Abstract
Collection efficiencies of four bioaerosol samplers (Andersen impactor, AGI-30 impinger, gelatin filter, and nuclepore filter) were evaluated for virus-containing aerosols. Four different bacteriophages were used as surrogates for the mammalian viruses. Results showed that the collection efficiency was significantly affected by the morphology of the virus particles. For hydrophilic viruses, the collection efficiencies of the Andersen impactor, impinger, and gelatin filter were 10 times higher than that of the nuclepore filter. For hydrophilic viruses, the collection efficiencies of all four samplers were 10–100 times higher than hydrophobic viruses. The infectivity of the virus in collected samples was also evaluated for an AGI-30 impinger. Results showed that the viruses retained more infectivity when the samples were refrigerated (up to 1 day) during storage than when stored at room temperature (up to 8 h). Therefore, even when refrigerated, airborne virus samples collected using an impinger should be processed as soon as possible to avoid loss of virus infectivity.
Keywords: Bioaerosols, Virus aerosol, Bacteriophage, Andersen impactor, AGI-30 Impinger, Gelatin filter, Nuclepore filter
1. Introduction
Viruses are obligate parasites and are pathogenic to humans and animals. Airborne and droplet transmission are the major routes for spreading viral diseases, such as smallpox, influenza, measles, and mumps. Recently, severe acute respiratory syndrome (SARS) virus and influenza virus have attracted public attention and are believed to be transmitted by aerosol. Virus droplets generated from sneezing or coughing typically range from 1 to in size and will evaporate to droplet nuclei that approach the size of the individual microbe (Kowalski & Bahnfleth, 1998). Such droplet nuclei remain airborne for long periods of time (up to 1 day) with potential for retention in the respiratory tract (Ijaz, Karim, Sattar, & Johnson-Lussenburg, 1987). When a virus is encased in a droplet, its infectivity is enhanced due to shielding from drying, temperature, and sunlight, compared to an isolated airborne virus (Tyrrell, 1967). Due to such droplet encasement, virus-containing aerosols less than in size have higher infectivity than those of the virus itself (Couch et al., 1965).
Relative humidity (RH), temperature, wind, light, irradiation, as well as suspending medium, influence the infectivity of airborne viruses (Benbough, 1971). Non-lipid viruses are stable at higher RH (), whereas lipid viruses are stable at lower RH () (Benbough, 1971). In addition, a virus loses its infectivity in the presence of an NaCl- or peptone-containing medium, whereas retains phenylalanine protects a virus from aerosol inactivation at various RH levels (Dubovi and Akers, 1970, Benbough, 1971, Trouwborst and de Jong, 1973).
The collection efficiency of different bioaerosol samplers differs significantly. Microbial collection and survival in bioaerosol samplers strongly depend on the type of sampler, microorganism hardiness, sampling time, and sampling flow rate (Macher and Willeke, 1992, Nevalainen et al., 1993; Lin and Li, 1998, Lin and Li, 1999a).
To evaluate collection efficiencies of bioaerosol samplers for virus aerosols, the commonly assessed virus targets have been harmful human/animal viruses, such as poliovirus, coronavirus, rotavirus, and adenovirus. For safety concerns during experiments, a single type of bacteriophage, MS2, has been typically used as a substitute for pathogenic viruses (Harstad, 1965, Hatch and Warren, 1969, Trouwborst and de Jong, 1972). However, MS2 cannot represent all types of viruses due to the wide range of structures and nucleic acids. Using an Andersen 6-STG sampler, Ijaz et al. (1987) demonstrated that 87% of aerosolized viruses have a particle size smaller than . Studies show that the collection efficiency of impingers for infective viruses is superior to that of filters (Hatch and Warren, 1969, Dubovi and Akers, 1970; Trouwborst, de Jong, & Winkler, 1972), and that RH, stress during sampling, and the extraction process strongly influence the collection efficiency of a biosampler for a virus (Harstad, 1965, Ijaz et al., 1987).
Interpretation of results from field studies is complicated by the delay caused by transport of samples to a laboratory before the samples are plated. Such delay gives the organisms an opportunity to multiply or die in transit (Thorne, Kiekhaefer, Whitten, & Donham, 1992). Although the effect of storage time and temperature on survival of bacterial and fungal aerosols collected using impingers has been reported (Li and Lin, 2001, Lin and Li, 2003), the effect on survival of virus aerosols has not yet been reported until our current study. Although a virus is an obligate parasite and cannot multiply without a host in the collection medium, virus infectivity might still be affected by the sampling method, storage temperature, storage duration, and sample processing. The results for collection efficiency from the previous sampling studies of aerosolized virus discussed above (Hatch and Warren, 1969, Dubovi and Akers, 1970, Trouwborst et al., 1972) could not be compared due to the use of different sampling devices, evaluation indexes, and virus targets. The collection efficiencies of different sampling devices for collecting infective viruses with different morphology and nucleic acid types needs further investigation.
In our current study, the collection efficiency was evaluated for four of the most commonly used bioaerosol samplers for virus aerosols (Andersen one-stage impactor, AGI-30 impingers, gelatin filter, and nuclepore polycarbonate filter). For safety concerns, bacteriophages with either single-strand DNA (phi X174), single-strand RNA (MS2), double-strand DNA (T7), or double-strand RNA (phi 6) were used as surrogates for mammalian viruses. Because RH strongly affects virus infectivity, collection was done at three RH levels (20%, 55%, and 85%). The effect of storage conditions (collection media, storage temperature, and storage time) and the kinetic decay curves of the virus aerosols at different storage temperatures were also evaluated for an AGI-30 impinger.
2. Materials and methods
2.1. Test viruses
In general, viruses with a lipid envelope are hydrophobic and viruses without a lipid envelope are hydrophilic (Vidaver, Koski, & Van Etten, 1973). In this study, the test viruses were four different bacteriophages, namely, those consisting of single-strand DNA (phi X174, ATCC 13706-B1), single-strand RNA (MS2, ATCC 15597-B1), double-strand DNA (T7, ATCC 11303-B1) and double-strand RNA (phi 6 with envelope lipid, ATCC 21781-B1). The host bacteria were Escherichia coli for coliphages phi X174, MS2, and T7 (ATCC 13706, 15597 and 11303, respectively) and Pseudomonas syringae (ATCC 21781) for phi 6. A high titer stock of bacteriophages (– PFU/mL) Plaque forming units (PFU) was prepared via plate lysis and elution. To allow the phage to attach to the host, the bacteriophages were mixed with their own respective host. First, 5 ml of top agar was added to a sterile tube of infected cells. Then, the contents of the tube were mixed by gentle tapping for 5 s and poured onto the center of a labeled agar plate. Finally, the plate was incubated for 24 h either at for coliphages or at for phi 6. After cultivation, 5 ml SM buffer (containing NaCl, , Tris, and gelatin) was pipetted onto a plate that showed confluent lysis. Then, the plate was slowly rocked for 40 min and the buffer was transferred to a tube for centrifugation at for 10 min. After the supernatant was removed, the resulting phage stock was stored at . Plaque assay described by Adams (1959) was then used to measure the bacteriophage concentrations.
2.2. Aerosol generation and test system
The test chamber for virus sampling was 29 cm in diameter and 32 cm in height. A Collision three-jet nebulizer (BGI Inc., Waltham, MA) was used to nebulize the bacteriophage stock in deionized water at 3 L/min with dry, filtered, and compressed laboratory air, then passed though a Kr-85 particle-charge neutralizer (model 3077, TSI). The aerosolized suspension was then diluted with filtered, compressed air at 57 L/min. The stock solutions of bacteriophages MS2, phi X174, and T7 were diluted in sterile, deionized water for nebulization, and that of phi 6 phage was diluted in sterile, deionized water containing 0.03% Tween 80 for preserving infectivity.
An aerodynamic particle sizer (APS, Model 3310A, TSI, Inc., St. Paul, MN) was used to measure the real-time number and size distribution of the total virus-containing aerosols in the range of 0.5–. An Andersen six-stage viable impactor (6-STG, Andersen Samplers, Inc., Atlanta, GA) was used to measure the size distributions of the virus aerosols that were to be evaluated by plaque assay.
2.3. Test samplers and sample processing
An Andersen 1-STG sampler is the sixth stage of the Andersen 6-STG sampler. This stage has 400 0.25-mm holes and has a sampling flow rate of 28.3 L/min (corresponding to a velocity of 24 m/s) when 20 mL LB (Luria-Bertani) broth is used with 3% gelatin plates. The measured and theoretical cut-point diameters of this stage are 0.57 and , respectively (Nevalainen et al., 1993).
The AGI-30 (Ace Glass Inc.) is an all-glass impinger with a 30-mm jet-to-plate distance, and was operated here at a sampling flow rate of 12.5 L/min for 5 min. To study the effect of the type of collection medium on the collection efficiency of an AGI-30 sampler reported by Crook (1995), three different collection media were used; sterile deionized water, nutrient broth (with 0.5% NaCl and 0.5% antifoam A purchased from Sigma Chemical Co., St. Louis, MO), and peptone broth (deionized water with 1% peptone, 0.01% Tween 80, and 0.005% antifoam A). These collection media are commonly used for phages and bacteria sampling (Forade et al., 1999, Li et al., 1999). From our preliminary evaluation (data not shown), no difference in collection efficiency was observed among these three collection media at 55% RH. Therefore, 20 mL sterile deionized water was chosen as the medium to evaluate the effect of RH on the collection efficiency of an AGI-30 sampler.
The gelatin filter used in this study had a 3.0- pore size and a 80-mm diameter (Sartorius, Gottingen, Germany). This filter was placed in a sterile filter holder by carefully letting the filter slide out of the cassette and onto the filter support of an aluminum filter holder. The filter was operated at 30 L/min at a sampling time of 5 min. After sampling, the filter was dissolved on an LB broth (with 3% gelatin) agar surface for further quantification by plaque assay.
The nuclepore filter (Costar, Cambridge, MA) used in this study consisted of a polycarbonate membrane with a 0.4- pore size and a 37-mm diameter supported by cellulose pads. Before the filter was loaded into an open-face, two-piece plastic cassette, both the filter and support pads were autoclaved and the plastic cassette was sterilized with ethylene oxide. The sampling flow rate was 2 L/min and the sampling time was 20 min.
After sampling, the plate with collection medium from the Andersen impactor was placed in an incubator at for 10 min. The nuclepore samples were eluted by rinsing in 5 ml sterile deionized water, and the suspension was slowly vortexed in a rotator (Vortex-2Genie, G-560, Scientific Industries Inc.) for 30 s. The filter was carefully placed into a 90-mm Petri dish and then dissolved in 20 mL LB broth with 3% gelatin in a incubator for 10 min. All of the viral samples were subjected to plaque assay for coliphages at and for phi 6 at . Then, the was calculated based on the dilution ratio, plated volume, sampling time, and flow rate.
2.4. Calculation of collection efficiency
The culturability and viability of aerosolized bacteria and fungi strongly affect the collection efficiency of a bioaerosol sampler (Lin and Li, 1998, Lin and Li, 1999a). The culturability and viability of these microorganisms, however, depend on the culture preparation process (Lin and Li, 1998, Lin and Li, 1999a). These bacterial and fungus results were also suitable for aerosolized virus. Therefore, total recovery (TR) is not a good indicator for collection efficiency of samplers; here, TR is defined as , where is , i.e., the number of infective virus particles per cubic meter of air passed through the sampler, and is the total number of virus particles per cubic meter of air passed through a particle counter. To better understand the collection efficiency for virus aerosols, the viability of microorganisms in liquid suspension used as the source of the generated bioaerosols should be evaluated. Therefore, in this study, we used a parameter called relative recovery (RR) as an indicator for collection efficiency of the samplers; here, RR is defined as /, where is the PFU/mL in the suspension, i.e., number of infective virus particles in the suspension.
2.5. Effect of virus storage conditions on collection efficiency
The effect of storage conditions of a virus collected by an impinger (AGI-30 sampler) were evaluated by determining the effect of medium type, storage temperature, and storage time on the variations in virus infectivity. Three different media were used, namely, deionized water, nutrient broth, and peptone broth. After virus air sampling by using the AGI-30, the first sample from the liquid collection medium was inoculated as soon as possible (5 min) for the initial concentration, . Each sample of collected virus was separated into two equal portions, one of which was then stored at (room temperature) and the other refrigerated at . To obtain a kinetic curve of concentration variation up to 1-day storage, each storage suspension was inoculated for plaque assay at storage times of 0, 1, 2, 4, 6, 8 and 24 h. The effect of storage time on the variation in virus infectivity was determined by calculating the ratio , where and are the PFU concentrations of the simultaneously collected samples stored for and 0 h, respectively.
3. Results and discussion
In this study, the collection efficiency of aerosolized viruses was evaluated for four different bioaerosol samplers (Andersen 1-STG impactor, AGI-30 impinger, gelatin filter, and nuclepore polycarbonate filter) using four bacteriophages (MS2, phi X174, T7 and phi 6). In addition, the effect of storage temperature, storage time, and collection media on the collection efficiency of an AGI-30 impinger was evaluated.
3.1. Characteristics of the aerosolized viruses
Based on our results, virus infectivity in the nebulizer suspension and aerosol phase (at RH=20%, 55% and 85%) could be maintained up to 90 min with a coefficient of concentration variation less than 25%. The measured geometric mean aerodynamic diameter of MS2 (Fig. 1 ), phi X174, T7, and phi 6 was 1.23, 1.25, 1.24, and , respectively, with a geometric standard deviation of 1.5. In the Andersen 6-STG sampler, more than 95% of recovered PFU plaques were less than in diameter. These measured size distributions for the four tested virus aerosols agree well with those previously reported (Couch et al., 1965; Akers, Prato, & Dubovi, 1973; Ijaz et al., 1987).
Fig. 1.
The particle size distributions of MS2 virus in the test chamber measured by (a) aerodynamic particle sizer and (b) Andersen six-stage impactor. Each particle size distribution represents the mean of at least three trials.
3.2. Collection efficiency of tested samplers
3.2.1. Andersen 1-STG sampler
For this sampler, the RR for MS2 and phi X174 (Fig. 2, Fig. 3 , respectively) were in the range at all three RH levels (20%, 55%, and 85%). The RR for T7 (Fig. 4 ) at RH 85% was similar to that for MS2 and phi X174, but was much lower (1 log decrease) at RH 20% and 55%. At all RH levels, the RR for phi 6 (Fig. 5 ) ranged between and , significantly lower than those for the other three viruses. These differences might be because MS2 and phi X174 phages are lipid-free icosahedral viruses, and are therefore more resistant to sampling stress compared to T7, which has a tail fiber, and to phi 6, which has a lipid envelope. The observed higher RR for the tailed phage T7 at high RH (85% RH) might be due to the formation of a moisture film that protects the delicate tail fibers of this phage from sampling stress (Hatch & Warren, 1969). Because the lipid content of phi 6 is extremely sensitive to environmental stress (Woolwine & Gerberding, 1995), the low RR of phi 6 might be related to the lipid content being affected by the sampling stress, such as impaction and dehydration. The lipid content of phi 6 reportedly is essential for infectivity (Woolwine & Gerberding, 1995).
Fig. 2.
The effects of relative humidity on RR of Andersen impactor, AGI-30 impinger, nuclepore and gelatin filter for MS2 virus. (; : by the evaluated sampler, the number of infective virus particles per of air by the evaluated sampler : PFU/mL in the suspension, the number of infective virus particles in the suspension.)
Fig. 3.
The effects of relative humidity on RR of Andersen impactor, AGI-30 impinger, nuclepore and gelatin filter for phi X174 virus. (; : by the evaluated sampler, the number of infective virus particles per of air by the evaluated sampler : PFU/mL in the suspension, the number of infective virus particles in the suspension.)
Fig. 4.
The effects of relative humidity on RR of Andersen impactor, AGI-30 impinger, nuclepore and gelatin filter for T7 virus. (; : by the evaluated sampler, the number of infective virus particles per of air by the evaluated sampler : PFU/mL in the suspension, the number of infective virus particles in the suspension.)
Fig. 5.
The effects of relative humidity on RR of Andersen impactor, AGI-30 impinger, nuclepore and gelatin filter for phi 6 virus. (; : by the evaluated sampler, the number of infective virus particles per of air by the evaluated sampler : PFU/mL in the suspension, the number of infective virus particles in the suspension.)
3.2.2. AGI-30 impinger
For this sampler, the RR for MS2 and phi X174 (Figs. 2 and 3, respectively) at all three RH levels and for T7 at 85% RH (Fig. 4) exceeded , similar to that for the Andersen sampler. The RR for T7 at RH 20% and 55%, however, was much lower than that of 85% RH (2 log decrease). These findings agree well with those reported for tailed phages T1 and T3 collected using an impinger (Harstad, 1965, Hatch and Warren, 1969). This significant lower recovery of T7 might be associated with the nature of the protein or nucleic acid of T7 to undergo instant reconstitution in impinger fluids and form into a molecular configuration that is not compatible with adsorption onto and penetration into a host for multiplication (Hatch & Warren, 1969). At all three RH levels, the RR for phi 6 (Fig. 5) was in the range, higher than that for the Andersen sampler.
3.2.3. Gelatin filter
For this sampler, the RR was in the range for MS2 (Fig. 2) and in the range – for phi X174 (Fig. 3) at all three RH levels. The RR for the two hydrophilic viruses (MS2 and phi X174) was similar for the Andersen 1-STG sampler, AGI-30, and gelatin filter, and was similar to those previously reported for hardy endospore B. subtilis and yeast cells (Li et al., 1999; Lin & Li, 1999b). Moreover, the RR for phi 6 (Fig. 5) at all three RH levels and T7 (Fig. 4) at RH 20% and 55% were low (), similar to those for fragile bacteria (Li et al., 1999). Previous studies show that a gelatin filter is not suitable for collecting airborne fragile bacteria because the gelatin dries out during extended sampling, thus placing additional dehydration stress on the collected microorganisms (Crook, 1995, Li et al., 1999). Similarly, filtration might induce dehydration stress in phi 6 and T7 because they are sensitive and fragile viruses.
3.2.4. Nuclepore filter
For this sampler, the average RR for MS2 and phi X174 (Figs. 2 and 3, respectively) was in the range, lower than the RR for the other three samplers. The RR was extremely low for both the phi 6 (Fig. 5) at all three RH levels () and for T7 (Fig. 4) at RH 20% and 55% (). The RR for T7 at RH 85% was , higher than those at RH 20% and 55%. Because all four of the test virus aerosols in this study were larger than the pore size of this nuclepore filter, penetration of the virus aerosols through the filter should be negligible. The observed loss of virus infectivity (reflected by the extremely low RR) in samples collected using a nuclepore filter is probably primarily related to the biological stress during filtration, dehydration during sampling, and extraction process (Li et al., 1999).
In summary, our results showed that both the morphology of the virus particles and the presence or absence of a lipid envelope significantly affected the collection efficiency of the four evaluated bioaerosol samplers and affected the infectivity of the collected virus sample. The Andersen impactor, impinger, and gelatin filter were found superior to the nuclepore filter for sampling hydrophilic viruses. In addition, the RR values of hydrophobic viruses (i.e., phi 6) (viruses with a lipid envelope) was lower than that of hydrophilic viruses (i.e., MS2, phi X174 and T7), because lipid is extremely sensitive to sampling stress, and virus recoveries of MS2, phi X174, and phi 6 did not depend on RH, whereas that of T7, which has a tail fiber, strongly depended on RH. These results are similar to those previously reported for bacteria, endospores, and fungal spores (Lin & Li, 1999b). Specifically, when the impactor or impinger were used for virus collection, the RR values for MS2 and phi X174 viruses were similar to those for E. coli and yeast cells, but lower than those for endospore B. subtilis and Penicillium (Li & Lin, 1999b; Li et al., 1999; Lin & Li, 1999a). When the gelatin filter was used, the RR values for MS2 and phi X174 virus were lower than that for Penicillium, were similar to those for B. subtilis and yeast, and were higher than that for E. coli (Lin & Li, 1998; Li & Lin, 1999a). When the nuclepore filter was used, the recoveries for MS2 and phi X174 virus were lower than those reported for Penicillium and B. subtilis and were similar to those for yeast and E. coli (Li et al., 1999; Lin & Li, 1999b).
3.2.5. Effect of storage conditions of sampled virus on collection efficiency of an impinger
The effect of storage temperature, storage time, and collection medium on the variation in virus infectivity was also evaluated for an impinger as the bioaerosol sampler. The results revealed that MS2 phage had an infectivity retention of more than 80% in all three collection media (deionized water, peptone broth, and nutrient broth) up to 8 h when the collected samples were stored at room temperature () or up to 1 day when refrigerated at (Fig. 6 ). For phi X174 (Fig. 7 ), the infectivity retention after 1 day was 80% when the sample was refrigerated compared with 50% when the sample was stored at room temperature.
Fig. 6.
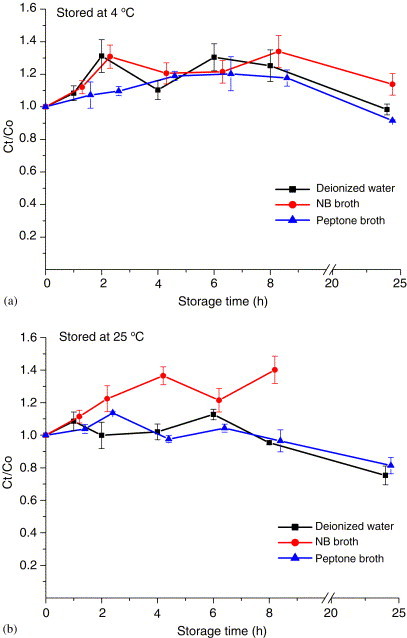
Effects of storage time of MS2 by AGI-30 impinger sampling at 12.5 L/min: (a) stored at , (b) stored at . and are the PFU concentrations of the simultaneously collected samples stored for and 0 h. Each error bar represents 1 S.D. on the mean of three replicates.
Fig. 7.
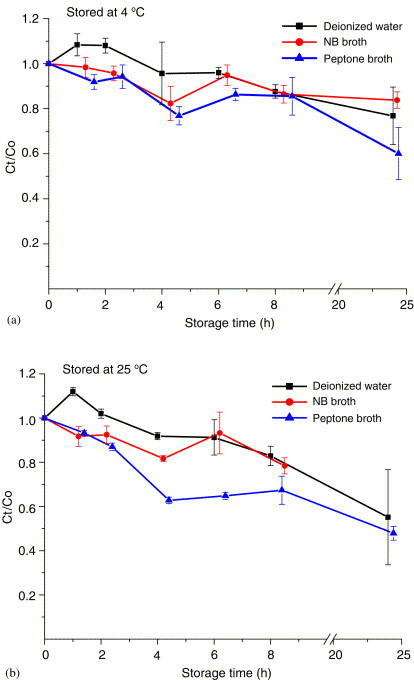
Effects of storage time of phi X174 by AGI-30 impinger sampling at 12.5 L/min: (a) stored at , (b) stored at . and are the PFU concentrations of the simultaneously collected samples stored for and 0 h. Each error bar represents 1 S.D. on the mean of three replicates.
In either deionized water or nutrient broth, the infectivity retention for T7 (Fig. 8 ) and MS2 was higher (), irrespective of storage temperature. In peptone broth, the infectivity retention for T7 at room temperature was significantly lower (40%) than that in either deionized water or nutrient broth. When stored at room temperature, Phi 6 retained little infectivity () in all three media (Fig. 9 ), but when refrigerated, it retained more than 80% infectivity in either deionized water or peptone broth. Based on previous studies indicating that salt solution might lower the infectivity of phage because of sampling stress of unbalanced forces at the air–water interface (Dubovi and Akers, 1970, Trouwborst and de Jong, 1973), the infectivity of phage phi 6 could be significantly affected by the salt in the nutrient broth during the impingement and thus cause the loss of virus infectivity. No plaque was found in the MS2, phi X174, and phi 6 samples in nutrient broth stored for 24 h at room temperature, possibly due to interference of plaque assay. Further study is needed, however, to confirm this interference.
Fig. 8.
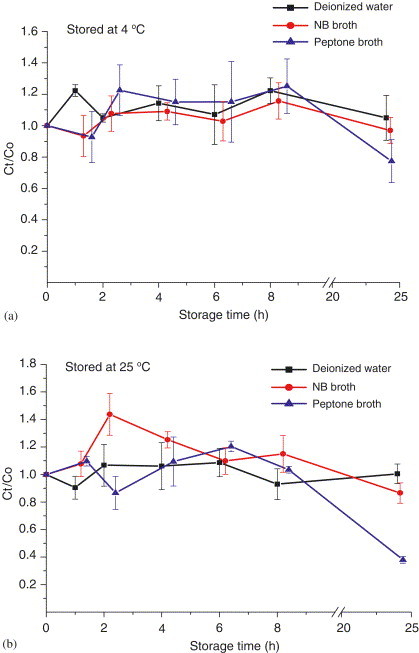
Effects of storage time of T7 by AGI-30 impinger sampling at 12.5 L/min: (a) stored at , (b) stored at . and are the PFU concentrations of the simultaneously collected samples stored for and 0 h. Each error bar represents 1 S.D. on the mean of three replicates.
Fig. 9.
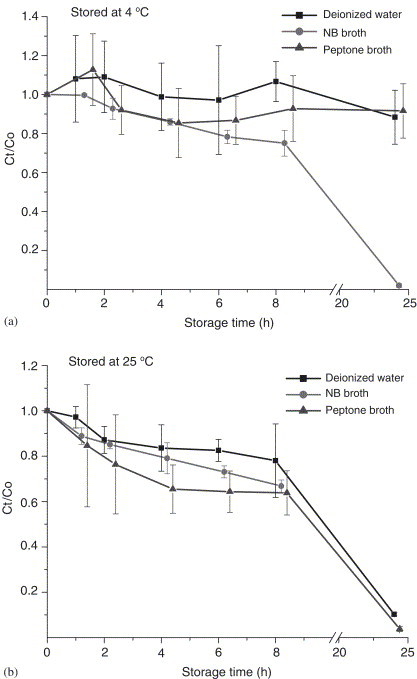
Effects of storage time of phi 6 by AGI-30 impinger sampling at 12.5 L/min: (a) stored at , (b) stored at . and are the PFU concentrations of the simultaneously collected samples stored for and 0 h. Each error bar represents 1 S.D. on the mean of three replicates.
Viruses refrigerated at had higher infectivity retention than those stored at room temperature (), except for phi 6 in peptone broth. Among the three evaluated collection media, deionized water provided better infectivity retention at and . Therefore, the loss of virus infectivity might be minimized by adjusting the storage temperature and composition of the collection medium. Our results also showed that the effects of storage conditions of MS2, phi X174, and T7 viruses on the collection efficiency were similar to those for endospore bacteria (B. subtilis) and fungi (yeast), and superior to those for fragile bacteria (E.coli). (Li et al., 1999; Lin & Li, 1999b). In conclusion, airborne virus samples collected using an impingement method should be processed as soon as possible to avoid loss of virus infectivity.
4. Conclusion
The collection efficiencies of four different bioaerosol samplers (Andersen impactor, AGI-30 impinger, Gelatin filter and nuclepore filter) for collecting different types of virus aerosols was evaluated. Results revealed that the collection efficiencies of these samplers for airborne viruses strongly depended on the virus morphology, the hydrophilic nature of the virus and relative humidity. For preserving higher virus infectivity, the Andersen impactor, impinger and gelatin filter were found more suitable than the nuclepore filter for collecting virus aerosols. The unsuitability of the nuclepore filter was possibly due to sampling stress during filtration, to dehydration during sampling, and to the extraction process. Results also revealed that the storage temperature and collection medium were the most critical factors in the storage of collected virus samples, suggesting that the loss of virus infectivity could be minimized by adjusting the storage temperature and composition of the collection medium. In conclusion, airborne virus samples collected using an impingement method should be processed as soon as possible to avoid loss of virus infectivity.
Acknowledgements
This work was supported by Grant NSC 91-2621-Z-002-025 from the National Science Council, Republic of China. Chun-Chieh Tseng was supported by a graduate scholarship from the same grant during part of this research effort.
References
- Adams, M. H. (1959). Bacteriophages (pp. 450–456). New York: Interscience Publishers Inc.
- Akers T.G., Prato C.M., Dubovi E.J. Airborne stability of simian virus 40. Applied Microbiology. 1973;26:146–148. doi: 10.1128/am.26.2.146-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbough J.E. Some factors affecting the survival of airborne viruses. Journal of General Virology. 1971;10:209–220. doi: 10.1099/0022-1317-10-3-209. [DOI] [PubMed] [Google Scholar]
- Couch, R. B., Gerone, P. J., Cate, T. R., Griffith, W. R., Alling, D. W., & Knight, V. (1965). Preparation and properties of a small-particle aerosol of Coxsackie A21. Proceedings of the Society for Experimental Biology and Medicine (Vol. 118, pp. 818–822). [DOI] [PubMed]
- Crook B. Non-inertial samplers: biological perspectives. In: Cox C.S., Wathes C.M., editors. Bioaerosols handbook. Lewis Publishers; Boca Raton, FL: 1995. pp. 269–283. [Google Scholar]
- Dubovi E.J., Akers T.G. Airborne stability of tailless bacterial viruses S-13 and MS-2. Applied Microbiology. 1970;19:624–628. doi: 10.1128/am.19.4.624-628.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forade K.K., Myers E.A., Hanley J.T., Ensor D.S., Roessler P.F. Methodology to perform clean air delivery rate type determinations with microbiological aerosols. Aerosol Science and Technology. 1999;30:235–245. [Google Scholar]
- Harstad J.B. Sampling submicron T1 bacteriophage aerosols. Applied Microbiology. 1965;13:899–908. doi: 10.1128/am.13.6.899-908.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M.T., Warren J.C. Enhanced recovery of airborne T3 coliphage and Pasteurella pestis bacteriophage by means of a presampling humidification technique. Applied Microbiology. 1969;17:685–689. doi: 10.1128/am.17.5.685-689.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M.K., Karim Y.G., Sattar S.A., Johnson-Lussenburg C.M. Development of methods to study the survival of airborne viruses. Journal of Virological Methods. 1987;18:87–106. doi: 10.1016/0166-0934(87)90114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski W.J., Bahnfleth W. Airborne respiratory diseases and mechanical systems for control of microbes. Heating/Piping/Air Conditioning HPAC Engineering. 1998;70:34–48. [Google Scholar]
- Li C.S., Hao M.L., Lin W.H., Chang C.W., Wang C.S. Evaluation of microbial samplers for bacterial microorganisms. Aerosol Science and Technology. 1999;30:100–108. [Google Scholar]
- Li C.S., Lin Y.C. Sampling performance of impactors for bacterial bioaerosols. Aerosol Science and Technology. 1999;30:280–287. [Google Scholar]
- Li C.S., Lin Y.C. Sampling performance of impactors for fungal spores and yeast cells. Aerosol Science and Technology. 1999;31:226–230. [Google Scholar]
- Li C.S., Lin Y.C. Storage effects on bacterial concentration: determination of impinger and filter samples. Science of the Total Environment. 2001;278:231–237. doi: 10.1016/s0048-9697(01)00654-4. [DOI] [PubMed] [Google Scholar]
- Lin W.H., Li C.S. The effect of sampling time and flow rates on the bioefficiency of three fungal spore sampling methods. Aerosol Science and Technology. 1998;28:511–522. [Google Scholar]
- Lin W.H., Li C.S. Collection efficiency and culturability of impingement into a liquid for bioaerosols of fungal spores and yeast cells. Aerosol Science and Technology. 1999;30:109–118. [Google Scholar]
- Lin W.H., Li C.S. Evaluation of impingement and filtration methods for yeast bioaerosol sampling. Aerosol Science and Technology. 1999;30:119–126. [Google Scholar]
- Lin W.H., Li C.S. Influence of storage on the fungal concentration determination of impinger and filter samples. American Industrial Hygiene Association Journal. 2003;64:102–107. doi: 10.1080/15428110308984798. [DOI] [PubMed] [Google Scholar]
- Macher J.M., Willeke K. Performance criteria for bioaerosol samplers. Journal of Aerosol Science. 1992;23:647–650. [Google Scholar]
- Nevalainen A., Willeke K., Liebhaber F., Pastuszka J., Burge H., Henningson E. Bioaerosol Sampling. In: Willeke K., Baron P.A., editors. Aerosol measurement: principles, techniques, and applications. Van Nostrand Reinhold; New York: 1993. pp. 471–492. [Google Scholar]
- Thorne P.S., Kiekhaefer M.S., Whitten P., Donham K. Comparison of bioaerosol sampling methods in barns housing swine. Applied and Environmental Microbiology. 1992;58:2543–2551. doi: 10.1128/aem.58.8.2543-2551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouwborst T., de Jong J.C. Mechanism of the inactivation of the bacteriophage T 1 in aerosols. Applied Microbiology. 1972;23:938–941. doi: 10.1128/am.23.5.938-941.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouwborst T., de Jong J.C. Interaction of some factors in the mechanism of inactivation of bacteriophage MS2 in aerosols. Applied Microbiology. 1973;26:252–257. doi: 10.1128/am.26.3.252-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouwborst T., de Jong J.C., Winkler K.C. Mechanism of inactivation in aerosols of bacteriophage T1. Journal of General Virology. 1972;15:235–242. doi: 10.1099/0022-1317-15-3-235. [DOI] [PubMed] [Google Scholar]
- Tyrrell D.A.J. The spread of viruses of the respiratory tract by the airborne route. Symposia of the Society for General Microbiology. 1967;17:286–306. [Google Scholar]
- Vidaver A.K., Koski R.K., Van Etten J.L. Bacteriophage phi 6: a lipid-containing virus of Pseudomonas phaseolicola. Journal of Virology. 1973;11:799–850. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolwine J.D., Gerberding J.L. Effect of testing method on apparent activities of antiviral disinfectants and antiseptics. Antimicrob Agents Chemother. 1995;39:921–923. doi: 10.1128/aac.39.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]


