous examination of the distribution of model residuals prior to hypothesis testing, and observations that were found to be overly influential outliers were eliminated from the analysis if the standardized residual was higher than 2 or lower than −2. For visualization, all individual data points are included in graphs, including those excluded from mixed-model calculations for 95% CIs. A 2-sided P < .05 was considered statistically significant. Statistical analyses were conducted from August 2018 to January 2019 using SAS software, version 9.4 (SAS Institute Inc).
Results
Structural alterations to the retina at the optic nerve head associated with spaceflight were observed in all but 1 crewmember in this cohort. The MRW, a measure of optic nerve head rim tissue thickness, increased from before spaceflight to flight day 10 by 4% (mean, 12.5 μm; 95% CI, 4.8-20.2 μm; P < .01) and increased from before spaceflight to flight day 150 by 10% (mean, 35.7 μm; 95% CI, 28.5-42.9 μm; P < .001) (Figure 2A). The MRW was also greater than before spaceflight 7 days after return to Earth (mean, 20.9 μm; 95% CI, 14.0-27.8 μm; P < .001) and 30 days after return to Earth (mean, 10.3 μm; 95% CI, 3.1-17.4 μm; P < .01) (Figure 2A), but was similar to preflight values by the 90th day.
Figure 2. Change in Minimum Rim Width (MRW) and Total Retinal Thickness (TRT).
The change in MRW (A) and global TRT (B) compared with before spaceflight (preflight) for both eyes of each participant (circles). The open circles represent data collected before spaceflight; gray symbols, data collected during spaceflight; beige symbols, data collected after spaceflight; and orange circles, 2 astronauts who had inflight optic disc edema findings. Bars indicate 95% CIs.
aP < .01.
bP < .001.
cNot significantly different compared with before spaceflight.
Similarly, the global TRT annulus at the optic nerve head (BMO to 250 μm) was greater than before spaceflight from flight day 10 (mean, 11.9 μm; 95% CI, 6.6-17.3 μm; P < .001) through flight day 150 (mean, 27.6 μm; 95% CI, 22.7-32.6 μm; P < .001) (Figure 2B). In addition, the global TRT annulus 250 to 500 μm from the optic nerve head was increased on flight day 10 (mean, 4.7 μm; 95% CI, 1.8-7.7 μm; P < .01) and remained greater than preflight levels through flight day 150 (mean, 12.9 μm; 95% CI, 10.0-15.7 μm; P < .001; eTable 1 in the Supplement). The TRT annuli from BMO to 250 μm and from 250 to 500 μm followed a similar recovery profile as the MRW; by 90 days after return to Earth, the values were not different from those before spaceflight. At greater distances from the optic nerve head, global TRT also increased on flight day 90 (annulus from 500 to 1000 μm; P < .05) and flight day 150 (annuli from 500 to 1000 μm and from 1000 to 1500 μm; P < .05) compared with before spaceflight (eTable 1 in the Supplement). However, this magnitude of change was less than the test-retest reliability for this measure. Similar TRT patterns of change associated with spaceflight were identified when divided by quadrant (eTable 2 in the Supplement). Fundus images of those astronauts with fundus grade optic disc edema are provided in the eFigure in the Supplement. Longer-duration missions appeared to be associated with optic disc edema propagating radially.
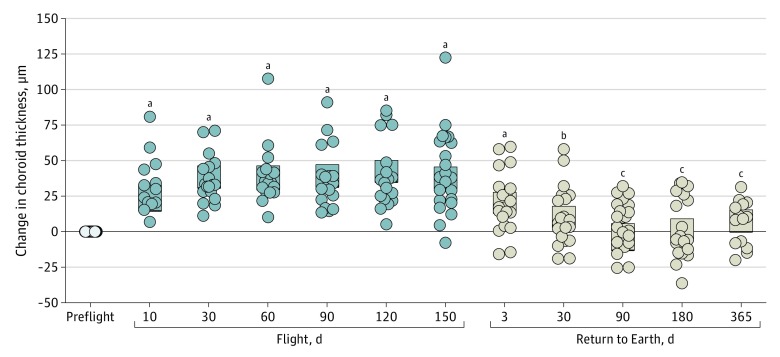
Peripapillary choroidal thickness increased as early as flight day 7 (mean, 24 μm; 95% CI, 15-33 μm; P < .001) (Figure 3) compared with measures before launch and progressively thickened during the duration of the mission. On flight day 150, choroid thickness was increased by 18% (mean, 43 μm; 95% CI, 35-46 μm; P < .001) compared with before spaceflight and remained increased 7 days after return to Earth (mean, 20 μm; 95% CI, 13-28 μm; P < .001) and 30 days after return to Earth (mean, 38 μm; 95% CI, 30-46 μm; P < .01). Peripapillary choroidal thickness values were not different from those before spaceflight 90 days after return.
Figure 3. Change in Choroid Thickness.
Change in global choroid thickness compared with before spaceflight (preflight) for both eyes of each participant (circles). The open circles represent data collected before spaceflight; gray symbols, data collected during spaceflight; and beige symbols, data collected after spaceflight. Bars indicate 95% CIs.
aP < .001.
bP < .01.
cNot significantly different compared with before spaceflight.
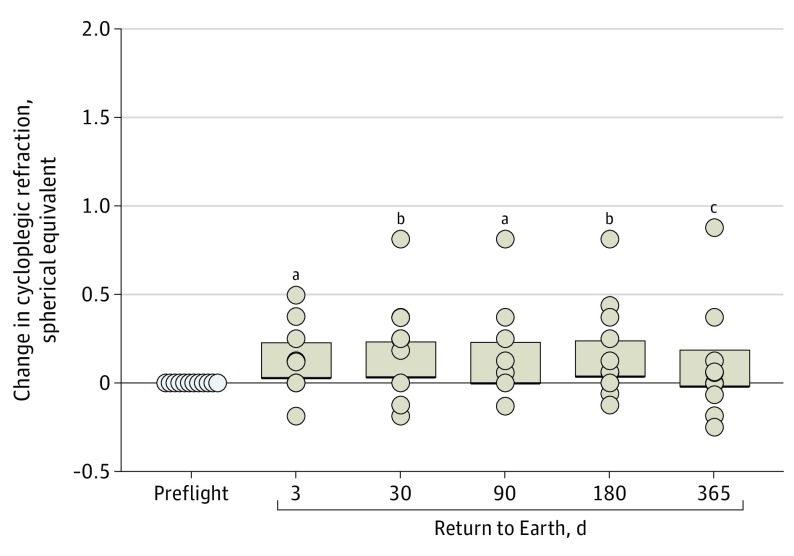
At the first postflight examination (return to Earth plus 7 days), axial length was decreased by 0.3% (mean, −0.08 mm; 95% CI, −0.10 to −0.07 mm; P < .001) (Figure 4A), and ACD was decreased by 3.0% (mean, −0.09 mm; 95% CI, −0.12 to −0.06 mm; P < .001) (Figure 4B). Both axial length (mean, −0.05 mm; 95% CI, −0.07 to −0.03 mm; P < .001) and ACD (mean, −0.10 mm; 95% CI, −0.13 to −0.06 mm; P < .001) remained lower than preflight values for up to 1 year after returning to Earth. These structural changes were associated with a hyperopic shift. On the return to Earth plus 7 days, the measured shift in spherical equivalent was 0.13 diopters (95% CI, 0.03-0.23 diopters; P < .05) compared with before spaceflight (eTable 3 in the Supplement). This scientifically meaningful change in refraction associated with spaceflight persisted through the examination 6 months following the return to Earth (Figure 5).
Figure 4. Change in Axial Length and Anterior Chamber Depth (ACD) Compared With Before Spaceflight.
Change from before spaceflight was measured in both eyes of each participant (circles) using optical biometry. Bars indicate 95% CIs. All P < .001 compared with preflight.
Figure 5. Change in Cycloplegic Refraction (Spherical Equivalent).
Change after returning to Earth compared with before spaceflight for both eyes of each participant (circles). Bars represent 95% CIs.
aP < .05.
bP < .01.
cNot significantly different.
Discussion
The primary finding of this study was that spaceflight was associated with peripapillary neural, retinal, and choroid tissue thickening in both eyes during early spaceflight that persisted throughout the mission. In addition, contrary to early anecdotal reports, we observed that peripapillary neural, retinal, and choroid tissue thickening occurred in a female astronaut. In the present cohort, peripapillary total retinal thickening expanded radially during the course of the mission as evidenced by increased thickness at the outer annuli late in spaceflight. Previous reports, limited to preflight and postflight measures only, have shown that, within a week of returning to Earth, a TRT annulus from BMO to 250 μm is elevated by 31 μm relative to before spaceflight.3 The present study found that between 30 and 90 days were required for the peripapillary retinal and choroid tissue thickening to return to values similar to those observed before flight.
One of the weaknesses of the current definition of SANS is that it has been primarily based on a subjective assessment using ophthalmoscopic imaging. This technique lacks the sensitivity to detect early ocular structural changes that might help explain the etiology of SANS and guide the development of countermeasures. Furthermore, this technique underestimates the number of astronauts affected by SANS with relevant TRT thickening. The present quantitative OCT-based measures of retinal thickness revealed early stages of optic disc edema and allowed for objective quantification of these structural changes compared with 2-dimensional color ophthalmoscopic images (Figure 1, Figure 2). For example, 2 participants in this cohort had mild disc edema documented by retinal photographs during spaceflight (eFigure in the Supplement). One of these participants presented with mild optic disc edema in the right eye on flight day 150 that was not present before spaceflight. This optic disc edema assessed by ophthalmoscopic imaging persisted until the last postflight data collection 356 days after return to Earth. The other participant had mild optic disc edema in the right eye that persisted for the duration of the mission and resolved 2 days after return to Earth. In contrast to ophthalmoscopic imaging, quantitative OCT-based measures of recovery suggest that approximately 45 to 90 days are required in the gravitational environment on Earth for the retinal thickness to return to preflight levels. Patients typically present with pathology without baseline imaging data prior to the development of disease. These unique data gathered in the present study from a healthy, normal population may provide important quantitative data to compare with the development and recovery among patients without baseline data to yield an understanding of the early structural changes of optic disc edema.
Current technology and data analysis methods do not permit determination of the factors that contribute to the development or recovery of TRT and choroidal thickness changes. However, the altered ocular morphology observed in association with spaceflight may be attributable to the chronic headward fluid shift that occurs immediately on entering weightlessness and remains throughout the duration of weightlessness.7,8 Removal of the gravitational fluid pressure gradient in weightlessness causes a cephalad fluid shift and may be associated with these ocular structural changes.9 Direct measures of ICP collected during brief weightlessness in parabolic flight suggest that ICP is at a level slightly less than that in a supine posture on Earth (16 vs 13 mm Hg), yet it is higher compared with that observed in a seated or standing posture.7 However, given the brevity of the weightlessness exposure in that experimental condition, caution must be used in extrapolating the results to the chronic weightlessness state. Currently, there are no direct measures of ICP during long-duration spaceflight; thus, data on ICP are lacking. Chronic but mild elevation in retrobulbar cerebrospinal fluid pressure, engorgement of the choroidal vasculature, or both may be associated with deformed tissues at the optic nerve head, as modeled recently.10,11 Moreover, the optic nerve head and optic nerve are densely vascularized tissues, and microvasculature hemodynamics may be altered when cerebrospinal fluid or tissue fluid are chronically elevated, limiting axoplasmic flow and resulting in neuronal swelling of retinal nerve fibers. In addition, if the headward fluid shift is associated with a chronic elevation in arterial or venous pressure in the vasculature at the optic nerve head, increased local capillary filtration may be associated with edema formation or direct compression of neural tissue by the vasculature that may impede axoplasmic flow.12 Although retinal and retrolaminar capillary endothelia contain tight junctions that create the retinal-blood barrier, the capillary endothelium in the prelaminar region of the optic nerve head lacks endothelial membrane proteins associated with the blood-brain barrier.13 Thus, the lack of a retinal-blood barrier in this region of the optic nerve head may be associated with a greater extravasation of fluid due to the headward fluid shift in weightlessness and may be a contributing factor to the retinal thickening quantified at this location. Therefore, this increased capillary filtration may be associated with tissue edema, limiting the oxygen diffusion distance affecting cell metabolism and reducing axoplasmic flow.
A previous case report noted choroid thickening during spaceflight; however, the measurement obtained approximately 1 week after spaceflight was not a significant increase.3,4 In the present report, increases in peripapillary choroid thickness were detected early during spaceflight, persisted throughout the mission, and required 45 to 90 days after returning to Earth to recover to preflight levels. Recent advancements in OCT technology may have enabled the detection of the deeper retinal choroid tissue structure not clearly visualized in the previous study.3 It has been hypothesized that the cephalad fluid shifts during weightlessness may cause decreased choroidal drainage and a relatively stagnant pooling of blood in the choroid leading to choroidal expansion.1 The slower recovery of choroid thickness in astronauts after spaceflight compared with the relatively rapid increase observed early during spaceflight (launch day to flight day 10) may suggest that chronic vascular congestion and expansion in this region may extend the delicate choroidal collagen lamella beyond its normal anatomic structural boundaries, resulting in localized choroidal remodeling associated with spaceflight or a yet-to-be-described fluid drainage pathway impairment.
In addition to changes of the optic nerve head, reductions in axial length were present after the mission and likely are associated with the observed hyperopic shift. By contrast, the reduction in ACD associated with spaceflight may have altered the optical path. This may explain why the hyperopic shift, as measured with cycloplegic refraction, was less than what would be generally predicted when based on the axial length change alone. It must also be determined whether this decrease in axial length poses any additional risk to that of developing acute angle-closure glaucoma during spaceflight. It is unlikely that standard ultrasonography would enable reliable detection of the presumed decrease in axial length during spaceflight of −0.08 mm owing to current ultrasonographic imaging resolution limitations. Optical biometry measures during spaceflight would help document the temporal profile of these changes in axial length and ACD.
Limitations
The primary limitations of this study include the limited numbers of participants. In addition, optical biometry and refraction measures were not conducted during spaceflight.
Conclusions
Although past publications have served an important purpose to inform the community and as the impetus for a new line of ophthalmology spaceflight research, the present work provides a comprehensive temporal profile of ocular structural change associated with long-duration spaceflight, both during and after the mission. This report documents that peripapillary optic disc edema and choroid thickening occurred in both the right and left eyes and in both sexes. In addition, we built on previous work to show for the first time that spaceflight was associated with axial length and ACD shortening. Finally, this report documents the postflight recovery of astronauts from optic disc edema and ocular structural changes that remained after spaceflight. Future work is required to determine whether these ocular structural findings are associated with diminished visual function or early visual changes later in life.
eTable 1. Global Total Retinal Thickness (TRT) Annuli.
eTable 2. Total Retinal Thickness (TRT) Annuli by Quadrant
eTable 3. Individual Refractive Data for Each Astronaut Before and After Spaceflight
eFigure. Fundus Images of Those Individual With Fundus Grade Optic Disc Edema
References
- 1.Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118(10):2058-2069. doi: 10.1016/j.ophtha.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 2.Stenger MB, Tarver WJ, Brunstetter T, et al. NASA Human Research Program evidence report: human health countermeasures element: risk of spaceflight associated neuro-ocular syndrome (SANS). Published November 30, 2017. Accessed February 15, 2018. https://humanresearchroadmap.nasa.gov/evidence/reports/SANS.pdf?rnd=0.434276635495143
- 3.Patel N, Pass A, Mason S, Gibson CR, Otto C. Optical coherence tomography analysis of the optic nerve head and surrounding structures in long-duration International Space Station astronauts. JAMA Ophthalmol. 2018;136(2):193-200. doi: 10.1001/jamaophthalmol.2017.6226 [DOI] [PubMed] [Google Scholar]
- 4.Mader TH, Gibson CR, Otto CA, et al. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol. 2017;37(2):133-139. doi: 10.1097/WNO.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 5.Law J, Van Baalen M, Foy M, et al. Relationship between carbon dioxide levels and reported headaches on the international space station. J Occup Environ Med. 2014;56(5):477-483. doi: 10.1097/JOM.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 6.Lifesciences Archive NASA. Medical operations: Space Medicine Division. Updated October 10, 2018. Accessed February 21, 2020. https://lsda.jsc.nasa.gov/MRID
- 7.Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595(6):2115-2127. doi: 10.1113/JP273557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton WE, Hoffler GW, Rummel J. Anthropometric changes and fluid shifts In: Johnston RS, Dietlein LF, eds. Biomedical Results From SKYLAB. NASA SP; 377. Scientific and Technical Information Office, National Aeronautics and Space Administration; 1977:330-339. [Google Scholar]
- 9.Marshall-Goebel K, Laurie SS, Alferova IV, et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open. 2019;2(11):e1915011. doi: 10.1001/jamanetworkopen.2019.15011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibony PA, Kupersmith MJ, Feldon SE, Wang JK, Garvin M; OCT Substudy Group for the NORDIC Idiopathic Intracranial Hypertension Treatment Trial . Retinal and choroidal folds in papilledema. Invest Ophthalmol Vis Sci. 2015;56(10):5670-5680. doi: 10.1167/iovs.15-17459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feola AJ, Nelson ES, Myers J, Ethier CR, Samuels BC. The impact of choroidal swelling on optic nerve head deformation. Invest Ophthalmol Vis Sci. 2018;59(10):4172-4181. doi: 10.1167/iovs.18-24463 [DOI] [PubMed] [Google Scholar]
- 12.Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19(4):312-326. doi: 10.1113/jphysiol.1896.sp000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofman P, Hoyng P, vanderWerf F, Vrensen GF, Schlingemann RO. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42(5):895-901. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Global Total Retinal Thickness (TRT) Annuli.
eTable 2. Total Retinal Thickness (TRT) Annuli by Quadrant
eTable 3. Individual Refractive Data for Each Astronaut Before and After Spaceflight
eFigure. Fundus Images of Those Individual With Fundus Grade Optic Disc Edema